The Feasibility of Using Computrition Software for Nutrition Research—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Satter House Trials of Reduced Sodium Study
2.2. Menu Design
2.3. Randomization and Blinding
2.4. Menu Implementation
2.5. Baseline Characteristics
2.6. Feasibility
2.7. Statistical Analysis
3. Results
3.1. Menus
3.2. Baseline Characteristics
3.3. Effectiveness
3.4. Compliance
3.5. Safety
3.6. Palatability
3.7. Sustainability
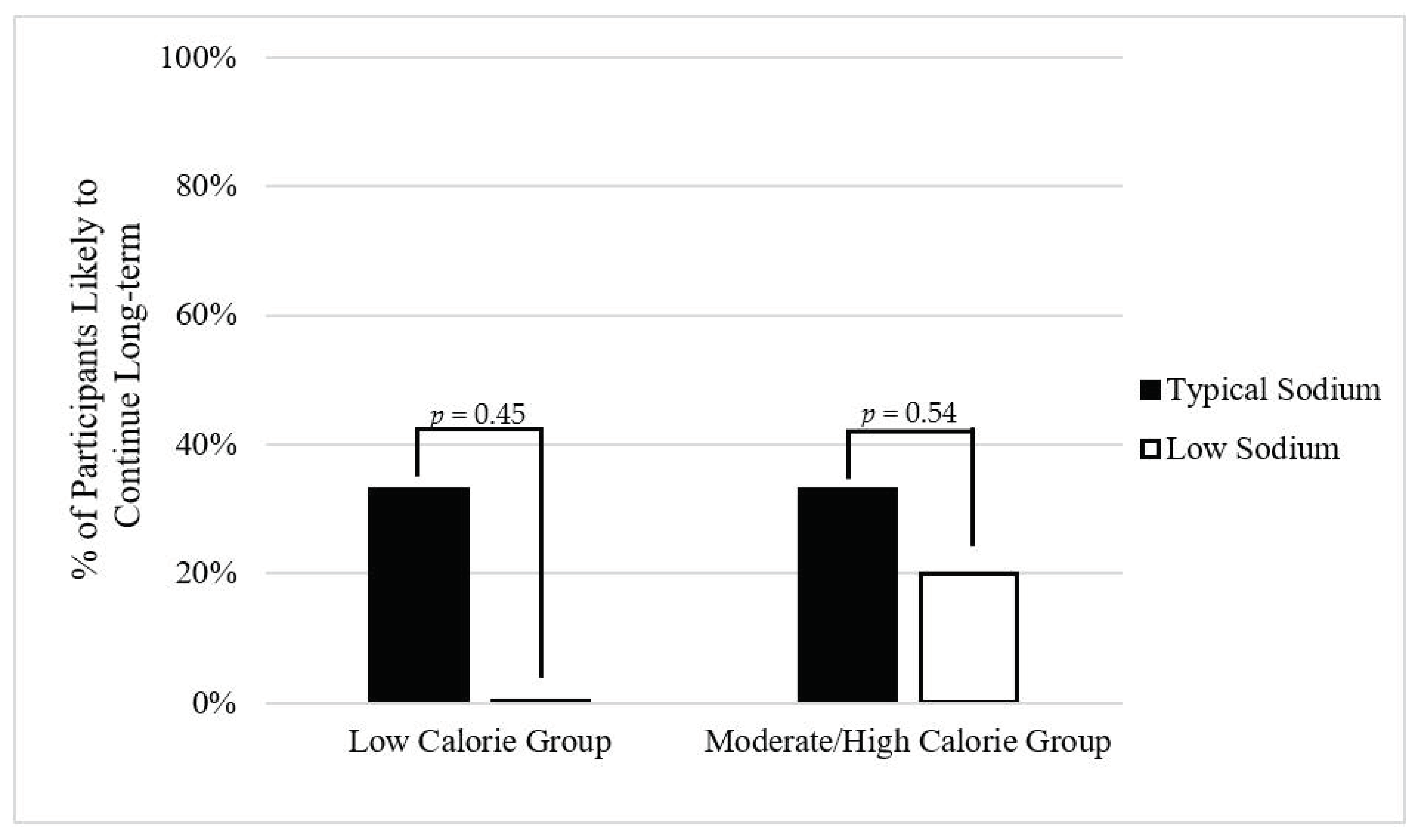
3.7.1. Low Calorie Group
3.7.2. Moderate/High calorie Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Krondl, M.; Coleman, P.; Lau, D. Helping older adults meet nutritional challenges. J. Nutr. Elder. 2008, 27, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A. Funding nutrition research: Where’s the money? Nutr. Clin. Pract. 2007, 22, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-term dietary intervention trials: Critical issues and challenges. Trials 2012, 13, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrides, J.; Collins, P.; Kowalski, A.; Sepede, J.; Vermeulen, M. Lifestyle Changes for Disease Prevention. Prim Care 2019, 46. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Computrition Foodservice Software Solutions. Available online: https://www.computrition.com/ (accessed on 3 June 2020).
