Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Measurement of Clinical and Metabolic Parameters
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
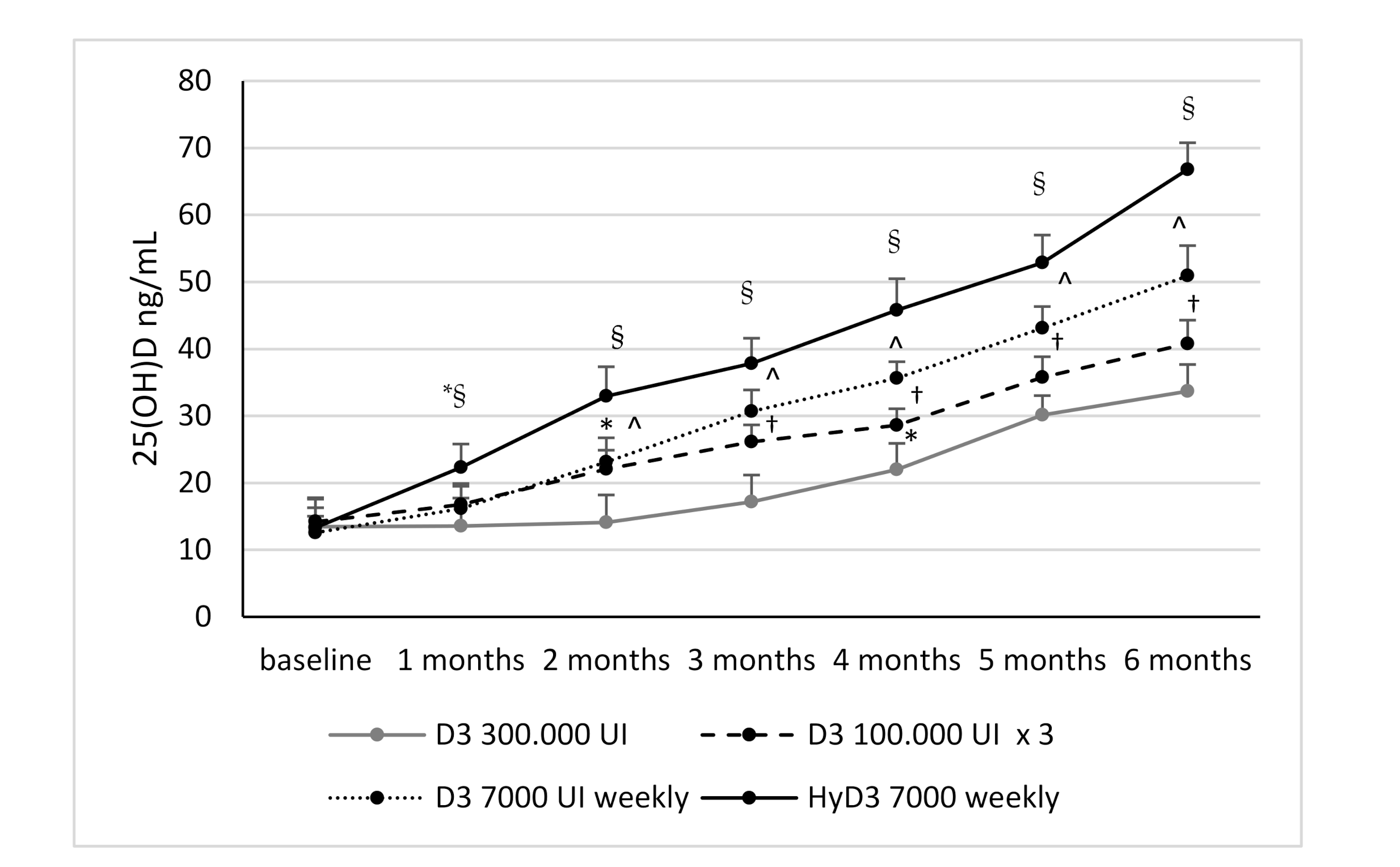
3.2. Effects of Vitamin D Supplementation on Serum 25(OH)D Levels and Serum 1,25-Dihydroxyvitamin D Levels and Biochemical Markers
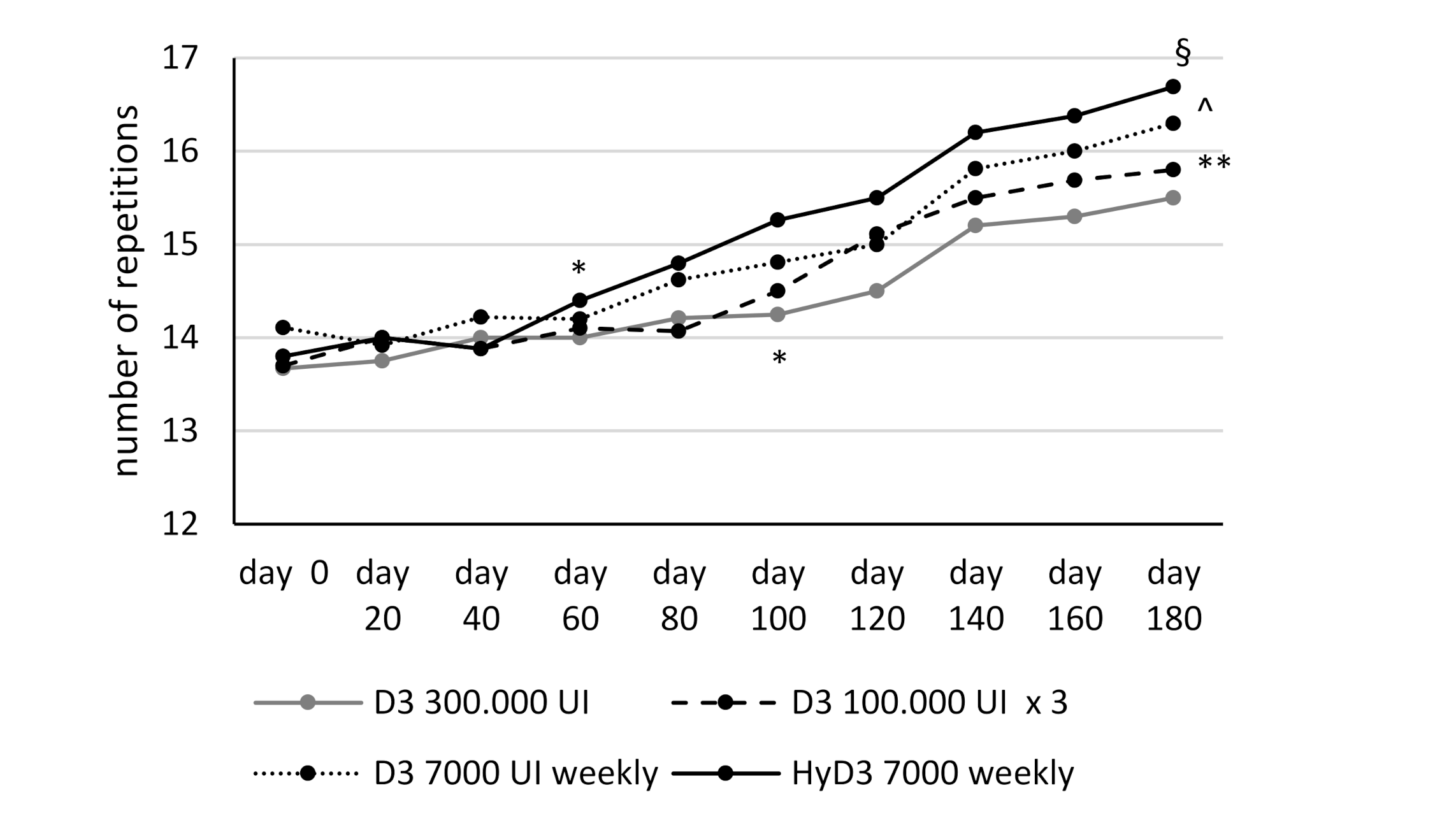
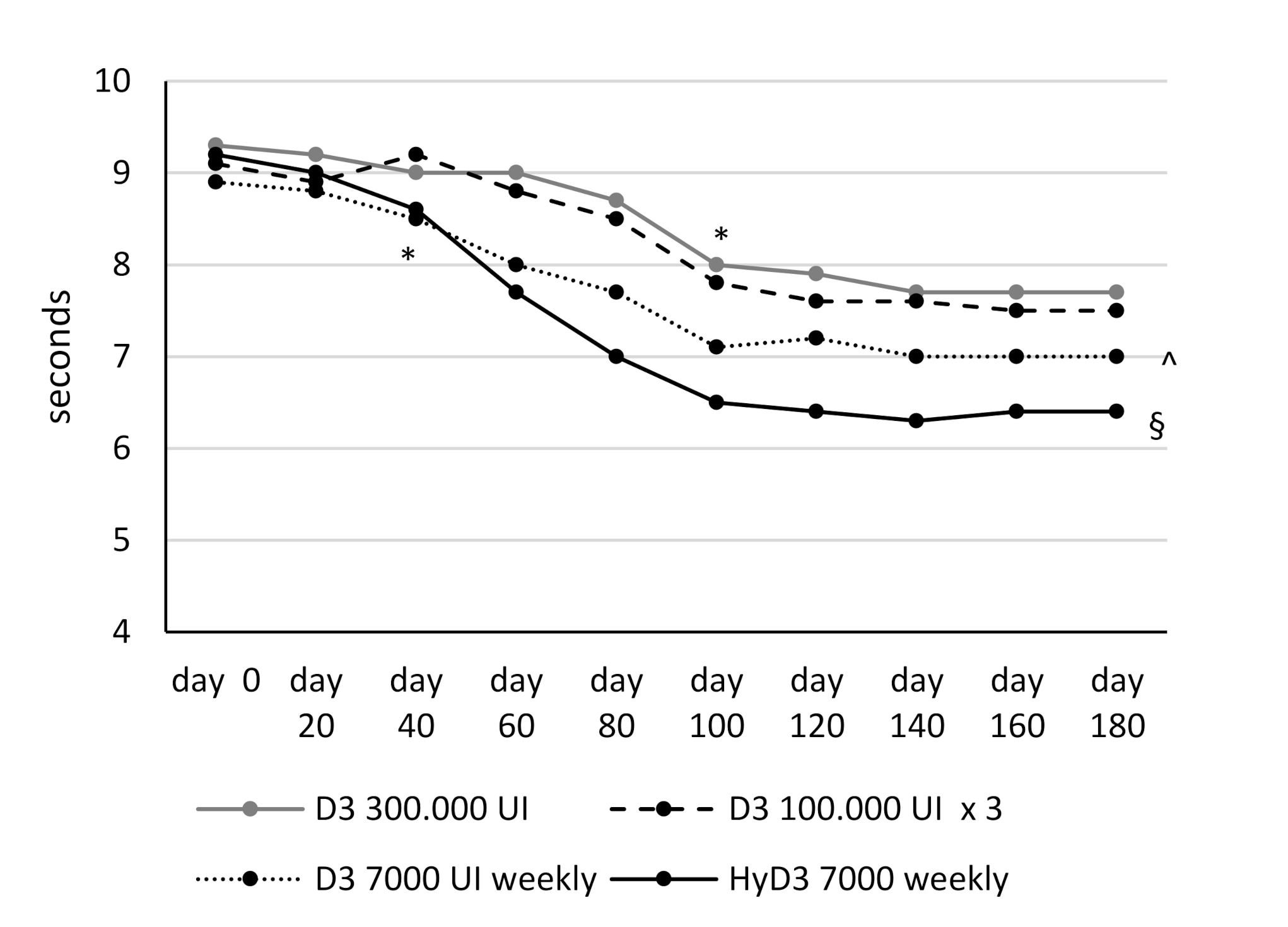
3.3. Effects of Vitamin D Supplementation on Muscular Lower Extremity Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Ooms, M.E.; Roos, J.C.; Bezemer, P.D.; van der Vijgh, W.J.; Bouter, L.M.; Lips, P. Prevention of bone loss by vitamin D supplementation in elderly women: A randomized double- blind trial. J. Clin. Endocrinol. Metab. 1995, 80, 1052–1258. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28, Erratum in: Am. J. Clin. Nutr. 2006, 84, 1253. [Google Scholar] [CrossRef]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Andersen, H.; Charles, P.; Eriksen, E.F. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif. Tissue Int. 2000, 66, 419–424. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Annweiler, C.; Henni, S.; Walrand, S.; Montero-Odasso, M.; Duque, G.; Duval, G.T. Vitamin D and walking speed in older adults: Systematic review and meta-analysis. Maturitas 2017, 106, 8–25. [Google Scholar] [CrossRef]
- Iolascon, G.; Mauro, G.L.; Fiore, P.; Cisari, C.; Benedetti, M.G.; Panella, L.; De Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicentre retrospective study. Eur. J. Phys. Rehabil. Med. 2018, 54, 676–682. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bouvard, B.; Annweiler, C.; Sallé, A.; Beauchet, O.; Chappard, D.; Audran, M.; Legrand, E. Extraskeletal effects of vitamin D: Facts, uncertainties, and controversies. Jt. Bone Spine 2011, 78, 10–16. [Google Scholar] [CrossRef]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A. Which vitamin D oral supplement is best for postmenopausal women? Curr. Osteoporos. Rep. 2012, 10, 251–257. [Google Scholar] [CrossRef]
