Providing Comprehensive Dietary Fatty Acid Profiling from Saturates to Polyunsaturates with the Malaysia Lipid Study-Food Frequency Questionnaire: Validation Using the Triads Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
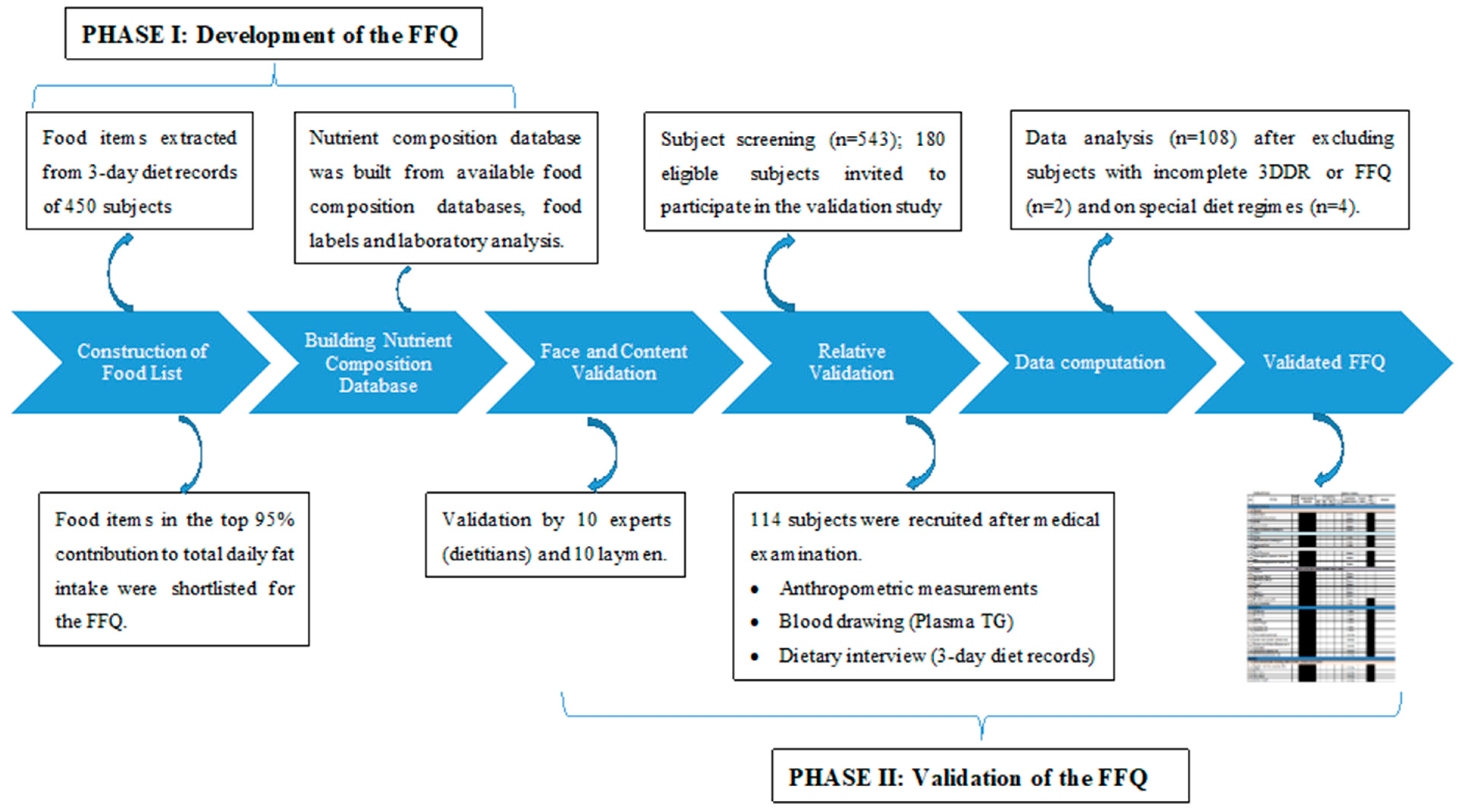
2.2. Phase 1—Development of the Food Frequency Questionnaire (FFQ)
Food Frequency Questionnaire Development
2.3. Phase 2—Validation of the FFQ
2.3.1. Face and Content Validation
2.3.2. Relative Validation in a Free-Living Population
2.3.3. Sampling Size
2.4. Fatty Acid Composition Analysis
2.5. Data Computation
2.6. Statistical Analysis
3. Results
3.1. Subjects’ Characteristics
3.2. Validity Test between FFQ and Reference Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Time to Deliver: Report of The WHO Independent High-Level Commission on Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Hyseni, L.; Bromley, H.; Kypridemos, C.; O’Flaherty, M.; Lloyd-Williams, F.; Guzman-Castillo, M.; Pearson-Stuttard, J.; Capewell, S. Systematic review of dietary trans-fat reduction interventions. Bull. World Health Organ. 2017, 95, 821G–830G. [Google Scholar] [CrossRef]
- Swierk, M.; Williams, P.G.; Wilcox, J.; Russell, K.G.; Meyer, B.J. Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake. Nutrition 2011, 27, 641–646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willett, W.; Stampfer, M.; Chu, N.F.; Spiegelman, D.; Holmes, M.; Rimm, E. Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am. J. Epidemiol. 2001, 154, 1107–1112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolk, A.; Furuheim, M.; Vessby, B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J. Nutr. 2001, 131, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products. Off. J. Int. Assoc. Study Obes. 2013, 14 (Suppl. S2), 21–28. [Google Scholar]
- Karupaiah, T.; Ismail, M.N.; Sundram, K. Dietary Fatty Acids and Their influence on Blood Lipids and Lipoproteins. In Healthful Lipids; Association of Official Analytical Chemists (AOAC) International: Champaign, IL, USA, 2005; pp. 171–203. [Google Scholar]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F.J.C. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [PubMed]
