Cortical Oxygenation Changes during Gastric Tube Feeding in Moderate- and Late-Preterm Babies: A NIRS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
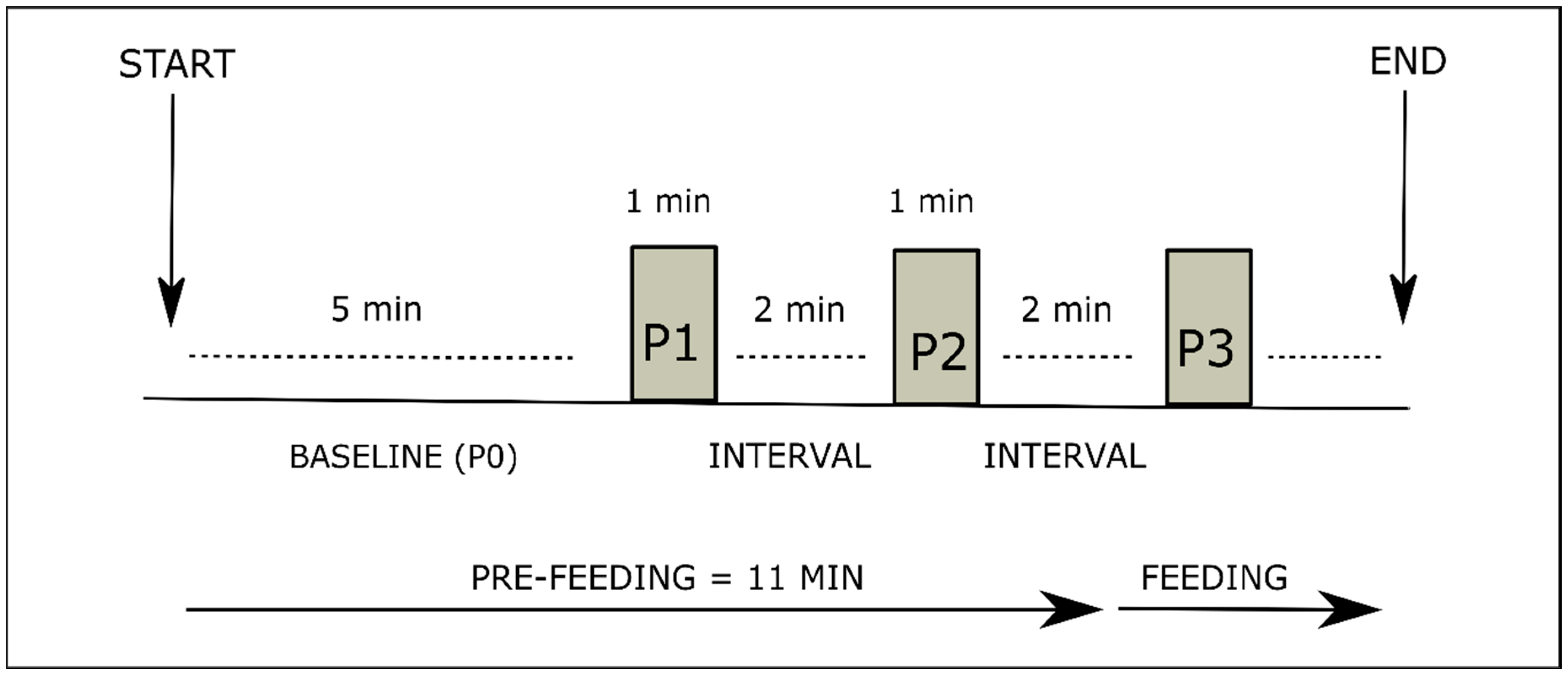
2.2. Assessment Procedure
2.3. Sensory Stimulation
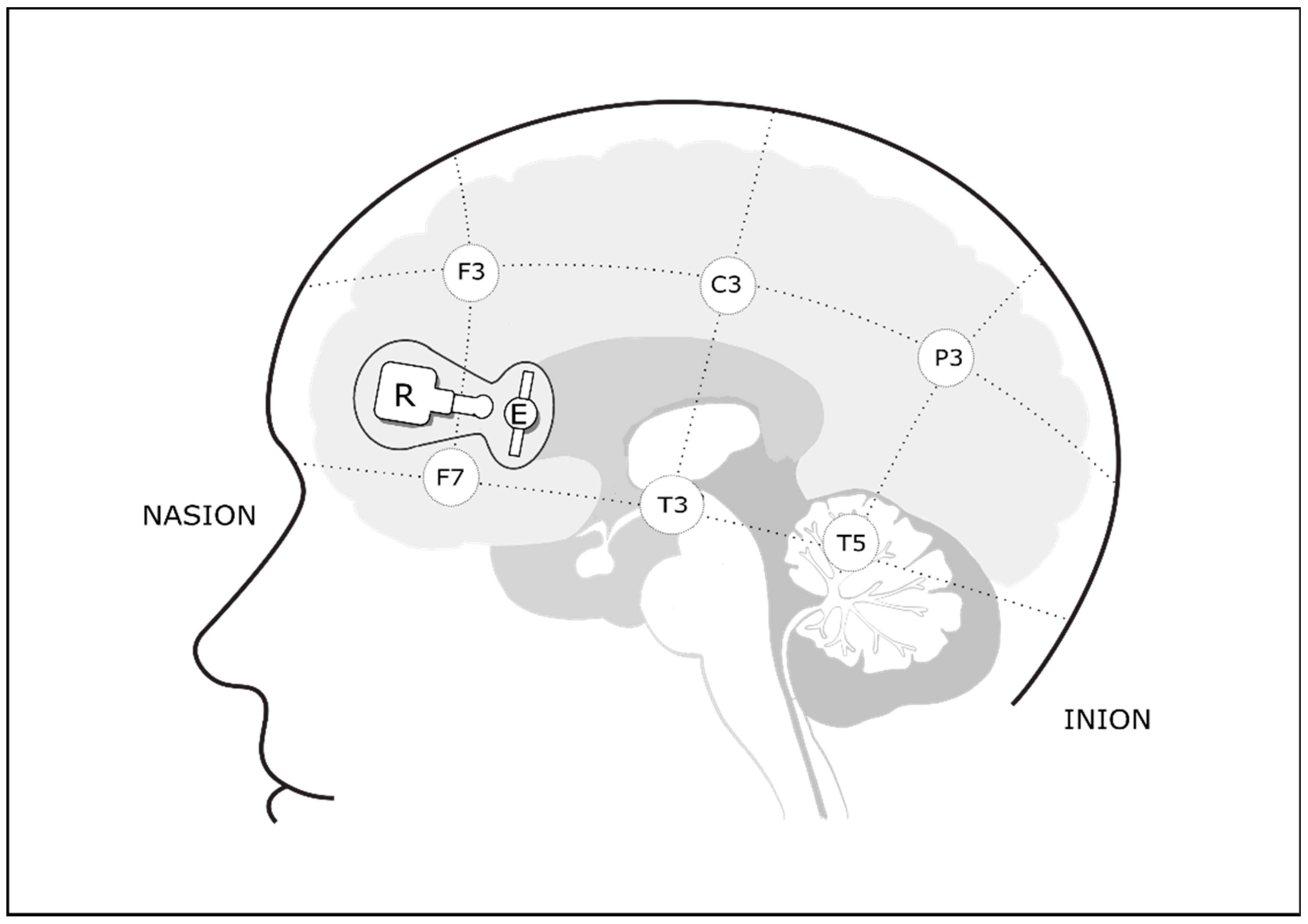
2.4. Cerebral Oxygenation Monitoring
2.5. Data Processing and Statistical Analysis
3. Results
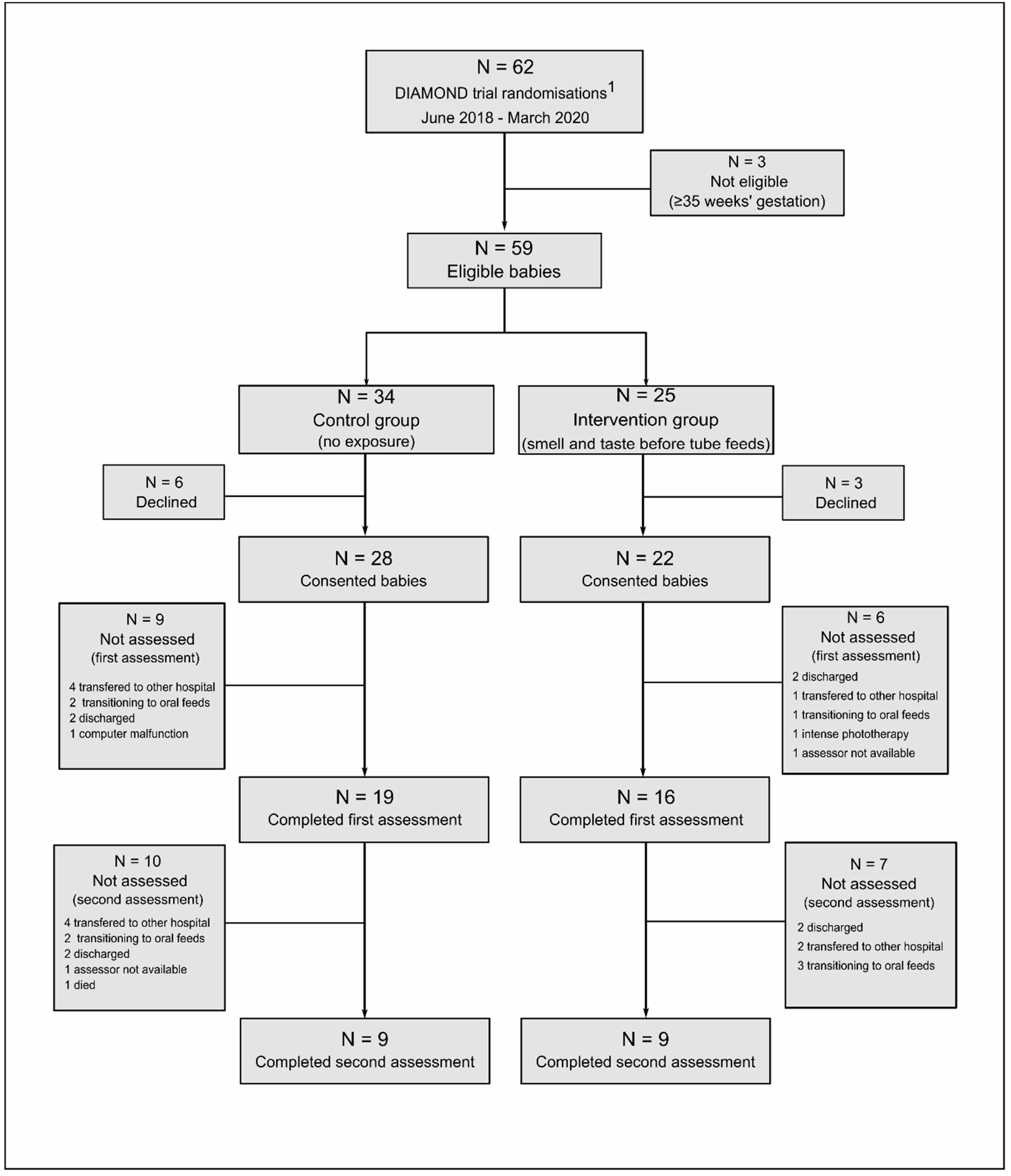
3.1. Study Population
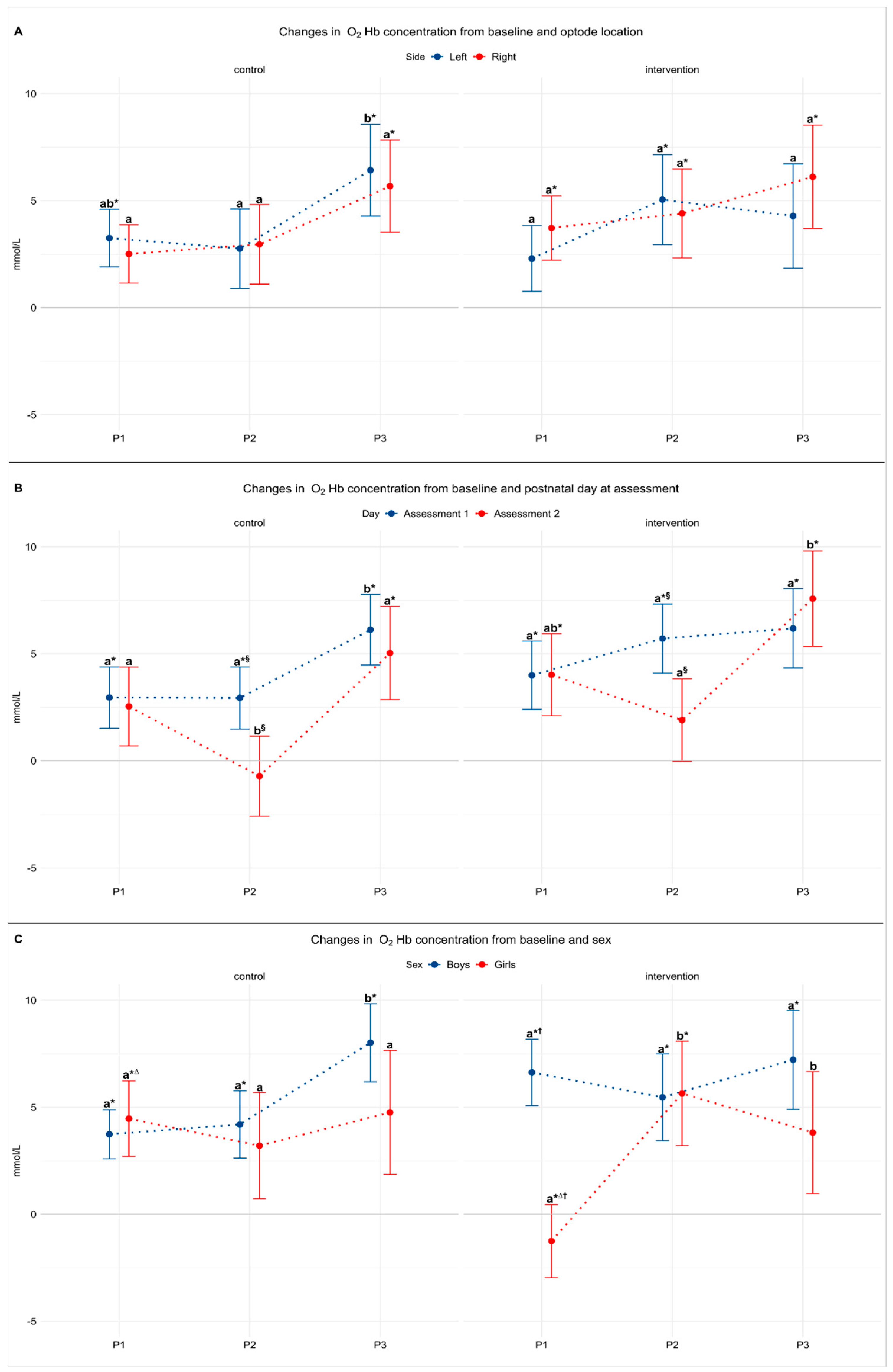
3.2. Model 1: The Effect of Laterality
3.3. Model 2: The Effect of Postnatal Day
3.4. Model 3: The Effect of Sex
4. Discussion
4.1. Main Findings
4.2. The Effect of Side
4.3. The Effect of Postnatal Day
4.4. The Effect of Sex
4.5. OFC Activation without Sensory Stimulation
4.6. The Effect of Feeding
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
National Health and Disability Ethics Committee Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipchock, S.; Reed, D.; Mennella, J. The gustatory and olfactory systems during infance: Implication for development of feeding behaviors in the high risk neonate. Clin. Perinatol. 2011, 38, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Knaapila, A. Genetics of taste and smell: Poisons and pleasures. Prog. Mol. Biol. Transl. Sci. 2010, 94, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Schulkin, J. Anticipatory physiological regulation in feeding biology: Cephalic phase responses. Appetite 2008, 50, 194–206. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, I.E.T.; Rolls, E.T.; Kringelbach, M.L.; McGlone, F.; Phillips, N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 2003, 18, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Dan, I. Functional near-infrared spectroscopy for human brain mapping of taste-related cognitive functions. J. Biosci. Bioeng. 2007, 103, 207–215. [Google Scholar] [CrossRef]