- Chao, Y.S.; Brunel, L.; Faris, P.; Veugelers, P.J. The importance of dose, frequency and duration of vitamin D supplementation for plasma 25-hydroxyvitamin D. Nutrients 2013, 5, 4067–4078. [Google Scholar] [CrossRef]
- Ish-Shalom, S.; Segal, E.; Salganik, T.; Raz, B.; Bromberg, I.L.; Vieth, R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J. Clin. Endocrinol. Metab. 2008, 93, 3430–3435. [Google Scholar] [CrossRef]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D.; Drezner, M.K. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J. Clin. Endocrinol. Metab. 2011, 96, 981–988. [Google Scholar] [CrossRef]
- Black, L.J.; Anderson, D.; Clarke, M.W.; Ponsonby, A.L.; Lucas, R.M. Ausimmune Investigator Group. Analytical Bias in the Measurement of Serum 25-Hydroxyvitamin D Concentrations Impairs Assessment of Vitamin D Status in Clinical and Research Settings. PLoS ONE 2015, 10, e0135478. [Google Scholar]
- Khant, N.; Dani, V.B.; Patel, P.; Rathod, R. Establishing the reference value for “timed up-and-go” test in healthy adults of Gujarat, India. J. Educ. Health Promot. 2018, 7, 62. [Google Scholar] [CrossRef]
- Stamp, T.C.; Haddad, J.G.; Twigg, C.A. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet 1977, 1, 1341–1343. [Google Scholar] [CrossRef]
- Barger-Lux, M.J.; Heaney, R.P.; Dowell, S.; Chen, T.C.; Holick, M.F. Vitamin D and its major metabolites: Serum levels after graded oral dosing in healthy men. Osteoporos. Int. 1998, 8, 222–230. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Stöcklin, E.; Sidelnikov, E.; Willett, W.C.; Edel, J.O.; Stähelin, H.B.; Wolfram, S.; Jetter, A.; Schwager, J.; et al. Oral supplementation with 25(OH)D3 versus vitamin D3: Effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J. Bone Miner. Res. 2012, 27, 160–169. [Google Scholar] [CrossRef]
- Mastaglia, S.R.; Mautalen, C.A.; Parisi, M.S.; Oliveri, B. Vitamin D2 dose required to rapidly increase 25(OH)D levels in osteoporotic women. Eur. J. Clin. Nutr 2006, 60, 681–687. [Google Scholar]