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, D.S.; Rimm, E.B.J.C. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005, 111, 157–164. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Kharroubi, W.; Kaoubaa, N.; Zarrouk, A.; Batbout, F.; Gamra, H.; Najjar, M.F.; Lizard, G.; Hininger-Favier, I.; Hammami, M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018, 17, 52. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation. GBD Compare Data Visualization. Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 16 July 2020).
- Riboli, E.; Ronnholm, H.; Saracci, R. Biological markers of diet. Cancer Surv. 1987, 6, 685–718. [Google Scholar] [PubMed]
- Institute for Public Health. National Health and Morbidity Survey 2014: Malaysian Adult Nutrition Survey (MANS); Institute for Public Health: St. Louis, MO, USA, 2014; Volume 2.
- Eng, J.Y.; Moy, F.M. Validation of a food frequency questionnaire to assess dietary cholesterol, total fat and different types of fat intakes among Malay adults. Asia Pac. J. Clin. Nutr. 2011, 20, 639–645. [Google Scholar] [PubMed]
- Raji, M.N.A.; Ab Karim, S.; Ishak, F.A.C.; Arshad, M.M. Past and present practices of the Malay food heritage and culture in Malaysia. J. Ethn. Foods 2017, 4, 221–231. [Google Scholar] [CrossRef]
- Karupaiah, T.; Chuah, K.A.; Chinna, K.; Pressman, P.; Clemens, R.A.; Hayes, A.W.; Sundram, K. A Cross-Sectional Study on the Dietary Pattern Impact on Cardiovascular Disease Biomarkers in Malaysia. Sci. Rep. 2019, 9, 13666. [Google Scholar] [CrossRef]
- Kabagambe, E.K.; Baylin, A.; Allan, D.A.; Siles, X.; Spiegelman, D.; Campos, H. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am. J. Epidemiol. 2001, 154, 1126–1135. [Google Scholar] [CrossRef]
- Ocke, M.C.; Kaaks, R.J. Biochemical markers as additional measurements in dietary validity studies: Application of the method of triads with examples from the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 1997, 65, 1240S–1245S. [Google Scholar] [CrossRef]
- Block, G.; Hartman, A.M.; Dresser, C.M.; Carroll, M.D.; Gannon, J.; Gardner, L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986, 124, 453–469. [Google Scholar] [CrossRef]
- Krejcie, R.V.; Morgan, D.W. Determining sample size for research activities. Educ. Psychol. Meas. 1970, 30, 607–610. [Google Scholar] [CrossRef]
- Department of Statistics Malaysia. Population Distribution and Basic Demographic Characteristics. 2010. Available online: https://www.dosm.gov.my/v1 (accessed on 25 July 2016).