- Avery, J.A.; Liu, A.G.; Ingeholm, J.E.; Riddell, C.D.; Gotts, S.J.; Martin, A. Taste quality representation in the human brain. J. Neurosci. 2020, 40, 1042–1052. [Google Scholar] [CrossRef]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef]
- Fox, P.; Raichle, M.; Mintun, M.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar] [CrossRef]
- Villringer, A.; Chance, B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997, 20, 435–442. [Google Scholar] [CrossRef]
- Brazy, J.E.; Lewis, V.; Mitnick, M.H.; vander Vliet, F.F. Noninvasive monitoring of cerebral oxygenation in preterm infants: Preliminary observations. Pedriatrics 1985, 75, 217. [Google Scholar]
- Dix, L.M.L.; van Bel, F.; Lemmers, P.M.A. Monitoring cerebral oxygenation in neonates: An update. Front. Pediatr. 2017, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aslin, R.N.; Mehler, J. Near-infrared spectroscopy for functional studies of brain activity in human infants: Promise, prospects, and challenges. J. Biomed. Opt. 2005, 10, 011009. [Google Scholar] [CrossRef] [PubMed]
- Bartocci, M.; Winberg, J.; Papendieck, G.; Mustica, T.; Serra, G.; Lagercrantz, H. Cerebral hemodynamic response to unpleasant odors in the preterm newborn measured by near-infrared spectroscopy. Pediatr. Res. 2001, 50, 324–330. [Google Scholar] [CrossRef]