- Chel, V.; Wijnhoven, H.A.H.; Smit, J.H.; Ooms, M.; Lips, P.T.A.M. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos. Int. 2008, 19, 663–671. [Google Scholar]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Quesada-Gómez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar]
- Jetter, A.; Egli, A.; Dawson-Hughes, B.; Staehelin, H.B.; Stoecklin, E.; Goessl, R.; Henschkowski, J.; Bischoff-Ferrari, H.A. Pharmacokinetics of oral vitamin D3 and calcifediol. Bone 2014, 59, 14–19. [Google Scholar]
- Rossini, M.; Viapiana, O.; Gatti, D.; James, G.; Girardello, S.; Adami, S. The long term correction of vitamin D deficiency: Comparison between di_erent treatments with vitamin D in clinical practice. Minerva Med. 2005, 96, 1–7. [Google Scholar]
- Navarro-Valverde, C.; Sosa-Henríquez, M.; Alhambra-Expósito, M.R.; Quesada-Gómez, J.M. Vitamin D3 and calcidiol are not equipotent. J. Steriod. Biochem. Mol. Biol. 2016, 164, 205–208. [Google Scholar]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef]
- Corachán, A.; Ferrero, H.; Aguilar, A.; Garcia, N.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil. Steril. 2019, 111, 397–407. [Google Scholar] [CrossRef]
- Rezagholizadeh, F.; Keshavarz, S.A.; Djalali, M.; Rad, E.Y.; Alizadeh, S.; Javanbakht, M.H. Vitamin D3 supplementation improves serum SFRP5 and Wnt5a levels in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Int. J. Vitam. Nutr. Res. 2018, 88, 73–79. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Xin, N.; Yuan, Y.; Zhang, Q.; Gong, P.; Wu, Y. 1α,25-Dihydroxyvitamin D3 promotes osteogenesis by promoting Wnt signaling pathway. J. Steroid Biochem. Mol. Biol. 2017, 174, 153–160. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Corrado, A.; Gaudio, A.; Ruggieri, S.; Ribatti, D. In vitro and in vivo angiogenic activity of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF and vitamin D3 treatment. Regul. Pept. 2013, 184, 81–84. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin. Exp. Med. 2014, 14, 275–283. [Google Scholar] [CrossRef]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef]