- Filippini, T.; Naska, A.; Kasdagli, M.I.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: A systematic review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The Godin-Shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef]
- Schoenfeld, D. Statistical considerations for pilot studies. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 371–374. [Google Scholar] [CrossRef]
- Campbell, A.; Williford, J.H.; Houston, S. Does Resident Participation in Planning Menus Improve Their Food Consumption? J. Am. Diet. Assoc. 1995, 95, A12. [Google Scholar] [CrossRef]
- Dollahite, J.; Franklinms, D.; McNew, R. Problems Encountered in Meeting the Recommended Dietary Allowances for Menus Designed According to the Dietary Guidelines for Americans. J. Am. Diet. Assoc. 1995, 95, 341–347. [Google Scholar] [CrossRef]
- Reguant-Closa, A.; Roesch, A.; Lansche, J.; Nemecek, T.; Lohman, T.G.; Meyer, N.L. The Environmental Impact of the Athlete’s Plate Nutrition Education Tool. Nutrients 2020, 12, 2484. [Google Scholar] [CrossRef] [PubMed]
- Chavent, G.; Bentivegna, B. Developing Marketable and Nutritious Menus for a New Children’s Museum Restaurant. J. Am. Diet. Assoc. 1997, 97, A42. [Google Scholar] [CrossRef]
- Prgomet, M.; Li, J.; Li, L.; Georgiou, A.; Westbrook, J.I. The impact of electronic meal ordering systems on hospital and patient outcomes: A systematic review. Int. J. Med. Inform. 2019, 129, 275–284. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Sodium and potassium in the pathogenesis of hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., III; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Friedberg, J.P.; DiGiovanni, A.; Wang, B.; Wylie-Rosett, J.; Hyoung, S.; Natarajan, S. A Tailored Behavioral Intervention to Promote Adherence to the DASH Diet. Am. J. Health Behav. 2019, 43, 659–670. [Google Scholar] [CrossRef]
- Mahdavi, R.; Bagheri Asl, A.; Abadi, M.A.J.; Namazi, N. Perceived Barriers to Following Dietary Recommendations in Hypertensive Patients. J. Am. Coll. Nutr. 2017, 36, 193–199. [Google Scholar] [CrossRef]
- Sergi, G.; Bano, G.; Pizzato, S.; Veronese, N.; Manzato, E. Taste loss in the elderly: Possible implications for dietary habits. Crit. Rev. Food Sci. Nutr. 2017, 57, 3684–3689. [Google Scholar] [CrossRef]
- Huang, H.-C.; Shanklin, C.W. An Integrated Model to Measure Service Management and Physical Constraints’ Effect on Food Consumption in Assisted-Living Facilities. J. Am. Diet. Assoc. 2008, 108, 785–792. [Google Scholar] [CrossRef]
- Edfors, E.; Westergren, A. Home-Living Elderly People’s Views on Food and Meals. J. Aging Res. 2012, 2012, 761291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cluskey, M. Offering Three-Meal Options in Continuing Care Retirement Communities May Improve Food Intake of Residents. J. Nutr. Elder. 2001, 20, 57–62. [Google Scholar] [CrossRef]