- Bartocci, M.; Winberg, J.; Ruggiero, C.; Bergqvist, L.L.; Serra, G.; Lagercrantz, H. Activation of olfactory cortex in newborn infants after odor stimulation: A functional near-infrared spectroscopy study. Pediatr. Res. 2000, 48, 18–23. [Google Scholar] [CrossRef]
- Aoyama, S.; Toshima, T.; Saito, Y.; Konishi, N.; Motoshige, K.; Ishikawa, N.; Nakamura, K.; Kobayashi, M. Maternal breast milk odour induces frontal lobe activation in neonates: A NIRS study. Early Hum. Dev. 2010, 86, 541–545. [Google Scholar] [CrossRef]
- Frie, J.; Bartocci, M.; Lagercrantz, H.; Kuhn, P. Cortical responses to alien odors in newborns: An fNIRS study. Cereb. Cortex 2018, 28, 3229–3240. [Google Scholar] [CrossRef]
- Frie, J.; Bartocci, M.; Kuhn, P. Neonatal cortical perceptions of maternal breast odours: A fNIRS study. Acta Paediatr. 2020, 109, 1330–1337. [Google Scholar] [CrossRef]
- Harada, H.; Tanaka, M.; Kato, T. Brain olfactory activation measured by near-infrared spectroscopy in humans. J. Laryngol. Otol. 2006, 120, 638–643. [Google Scholar] [CrossRef]
- Okamoto, M.; Dan, H.; Clowney, L.; Yamaguchi, Y.; Dan, I. Activation in ventro-lateral prefrontal cortex during the act of tasting: An fNIRS study. Neurosci. Lett. 2009, 451, 129–133. [Google Scholar] [CrossRef]
- Bingham, P.M.; Churchill, D.; Ashikaga, T. Breast milk odor via olfactometer for tube-fed, premature infants. Behav. Res. Methods 2007, 39, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Raimbault, C.; Saliba, E.; Porter, R.H. The effect of the odour of mother’s milk on breastfeeding behaviour of premature neonates. Acta Paediatr. Int. J. Paediatr. 