- Skaria, J.; Katiyar, B.C.; Srivastava, T.P.; Dube, B. Myopathy and neuropathy associated with osteomalacia. Acta Neurol. Scand. 1975, 51, 37–58. [Google Scholar] [CrossRef]
- Young, A.; Edwards, R.H.T.; Jones, D.A.; Brenton, D.P. Quadriceps muscle strength and fibre size during the treatment of osteomalacia. In Stokes IAF, Mechanical Factors and the Skeleton; Libbey: London, UK, 1981; Volume 12, pp. 137–145. [Google Scholar]
- Glerup, H.; Eriksen, E.F. Osteomalacia and servere vitamin D deficiency—A review of the clinical and paraclinical findings and guidelines for the treatment with vitamin D. Ugeskr. Laeger. 1999, 161, 2515–2521. [Google Scholar]
- Young, A.; Brenton, D.P.; Edwards, R. Analysis of muscle weakness in osteomalacia. Clin. Sci. Mol. Med. 1978, 54, 31. [Google Scholar]
- Tanner, S.B.; Harwell, S.A. More than healthy bones: A review of vitamin D in muscle health. Ther. Adv. Musculoskelet. Dis. 2015, 7, 152–159. [Google Scholar] [CrossRef]
- Annweiler, C.; Montero-Odasso, M.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Fall prevention and vitamin D in the elderly: An overview of the key role of the non-bone effects. J. Neuroeng. Rehabil. 2010, 7, 50. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Staehelin, H.B.; Orav, J.E.; Stuck, A.E.; Theiler, R.; Wong, J.B.; Egli, A.; Kiel, D.P.; Henschkowski, J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomized controlled trials. BMJ 2009, 339, b3692. [Google Scholar] [CrossRef]
- Murad, M.H.; Elamin, K.B.; Abu Elnour, N.O.; Elamin, M.B.; Alkatib, A.A.; Fatourechi, M.M.; Almandoz, J.P.; Mullan, R.J.; Lane, M.A.; Liu, H.; et al. Clinical review: The effect of vitamin D on falls: A systematic review and meta- analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2997–3006. [Google Scholar] [CrossRef]
