Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Randomized Controlled Trial Design and Setting
2.2. Participants
2.3. Inclusion and Exclusion Criteria
2.4. Sample Size Calculation
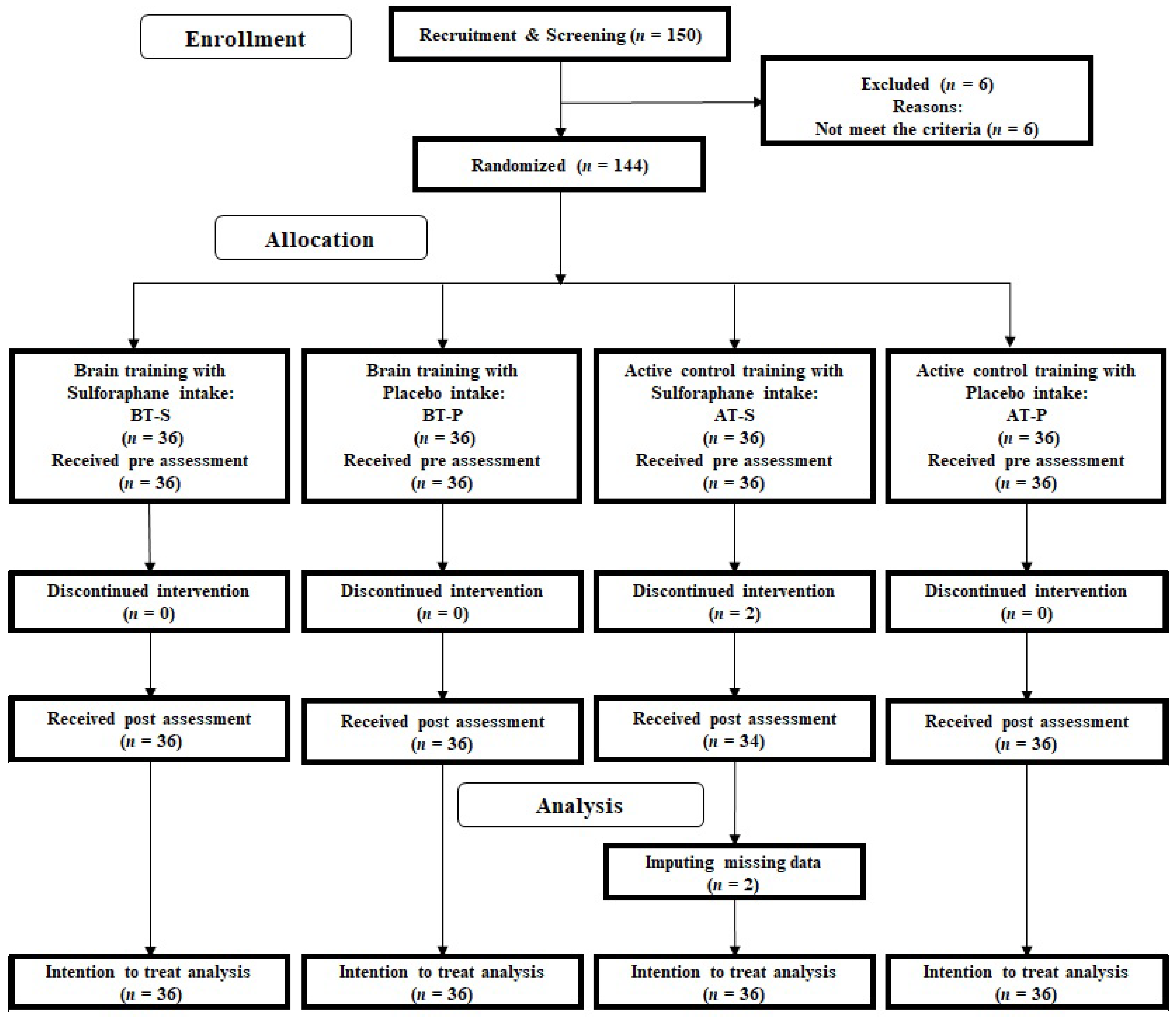
2.5. Randomization
2.6. Overview of the Intervention
2.7. Video Game Training
2.8. Sulforaphane and Placebo Supplements
2.9. Cognitive Function Measurement
2.10. Emotional State Measurement
2.11. Urine Analysis
2.12. Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, R.S.; Wang, T.; Yu, L.; Bennett, D.A.; Boyle, P.A. Normative Cognitive Decline in Old Age. Ann. Neurol. 2020, 87, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Njegovan, V.; Man-Son-Hing, M.; Mitchell, S.L.; Molnar, F. The Hierarchy of Functional Loss Associated With Cognitive Decline in Older Persons. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M638–M643. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-L.; Chu, H.; Tsai, J.-C.; Liu, D.; Chen, Y.-R.; Yang, H.-L.; Chou, K.-R. The effect of cognitive-based training for the healthy older people: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0176742. [Google Scholar] [CrossRef]
- Klimova, B.; Valis, M. Nutritional Interventions as Beneficial Strategies to Delay Cognitive Decline in Healthy Older Individuals. Nutrients 2018, 10, 905. [Google Scholar] [CrossRef]
- Toman, J.; Klímová, B.; Vališ, M. Multidomain Lifestyle Intervention Strategies for the Delay of Cognitive Impairment in Healthy Aging. Nutrients 2018, 10, 1560. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Noëlle-Cuffi, M.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Bell, K.E.; Fang, H.; Snijders, T.; Allison, D.J.; Zulyniak, M.A.; Chabowski, A.; Parise, G.; Phillips, S.M.; Heisz, J.J. A Multi-Ingredient Nutritional Supplement in Combination With Resistance Exercise and High-Intensity Interval Training Improves Cognitive Function and Increases N-3 Index in Healthy Older Men: A Randomized Controlled Trial. Front. Aging Neurosci. 2019, 11, 107. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Smith, P.J.; Mabe, S.; Hinderliter, A.; Lin, P.H.; Liao, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Kraus, W.E.; Doraiswamy, P.M.; et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 2019, 92, E212–E223. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Akitsuki, Y.; Shigemune, Y.; Sekiguchi, A.; Kotozaki, Y.; Tsukiura, T.; Yomogida, Y.; et al. Brain Training Game Improves Executive Functions and Processing Speed in the Elderly: A Randomized Controlled Trial. PLoS ONE 2012, 7, e29676. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Kambara, T.; Sekiguchi, A.; Miyauchi, C.M.; Kotozaki, Y.; Nouchi, H.; et al. Brain Training Game Boosts Executive Functions, Working Memory and Processing Speed in the Young Adults: A Randomized Controlled Trial. PLoS ONE 2013, 8, e55518. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M.H. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland Health Study. Br. J. Nutr. 2010, 104, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Zhao, J.; Orsi, S.A.; Zhang, M.; Moore, A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009, 460, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Shiina, A.; Kanahara, N.; Sasaki, T.; Oda, Y.; Hashimoto, T.; Hasegawa, T.; Yoshida, T.; Iyo, M.; Hashimoto, K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015, 13, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Kawata, N.Y.D.S.; Saito, T.; Himmelmeier, R.M.; Nakamura, R.; Nouchi, H.; Kawashima, R. Dorsolateral Prefrontal Cortex Activity during a Brain Training Game Predicts Cognitive Improvements after Four Weeks’ Brain Training Game Intervention: Evidence from a Randomized Controlled Trial. Brain Sci. 2020, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A frontal assessment battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Sugishita, K.; Sugishita, M.; Hemmi, I.; Asada, T.; Tanigawa, T. A Validity and Reliability Study of the Japanese Version of the Geriatric Depression Scale 15 (GDS-15-J). Clin. Gerontol. 2017, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, T.; Taki, Y.; Kanno, A.; Akimoto, Y.; Ihara, M.; Yokoyama, R.; Kotozaki, Y.; Nouchi, R.; Sekiguchi, A.; Takeuchi, H.; et al. Effects of Different Types of Cognitive Training on Cognitive Function, Brain Structure, and Driving Safety in Senior Daily Drivers: A Pilot Study. Behav. Neurol. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Nozawa, T.; Sekiguchi, A.; Kawashima, R. Reading Aloud and Solving Simple Arithmetic Calculation Intervention (Learning Therapy) Improves Inhibition, Verbal Episodic Memory, Focus Attention and Processing Speed in Healthy Elderly People: Evidence from a Randomized Controlled Trial. Front. Hum. Neurosci. 2016, 10, 217. [Google Scholar] [CrossRef]
- Nouchi, R.; Saito, T.; Nouchi, H.; Kawashima, R. Small Acute Benefits of 4 Weeks Processing Speed Training Games on Processing Speed and Inhibition Performance and Depressive Mood in the Healthy Elderly People: Evidence from a Randomized Control Trial. Front. Aging Neurosci. 2016, 8, 302. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- McCarrey, A.C.; An, Y.; Kitner-Triolo, M.H.; Ferrucci, L.; Resnick, S.M. Sex differences in cognitive trajectories in clinically normal older adults. Psychol. Aging 2016, 31, 166–175. [Google Scholar] [CrossRef]