2007, 96, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Muelbert, M.; Lin, L.; Bloomfield, F.H.; Harding, J.E. Exposure to the smell and taste of milk to accelerate feeding in preterm infants. Cochrane Database Syst. Rev. 2019, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Nasuf, A.W.A.; Ojha, S.; Dorling, J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Bloomfield, F.H.; Harding, J.E.; Meyer, M.P.; Alsweiler, J.M.; Jiang, Y.; Wall, C.R.; Alexander, T.; Asadi, S.; Beker, F.; Cameron-Smith, D.; et al. The DIAMOND trial—DIfferent Approaches to MOderate & late preterm Nutrition: Determinants of feed tolerance, body composition and development: Protocol of a randomised trial. BMC Pediatr. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Kabdebon, C.; Leroy, F.; Simmonet, H.; Perrot, M.; Dubois, J.; Dehaene-Lambertz, G. Anatomical correlations of the international 10–20 sensor placement system in infants. Neuroimage 2014, 99, 342–356. [Google Scholar] [CrossRef]
- Epstein, L.H.; Temple, J.L.; Roemmich, J.N.; Bouton, M.E. Habituation as a determinant of human food intake. Psychol. Rev. 2009, 116, 384–407. [Google Scholar] [CrossRef]
- Doty, R.L.; Cameron, E.L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009, 97, 213–228. [Google Scholar] [CrossRef]
- Bröring, T.; Oostrom, K.J.; Lafeber, H.N.; Jansma, E.P.; Oosterlaan, J. Sensory modulation in preterm children: Theoretical perspective and systematic review. PLoS ONE 2017, 12, 1–23. [Google Scholar] [CrossRef]
- VandenBerg, K.A. Individualized developmental care for high risk newborns in the NICU: A practice guideline. Early Hum. Dev. 2007, 83, 433–442. [Google Scholar] [CrossRef]