- Annweiler, C.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Vitamin D-related changes in physical performance: A systematic review. J. Nutr. Health Aging 2009, 13, 893–898. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta- analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic. Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef]
- Rosendahl-Riise, H.; Spielau, U.; Ranhoff, A.H.; Gudbrandsen, O.A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 3–15. [Google Scholar] [CrossRef]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar] [CrossRef]

| Group 1 (28) D3 300,000 IU Single Dose | Group 2 (26) D3 100,000 IU Monthly | Group 3 (27) D3 7000 IU Weekly | Group 4 (26) HyD3 7000 IU Weekly | p | |
|---|---|---|---|---|---|
| Age | 60.51 ± 5.1 | 58.3 ± 7.4 | 63.4 ± 5.5 | 60.9 ± 8.1 | ns |
| BMI | 24.8 ± 2.3 | 25.9 ± 0.45 | 23.8 ± 1.5 | 23.3 ± 1.2 | ns |
| SST (n) | 13.6 ± 1.07 | 13.7 ± 1.19 | 14.1 ± 0.8 | 13.8 ± 1.14 | ns |
| TUG (seconds) | 9.36 ± 1 | 9.19 ± 1.03 | 8.9 ± 1.01 | 9.2 ± 1.04 | ns |
| Serum 25(OH)D | 13.46 ± 4.3 | 14.2 ± 3.3 | 12.5 ± 2.46 | 13.3 ± 2.9 | ns |
| Serum Ca | 9.9 (0.93) | 9.9 (0.60) | 9.4 (1.8) | 9.1 (1.5) | ns |
| Serum P | 3.35 (0.3) | 3.3 (0.7) | 3.3 (0.28) | 3.6 (0.35) | ns |
| PTH | 33.5 (18.28) | 37 (6.27) | 35.5(4.1) | 28.3 (8.08) * | 0.0001 |
| ALP | 50.5 (12.17) | 50.1 (8.45) | 73.2 (9.4) ** | 70.5 (7.3) ** | 0.0001 |
| Baseline | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | ||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Ca | 9.9 (0.9) | 9.7 (0.8) | 9.6 (0.8) | 9.7 (0.5) | 9.6 (0.6) | 9.6 (0.6) | 9.4 (0.5) |
| P | 3.3 (0.3) | 3.5 (0.4) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.6 (0.5) | 3.5 (0.4) | |
| PTH | 33.5 (18.3) | 30.6 (9.7) | 30 (6.3) | 33.1 (11.7) | 32.1 (8.4) | 33.4 (7.8) | 32.8 (10.4) | |
| ALP | 50.5 (12.2) | 66.8 (12.8) | 67 (8.2) | 67.3 (5.1) | 69.2 (6.4) | 69.2 (9.7) | 70.3 (10.3) | |
| Group 2 | Ca | 9.9 (0.6) | 9.7 (0.5) | 9.4 (0.5) | 9.4 (0.6) | 9.6 (0.6) | 9.5 (0.4) | 9.5 (0.5) |