- Nouchi, R.; Kobayashi, A.; Nouchi, H.; Kawashima, R. Newly Developed TV-Based Cognitive Training Games Improve Car Driving Skills, Cognitive Functions, and Mood in Healthy Older Adults: Evidence From a Randomized Controlled Trial. Front. Aging Neurosci. 2019, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of Humans by Plant Glucosinolates: Efficiency of Conversion of Glucosinolates to Isothiocyanates by the Gastrointestinal Microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Uno, M.; Kasai, K.; Koyama, K.; Kim, Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin. Neurosci. 2006, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D.A. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Sekiguchi, A.; Nouchi, H.; Kawashima, R. Beneficial effects of reading aloud and solving simple arithmetic calculations (learning therapy) on a wide range of cognitive functions in the healthy elderly: Study protocol for a randomized controlled trial. Trials 2012, 13, 32. [Google Scholar] [CrossRef]
- Hakoda, Y.; Watanabe, M. Manual for New Stroop Test II; Toyo Physical: Fukuoka, Japan, 2004. [Google Scholar]
- Wechsler, D.A. Wechsler Memory Scale Revised; The Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Peters, M.; Laeng, B.; Latham, K.; Jackson, M.; Zaiyouna, R.; Richardson, C. A Redrawn Vandenberg and Kuse Mental Rotations Test—Different Versions and Factors That Affect Performance. Brain Cogn. 1995, 28, 39–58. [Google Scholar] [CrossRef]
- Heuchert, J.P.; McNair, D.M. POMS-2 Manual: A Profile of Mood States, 2nd ed.; Multi-Health Systems Inc.: North Tonawanda, NY, USA, 2012. [Google Scholar]
- Yokoyama, K.; Watanabe, K. Japanese Version POMS-2 Manual: A Profile of Mood States, 2nd ed.; Kaneko Shobo: Tokyo, Japan, 2015. [Google Scholar]
- Radloff, L.S. The CES-D Scale. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M. Factor structure of the 12-item General Health Questionnaire in the Japanese general adult population. Psychiatry Clin. Neurosci. 2003, 57, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Kensler, T.W.; Chen, J.-G.; Gange, S.J.; Groopman, J.D.; Friesen, M.D. Quantification of Sulforaphane Mercapturic Acid Pathway Conjugates in Human Urine by High-Performance Liquid Chromatography and Isotope-Dilution Tandem Mass Spectrometry. Chem. Res. Toxicol. 2008, 21, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing With Independent Statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef]
- De Giglio, L.; De Luca, F.; Prosperini, L.; Borriello, G.; Bianchi, V.; Pantano, P.; Pozzilli, C. A low-cost cognitive rehabilitation with a commercial video game improves sustained attention and executive functions in multiple sclerosis: A pilot study. Neurorehabilit. Neura. Repair. 2015, 29, 453–461. [Google Scholar] [CrossRef]