- Hummel, S.L.; Karmally, W.; Gillespie, B.W.; Helmke, S.; Teruya, S.; Wells, J.; Trumble, E.; Jimenez, O.; Marolt, C.; Wessler, J.D.; et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ. Heart Fail. 2018, 11, e004886. [Google Scholar] [CrossRef] [PubMed]
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|
| Breakfast | Eggs Bread Orange Juice | English Muffin Peanut Butter and Jelly Egg Fruit cup Juice | Cereal Yogurt Banana Juice | Muffin Cottage Cheese Banana Juice | Bagel Cream CheeseYogurt Juice | Muffin Cottage Cheese Fruit Juice | Omelet Bagel Cream Cheese Yogurt Banana Juice |
| Lunch | Turkey Burger Potato Salad Pear | Pasta Primavera Breadstick Salad w/dressing | Tuna Salad Wrap Pasta Salad Cookies Mandarin Orange | Whitefish Salad Plate w/dressing Pita Bread Israeli Salad | Chicken Salad Sandwich Pasta Salad Peaches Applesauce | Quiche Homefries Mandarin Oranges | Grilled Chicken Salad Plate w/dressing Potato Chips |
| Snack 1 | Cookies Water | Pudding Water | Crackers Water | Pudding Water | Crackers w/peanut butter Water | Pudding Water | Cookie Water |
| Snack 2 | Cookies Water | Peaches Water | Cookie Water | Cookie Water | Cookie Water | Jello Water | Fruit Water |
| Dinner | Salad w/dressing Baked Fish Rice Spinach | Salad w/dressing Brisket Potatoes Carrots | Salad w/dressing Chicken Kabob Rice | Salad w/dressing Roast Chicken Mashed Sweet Potato Corn Apple | Salad w/dressing Spaghetti and Meatballs Roll Fruit | Salad w/dressing Salmon Burger Asparagus | Salad w/dressing Meatloaf w/gravy Potatoes Green Beans |
| Low Calorie Group (n = 12) | Moderate/High Calorie Group (n = 8) | |||||
|---|---|---|---|---|---|---|
| Typical Sodium (n = 6) | Low Sodium (n = 6) | p-Value | Typical Sodium (n = 3) | Low Sodium (n = 5) | p-Value | |
| Age (year, range 64–91) | 82.2 ± 9.4 | 80.6 ± 5.3 | 0.74 | 74.3 ± 8.5 | 72.6 ± 4.2 | 0.71 |
| Female, n (% a) | 6 (100) | 6 (100) | 1.00 | 3 (100) | 4 (80) | 0.41 |
| European ancestry, n (%) | 6 (100) | 5 (83) | 0.29 | 3 (100) | 5 (100) | 1.00 |
| Height (cm) | 152.3 ± 2.5 | 159.2 ± 6.0 | 0.03 * | 158.6 ± 7.0 | 156.1 ± 6.6 | 0.64 |
| Weight (kg) | 65.6 ± 13.4 | 70.0 ± 7.8 | 0.50 | 99.0 ± 13.4 | 92.3 ± 16.5 | 0.63 |
| BMI (kg/m2) | 28.3 ± 5.9 | 27.6 ± 2.6 | 0.80 | 40.0 ± 6.8 | 38.4 ± 7.3 | 0.82 |
| Caloric Intake (kcal/day) | 1599 ± 152 | 1669 ± 111 | 0.34 | 2158 ± 130 | 2065 ± 163 | 0.85 |
| Physical Activity Score | 27.5 ± 12.5 | 16.3 ± 14.0 | 0.18 * | 13.3 ± 12.6 | 15.4 ± 18.0 | 0.87 |
| Current Smoking, n (%) | 0 (0) | 2 (33) | 0.12 * | 0 (0) | 0 (0) | 1.00 |
| Hypertensive Medication, n (%) | 2 (33) | 3 (50) | 0.56 | 3 (100) | 4 (80) | 0.41 |
| CVD Conditions, n (%) | 5 (84) | 4 (67) | 0.51 | 1 (33) | 3 (60) | 0.47 |
| GI Conditions, n (%) | 0 (0) | 1 (17) | 0.29 | 1 (33) | 2 (40) | 0.85 |
| Diabetes, n (%) | 2 (33) | 0 (0) | 0.12 * | 1 (33) | 3 (60) | 0.47 |
| Cancer, n (%) | 2 (33) | 0 (0) | 0.12 * | 0 (0) | 1 (20) | 0.41 |