| P | 3.3 (0.7) | 3.3 (0.5) | 3.6 (0.2) | 3.5 (0.3) | 3.5 (0.4) | 3.6 (0.4) | 3.6 (0.6) | |
| PTH | 37 (6.3) | 40.1 (7.7) | 35.7 (7.5) | 39.5 (8.3) | 44.4 (7.8) | 41.4 (6.3) | 42.7 (13.1) | |
| ALP | 50.1 (8.4) | 71.4 (11.1) | 64.8 (9.2) | 69.7 (9.9) | 62.2 (8.1) | 61.3 (7.6) | 66.4 (7.5) | |
| Group 3 | Ca | 9.4 (1.8) | 9.4 (0.7) | 9.5 (0.7) | 9.6 (0.4) | 9.4 (0.6) | 9.6 (0.7) | 9.5 (0.5) |
| P | 3.3 (0.4) | 3.4 (0.5) | 3.5 (0.6) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | |
| PTH | 35.5 (4.1) | 40.2 (8.3) | 40.2 (8.3) | 40 (12) | 41.3 (7.5) | 38.4 (5.8) | 34.2 (7.7) | |
| ALP | 73.2 (9.4) | 68.9 (13.9) | 70.5 (9.4) | 71.2 (8.9) | 71.9 (6.3) | 70.2 (6.6) | 70.2 (8.5) | |
| Group 4 | Ca | 9.1 (1.5) | 9.4 (0.7) | 9.1 (0.8) | 9.2 (0.5) | 9.1 (0.7) | 9.4 (0.5) | 9.3 (0.6) |
| P | 3.6 (0.4) | 3.6 (0.5) | 3.6 (0.6) | 3.7 (0.8) | 3.7 (0.7) | 3.6 (0.7) | 3.7 (0.9) | |
| PTH | 28.3 (8.1) | 30.2 (7.3) | 30.1 (9) | 30 (6.2) | 32.9 (7.1) | 33.5 (8.2) | 31.2 (6.7) | |
| ALP | 70.5 (7.3) | 75.9 (10.3) | 69.5 (6.1) | 70.1 (9.1) | 70.1 (4.8) | 70.3 (12.4) | 69.5 (12.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrado, A.; Rotondo, C.; Cici, D.; Berardi, S.; Cantatore, F.P. Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study. Nutrients 2021, 13, 380. https://doi.org/10.3390/nu13020380
Corrado A, Rotondo C, Cici D, Berardi S, Cantatore FP. Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study. Nutrients. 2021; 13(2):380. https://doi.org/10.3390/nu13020380
Chicago/Turabian StyleCorrado, Addolorata, Cinzia Rotondo, Daniela Cici, Stefano Berardi, and Francesco Paolo Cantatore. 2021. "Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study" Nutrients 13, no. 2: 380. https://doi.org/10.3390/nu13020380
APA StyleCorrado, A., Rotondo, C., Cici, D., Berardi, S., & Cantatore, F. P. (2021). Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study. Nutrients, 13(2), 380. https://doi.org/10.3390/nu13020380