- Maßberg, D.; Hatt, H. Human olfactory receptors: Novel cellular functions outside of the nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin cells of the human gut: Sensors for spices and odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Calvo, S.S.C.; Egan, J.M. The endocrinology of taste receptors. Nat. Rev. Endocrinol. 2015, 11, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Omari, T.I.; Barnett, C.P.; Benninga, M.A.; Lontis, R.; Goodchild, L.; Haslam, R.R.; Dent, J.; Davidson, G.P. Mechanisms of gastro-oesophageal reflux in preterm and term infants with reflux disease. Gut 2002, 51, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Vandenplas, Y.; Singendonk, M.; Cabana, M.; Dilorenzo, C.; Gottrand, F.; Gupta, S.; Langendam, M.; Staiano, A.; Thapar, N.; et al. Pediatric gastroesophageal reflux clinical practice guidelines: Joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2018, 66, 516–554. [Google Scholar] [CrossRef]
| Study Group | |||
|---|---|---|---|
| Control (n = 19) | Intervention (n = 16) | p | |
| Participant characteristics: | |||
| Boys | 14 (74) | 10 (63) | 0.59 |
| Gestational age, weeks | 33 (32–34) | 33 (32–34) | 0.93 |
| Birth weight, grams | 1883 (306) | 1909 (393) | 0.81 |
| Birth length, cm | 43.6 (2.7) | 43 (3.1) | 0.50 |
| Birth head circumference, cm | 30.6 (1.2) | 30.4 (2.0) | 0.81 |
| Gestational age, weeks | 33 (32–34) | 33 (32–34) | 0.93 |
| Discharge weight, grams | 2557 (299) | 2411 (375) | 0.16 |
| Duration of NICU stay, days | 28 (11–51) | 24 (10–36) | 0.30 |
| In-hospital weight gain, g/Kg·day | 10.8 (4.1) | 9.0 (4.6) | 0.28 |
| Maternal age, years | 35 (21–50) | 32 (18–45) | 0.20 |
| Birth by Cesarean section | 14 (74) | 13 (81) | 0.71 |