- De Giglio, L.; Tona, F.; De Luca, F.; Petsas, N.; Prosperini, L.; Bianchi, V.; Pozzilli, C.; Pantano, P. Multiple Sclerosis: Changes in Thalamic Resting-State Functional Connectivity Induced by a Home-based Cognitive Rehabilitation Program. Radiology 2016, 280, 202–211. [Google Scholar] [CrossRef]
- Schneider, W.J.; McGrew, K. The Cattell-Horn-Carroll model of intelligence. In Contemporary Intellectual Assessment: Theories, Tests, and Issues; Flanagan, D., Harrison, P., Eds.; Guilford: New York, NY, USA, 2012. [Google Scholar]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between Inflammation and Oxidative Stress and Cognitive Decline in the Institutionalized Elderly. Oxidative Med. Cell. Longev. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K. The Role of Selected Bioactive Compounds in the Prevention of Alzheimer’s Disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef]
- Kim, H.V.; Kim, H.Y.; Ehrlich, H.Y.; Choi, S.Y.; Kim, D.J.; Kim, Y. Amelioration of Alzheimer’s disease by neuroprotective effect of sulforaphane in animal model. Amyloid 2012, 20, 7–12. [Google Scholar] [CrossRef]
- Huang, C.; Huang, C.; Chen, D.; Jin, J.; Wu, Y.; Chen, Z. Effects of sulforaphane in the central nervous system. Eur. J. Pharmacol. 2019, 853, 153–168. [Google Scholar] [CrossRef]
| Cognitive Training | BT | AT | ||
|---|---|---|---|---|
| Nutrition | SFN | P | SFN | P |
| Age (years) | 67.97 | 67.42 | 67.59 | 67.86 |
| (3.12) | (4.78) | (4.58) | (4.92) | |
| Sex | Male = 12 Female = 24 | Male = 12 Female = 24 | Male = 12 Female = 24 | Male = 12 Female = 24 |
| MMSE | 28.36 | 28.42 | 28.50 | 28.56 |
| (1.29) | (1.44) | (1.29) | (1.21) | |
| FAB | 14.53 | 14.72 | 14.18 | 14.25 |
| (1.73) | (1.67) | (1.75) | (1.75) | |
| JART | 19.44 | 19.22 | 20.71 | 20.19 |
| (3.64) | (4.75) | (3.28) | (3.55) | |
| GDS | 3.12 | 3.45 | 3.31 | 3.21 |
| (1.04) | (1.21) | (0.91) | (1.24) | |
| WHO-5 | 15.75 | 16.39 | 15.44 | 16.56 |
| (4.59) | (4.67) | (4.31) | (3.04) | |
| GHQ12 | 21.92 | 22.58 | 21.24 | 21.19 |
| (4.28) | (4.74) | (3.69) | (3.85) | |
| POMS | 21.31 | 22.92 | 24.09 | 22.75 |
| (12.47) | (10.42) | (12.05) | (12.62) | |
| CES-D | 11.06 | 11.08 | 9.68 | 9.64 |
| (7.58) | (5.74) | (6.34) | (5.14) | |
| Cognitive Training | BT | AT | ||
|---|---|---|---|---|
| Nutrition | SFN | P | SFN | P |
| LM-immediate | 10.78 | 10.78 | 10.53 | 11.69 |
| (4.57) | (3.60) | (3.42) | (4.63) | |
| LM-delayed | 10.44 | 9.69 | 9.65 | 10.86 |
| (4.36) | (3.55) | (3.27) | (4.54) | |
| Cd | 72.03 | 72.81 | 75.24 | 72.89 |
| (13.48) | (12.27) | (15.81) | (12.46) | |
| SS | 34.39 | 37.19 | 36.29 | 36.44 |