- Kim, J.; Chan, M.M.; Shore, R.E. Development and validation of a food frequency questionnaire for Korean Americans. Int. J. Food Sci. Nutr. 2002, 53, 129–142. [Google Scholar] [CrossRef]
- Tee, E.S.; Ismail, M.N.; Nasir, A.; Khatijah, I. Nutrient Composition of Malaysian Foods, 4th ed.; Institute for Medical Research: Kuala Lumpur, Malaysia, 1997.
- Health Promotion Board. Food Composition Guide Singapore; Health Promotion Board: Singapore, 2003.
- US Department of Agriculture. Food Data Central; USDA: Washington, DC, USA, 2019.
- Puwastien, P.; Burlingame, B.; Raroengwichit, M. Food Composition Tables; Mahidol University: Bangkok, Thailand, 2000. [Google Scholar]
- Sundram, K.; Karupaiah, T.; Hayes, K.C. Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr. Metab. 2007, 4, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karupaiah, T.; Sundram, K. Modulation of human postprandial lipemia by changing ratios of polyunsaturated to saturated (P/S) fatty acid content of blended dietary fats: A cross-over design with repeated measures. Nutr. J. 2013, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Karupaiah, T.; Tan, H.K.; Ong, W.W.; Tan, C.H.; Sundram, K. Trans fatty acid content in Malaysian supermarket foods: A field-to-laboratory approach in assessing food risk. Food Addit. Contam. 2014, 31, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Tee, E.; Mizura, S.S.; Anuar, A.; Kuladevan, R.; Young, S.; Khor, S.; Chin, S.J.P. Nutrient composition of selected cooked and processed snack foods. Pertanika 1989, 12, 15–25. [Google Scholar]
- Arlington, V. Official Methods of Analysis of Official Analytical Chemists International; Association of Official Analytical Chemists (AOAC) International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Hitchcock, C.; Hammond, E.W. The Determination of Lipids in Foods. In Developments in Food Analysis Techniques; King, R.D., Ed.; Elsevier Applied Science Publishers Ltd.: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Karupaiah, T.; Wong, K.; Chinna, K.; Arasu, K.; Chee, W.S. Metering Self-Reported Adherence to Clinical Outcomes in Malaysian Patients With Hypertension: Applying the Stages of Change Model to Healthful Behaviors in the CORFIS Study. Health Educ. Behav. 2015, 42, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Fatihah, F.; Ng, B.K.; Hazwanie, H.; Norimah, A.K.; Shanita, S.N.; Ruzita, A.T.; Poh, B.K. Development and validation of a food frequency questionnaire for dietary intake assessment among multi-ethnic primary school-aged children. Singap. Med. J. 2015, 56, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.G.; Newman, T.B. Designing Clinical Research, 4th ed.; Lippincott: Philadelphia, PA, USA, 2013. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Black, A.E.; Cole, T.J. Biased over-or under-reporting is characteristic of individuals whether over time or by different assessment methods. J. Am. Diet. Assoc. 2001, 101, 70–80. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F. Dietary assessment methodology. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 5–48. [Google Scholar]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2012. [Google Scholar]
- Norimah, A.K., Jr.; Safiah, M.; Jamal, K.; Haslinda, S.; Zuhaida, H.; Rohida, S.; Fatimah, S.; Norazlin, S.; Poh, B.K.; Kandiah, M.; et al. Food Consumption Patterns: Findings from the Malaysian Adult Nutrition Survey (MANS). Malays. J. Nutr. 2008, 14, 25–39. [Google Scholar]