| Antenatal corticosteroid received | 18 (95) | 13 (81) | 0.57 |
| At assessment 1: | |||
| Postnatal age at assessment, days | 5 (3–6) | 4 (3–5) | 0.15 |
| Volume of tube feed, mL | 15 (2–43) | 18 (4–38) | 0.61 |
| Duration of assessment, min | 20 (14–31) | 21 (11–41) | 0.91 |
| Milk fed during assessment | 0.61 | ||
| Breastmilk only | 16 (84.2) | 12 (75) | |
| Mixed feeding A | 1 (5.3) | 3 (18.7) | |
| Infant formula | 2 (10.5) | 1 (6.3) | |
| At assessment 2: | |||
| Total participants | 9 (47) | 9 (56) | |
| Boys | 8 (89) | 8 (89) | |
| Postnatal age at assessment, days | 10 (8–11) | 10 (8–12) | 0.86 |
| Volume of tube feed, mL | 40 (26–50) | 29 (16–48) | 0.30 |
| Duration of assessment, min | 27 (24–35) | 26 (22–44) | 0.80 |
| Milk fed during assessment | 0.38 | ||
| Breastmilk only | 8 (88.9) | 7 (77.8) | |
| Mixed feeding A | 0 | 0 | |
| Infant formula | 1 (11.1) | 2 (22.2) | |
| Control Group (n = 19) | Intervention Group (n = 16) | |||||
|---|---|---|---|---|---|---|
| Period | Period | |||||
| Model Estimates | P1 | P2 | P3 | P1 (Smell) | P2 (Taste) | P3 |
| (A) Laterality:F(2,59) = 0.88 p = 0.4 | ||||||
| Left side | 3.2 *,a,b (0.5–6.0) | 2.8 a (−0.9–6.5) | 6.4 *,b (2.1–10.7) | 2.3 a (−0.8–5.8) | 5.0 *,a (0.8–9.2) | 4.3 a (−0.6–9.2) |
| Right side | 2.2 a (−0.2–5.3) | 2.9 a (−0.8–6.7) | 5.7 *,a (1.4–10.0) | 3.7 *,a (0.7–6.7) | 4.4*,a (0.2–8.6) | 6.1 *,a (1.3–10.9) |
| (B) Postnatal day:F(2,95) = 0.41 p = 0.7 | ||||||
| Assessment 1 | 2.9 *,a (0.05–5.8) | 2.9 *,a,§ (0.01–5.9) | 6.1 *,b (2.8–9.4) | 4.0 *,a (0.8–7.2) | 5.7 *,a,§ (2.4–9.0) | 6.2 *,a (2.5–9.9) |