| (4.96) | (6.04) | (6.53) | (6.72) | |
| D-CAT | 45.78 | 46.44 | 47.21 | 47.03 |
| (7.30) | (12.13) | (11.51) | (9.71) | |
| rST | 45.47 | 46.97 | 49.32 | 45.86 |
| (9.07) | (7.69) | (7.21) | (7.54) | |
| ST | 32.67 | 31.97 | 32.85 | 33.67 |
| (8.71) | (7.82) | (8.36) | (7.94) | |
| DS-F | 5.67 | 5.56 | 5.38 | 5.64 |
| (0.83) | (1.05) | (1.02) | (1.07) | |
| DS-B | 4.25 | 4.31 | 4.32 | 4.47 |
| (0.94) | (0.92) | (1.20) | (1.11) | |
| MR | 18.36 | 16.83 | 18.06 | 17.83 |
| (4.65) | (6.10) | (5.83) | (4.66) | |
| Cognitive Training | BT | AT | ||
|---|---|---|---|---|
| Nutrition | SFN | P | SFN | P |
| LM-immediate | −0.33 | −0.33 | 0.19 | −0.89 |
| (3.46) | (2.61) | (4.01) | (3.67) | |
| LM-delayed | 0.39 | 0.64 | 0.97 | −0.39 |
| (3.40) | (2.74) | (3.64) | (3.16) | |
| Cd | 4.94 | 2.78 | 2.42 | 1.33 |
| (4.88) | (5.25) | (6.54) | (4.54) | |
| SS | 3.47 | 1.39 | 1.47 | 0.08 |
| (2.95) | (3.50) | (3.80) | (4.12) | |
| D-CAT | 0.67 | −2.97 | 6.56 | 10.53 |
| (21.66) | (24.49) | (29.28) | (18.34) | |
| rST | 0.81 | −0.06 | 0.64 | 0.67 |
| (4.57) | (4.90) | (4.40) | (4.08) | |
| ST | −0.11 | 0.67 | −0.81 | 1.00 |
| (3.73) | (3.49) | (7.09) | (5.54) | |
| DS-F | 0.31 | 0.11 | 0.44 | 0.56 |
| (1.45) | (1.53) | (1.50) | (1.42) | |
| DS-B | 0.92 | 0.36 | 0.81 | −0.11 |
| (1.56) | (1.50) | (1.55) | (1.75) | |
| MR | 1.06 | 0.56 | 1.06 | 0.08 |
| (5.36) | (4.31) | (5.39) | (3.62) | |
| Cognitive Training | BT | AT | ||
|---|---|---|---|---|
| Nutrition | SFN | P | SFN | P |
| WHO-5 | −0.19 | 0.03 | −0.24 | −1.58 |
| (3.75) | (3.68) | (3.46) | (4.23) | |
| GHQ12 | 0.03 | −1.25 | 0.50 | 0.22 |
| (3.78) | (4.26) | (5.61) | (5.06) | |
| POMS | −2.47 | 0.25 | 0.41 | −0.72 |
| (9.96) | (10.07) | (8.54) | (10.86) | |
| CES-D | −1.81 | -0.39 | −0.32 | −0.50 |
| (5.56) | (5.92) | (5.41) | (5.30) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouchi, R.; Hu, Q.; Saito, T.; Kawata, N.Y.d.S.; Nouchi, H.; Kawashima, R. Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients 2021, 13, 352. https://doi.org/10.3390/nu13020352
Nouchi R, Hu Q, Saito T, Kawata NYdS, Nouchi H, Kawashima R. Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients. 2021; 13(2):352. https://doi.org/10.3390/nu13020352
Chicago/Turabian StyleNouchi, Rui, Qingqiang Hu, Toshiki Saito, Natasha Yuriko dos Santos Kawata, Haruka Nouchi, and Ryuta Kawashima. 2021. "Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial" Nutrients 13, no. 2: 352. https://doi.org/10.3390/nu13020352
APA StyleNouchi, R., Hu, Q., Saito, T., Kawata, N. Y. d. S., Nouchi, H., & Kawashima, R. (2021). Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients, 13(2), 352. https://doi.org/10.3390/nu13020352