| Typical Sodium | Low Sodium | |||||
|---|---|---|---|---|---|---|
| Targeted | Prepared | % Difference | Targeted | Prepared | % Difference | |
| Low Calorie Group | ||||||
| Energy, kcal/day | 1750 | 1861 | 6.3 | 1750 | 1842 | 5.3 |
| Carbohydrates, % 1 | 50 | 52 | 4.0 | 50 | 49 | −2.0 |
| Sodium, mg/day | 3500 | 3589 | 2.5 | 1650 | 1643 | −0.4 |
| Sodium Density, mg/kcal | 2.00 | 1.93 | −3.5 | 0.95 | 0.98 | 3.2 |
| Potassium, mg/day | 3500 | 2953 | −15.6 | 3500 | 2966 | −15.3 |
| Moderate/High calorie Group | ||||||
| Energy, kcal/day | 2125 | 2177 | 2.4 | 2125 | 2072 | −2.5 |
| Carbohydrates, % 1 | 50 | 52 | 4.0 | 50 | 50 | −1.0 |
| Sodium, mg/day | 4250 | 4233 | −0.4 | 2000 | 2008 | 0.4 |
| Sodium Density, mg/kcal | 2 | 2 | −2.8 | 1 | 1 | 2.1 |
| Potassium, mg/day | 4250 | 3271 | −23.0 | 4250 | 3140 | −26.1 |
| Low Calorie Group | Moderate/High Calorie Group | |||||
|---|---|---|---|---|---|---|
| Compliance Measure | Typical Sodium (n = 6) | Low Sodium (n = 6) | p-Value | Typical Sodium (n = 3) | Low Sodium (n = 5) | p-Value |
| Percentage of Provided Sodium Consumed (%) | 61.1 ± 24.3 | 73.6 ± 22.4 | 0.37 | 54.0 ± 40.9 | 68.2 ± 22.5 | 0.54 |
| Δ Sodium (mmol/L) | −2.3 ± 22.2 | −18.3 ± 12.6 | 0.16* | 0.0 ± 12.5 | −22.0 ± 28.2 | 0.26 |
| Δ Potassium (mmol/L) | −1.0 ± 25.9 | 4.0 ± 33.4 | 0.78 | 5.0 ± 18.4 | 7.6 ± 24.6 | 0.88 |
| Δ Creatinine (mg/dL) | −5.83 ± 73.2 | 6.7 ± 19.0 | 0.69 | −27.7 ± 40.1 | −1.4 ± 38.3 | 0.39 |
| Low Calorie Group | Moderate/High Calorie Group | |||||
|---|---|---|---|---|---|---|
| Typical Sodium (n = 6) | Low Sodium (n = 6) | p-Value | Typical Sodium (n = 3) | Low Sodium (n = 5) | p-Value | |
| Likely to Waste Food (%) | 0% | 16.7% | 0.55 | 0% | 20% | 0.71 |
| Guessed Low Sodium Diet Assignment (%) | 67% | 67% | 1.00 | 0% | 60% | 0.09 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millar, C.L.; Cohen, A.; Juraschek, S.P.; Foley, A.; Shtivelman, M.; Mukamal, K.J.; Sahni, S. The Feasibility of Using Computrition Software for Nutrition Research—A Pilot Study. Nutrients 2021, 13, 329. https://doi.org/10.3390/nu13020329
Millar CL, Cohen A, Juraschek SP, Foley A, Shtivelman M, Mukamal KJ, Sahni S. The Feasibility of Using Computrition Software for Nutrition Research—A Pilot Study. Nutrients. 2021; 13(2):329. https://doi.org/10.3390/nu13020329
Chicago/Turabian StyleMillar, Courtney L., Alegria Cohen, Stephen P. Juraschek, Abby Foley, Misha Shtivelman, Kenneth J. Mukamal, and Shivani Sahni. 2021. "The Feasibility of Using Computrition Software for Nutrition Research—A Pilot Study" Nutrients 13, no. 2: 329. https://doi.org/10.3390/nu13020329
APA StyleMillar, C. L., Cohen, A., Juraschek, S. P., Foley, A., Shtivelman, M., Mukamal, K. J., & Sahni, S. (2021). The Feasibility of Using Computrition Software for Nutrition Research—A Pilot Study. Nutrients, 13(2), 329. https://doi.org/10.3390/nu13020329