| Assessment 2 | 2.5 a (−1.1–6.2) | −0.7 b,§ (−4.4–3.0) | 5.0 *,a (0.7–9.4) | 4.0 *,ab (0.2–7.8) | 1.9 a,§ (−2.0–5.8) | 7.6 *,b (3.1–12.0) |
| (C) Sex:F(2,59) = 4.94 p = 0.01 | ||||||
| Boys | 3.7 *,a (1.4–6.1) | 4.2 *,a (1.0–7.4) | 8.0 *,b (4.3–11.7) | 6.6 *,a,† (3.4–9.8) | 5.5 *,a (1.4–9.5) | 7.2 *,a (2.6–11.8) |
| Girls | 4.5 *,a,∆ (0.8–8.1) | 3.2 a (−1.8–8.2) | 4.7 a (−1.0–10.6) | −1.2 a,∆,† (−4.8–2.3) | 5.6 *,b (0.7–10.5) | 3.8 b (−1.9–9.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muelbert, M.; Alexander, T.; Pook, C.; Jiang, Y.; Harding, J.E.; Bloomfield, F.H., for the DIAMOND Study Group. Cortical Oxygenation Changes during Gastric Tube Feeding in Moderate- and Late-Preterm Babies: A NIRS Study. Nutrients 2021, 13, 350. https://doi.org/10.3390/nu13020350
Muelbert M, Alexander T, Pook C, Jiang Y, Harding JE, Bloomfield FH for the DIAMOND Study Group. Cortical Oxygenation Changes during Gastric Tube Feeding in Moderate- and Late-Preterm Babies: A NIRS Study. Nutrients. 2021; 13(2):350. https://doi.org/10.3390/nu13020350
Chicago/Turabian StyleMuelbert, Mariana, Tanith Alexander, Chris Pook, Yannan Jiang, Jane Elizabeth Harding, and Frank Harry Bloomfield for the DIAMOND Study Group. 2021. "Cortical Oxygenation Changes during Gastric Tube Feeding in Moderate- and Late-Preterm Babies: A NIRS Study" Nutrients 13, no. 2: 350. https://doi.org/10.3390/nu13020350
APA StyleMuelbert, M., Alexander, T., Pook, C., Jiang, Y., Harding, J. E., & Bloomfield, F. H., for the DIAMOND Study Group. (2021). Cortical Oxygenation Changes during Gastric Tube Feeding in Moderate- and Late-Preterm Babies: A NIRS Study. Nutrients, 13(2), 350. https://doi.org/10.3390/nu13020350