- Deurenberg-Yap, M.; Li, T.; Tan, W.L.; van Staveren, W.A.; Deurenberg, P. Validation of a semiquantitative food frequency questionnaire for estimation of intakes of energy, fats and cholesterol among Singaporeans. Asia Pac. J. Clin. Nutr. 2000, 9, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Thompson, F.E.; Smith, A.F.; Jobe, J.B.; Ziegler, R.G.; Potischman, N.; Schatzkin, A.; Hartman, A.; Swanson, C.; Kruse, L. Improving food frequency questionnaires: A qualitative approach using cognitive interviewing. J. Am. Diet. Assoc. 1995, 95, 781–788. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Horwath, C.C. Validity of a short food frequency questionnaire for estimating nutrient intake in elderly people. Br. J. Nutr. 1993, 70, 3–14. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Dujardin, B.; Kirwan, L. The raw primary commodity (RPC) model: Strengthening EFSA’s capacity to assess dietary exposure at different levels of the food chain, from raw primary commodities to foods as consumed. EFSA Suppor. Publ. 2019, 16, 1532E. [Google Scholar]
- Margetts, B.M.; Nelson, M. Design Concepts in Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Karupaiah, T.; Mutalib, S.A.; Shahril, M.R.; Chuah, K.A.; Norizan, N.A. Fats. In Recommended Nutrient Intakes for Malaysia; Ministry of Health: Putrajaya, Malaysia, 2017. [Google Scholar]
- Cantwell, M.M.; Gibney, M.J.; Cronin, D.; Younger, K.M.; O’Neill, J.P.; Hogan, L.; Flynn, M.A. Development and validation of a food-frequency questionnaire for the determination of detailed fatty acid intakes. Public Health Nutr. 2005, 8, 97–107. [Google Scholar] [CrossRef][Green Version]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- James, P.; Seward, M.W.; James O’Malley, A.; Subramanian, S.V.; Block, J.P. Changes in the food environment over time: Examining 40 years of data in the Framingham Heart Study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 84. [Google Scholar] [CrossRef]
- Welch, A.A.; Luben, R.; Khaw, K.T.; Bingham, S.A. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J. Hum. Nutr. Diet. 2005, 18, 99–116. [Google Scholar] [CrossRef]
- Md Ali, M.S.; Yeak, Z.-W.; Khor, B.-H.; Sahathevan, S.; Sualeheen, A.; Lim, J.-H.; Adanan, N.I.H.; Abdul Gafor, A.H.; Khosla, P.; Karupaiah, T. HD-FFQ to Detect Nutrient Deficiencies and Toxicities for a Multiethnic Asian Dialysis Population. Nutrients 2020, 12, 1585. [Google Scholar] [CrossRef]
- Greenfield, H.; Southgate, D.A. Food Composition Data: Production, Management, and Use; Food & Agriculture Org.: Rome, Italy, 2003. [Google Scholar]
| Characteristics (n = 108) | Values |
|---|---|
| Age, years | 35.4 ± 9.2 |
| Gender, male/female, n | 44/64 |
| Ethnicity, Malay/Chinese/ndian, n | 36/35/37 |
| Body Mass Index, kg/m2 | 26.5 ± 5.6 |
| Mean energy intake by gender, kcal | |
| DR | |
| Male | 2066 ± 410 |
| Female | 1499 ± 256 |
| FFQ | |
| Male | 2042 ± 553 |
| Female | 1616 ± 495 |
| Mean energy intake by ethnicity, kcal | |
| DR | |
| Malay | 1720 ± 365 |
| Chinese | 1908 ± 475 |
| Indian | 1571 ± 386 |
| FFQ | |
| Malay | 1812 ± 525 |
| Chinese | 1775 ± 597 |
| Indian | 1782 ± 566 |
| Dietary Intake | DR | FFQ | Mean Difference (%) | p-Value * | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |||
| Total Fat (g/day) | 59.1 ± 20.4 | 54.9 (45.4, 71.7) | 62.6 ± 23.4 | 58.1 (47.5, 74.7) | −5.9 | 0.230 |
| SFA (g/day) | 26.4 ± 9.1 | 24.5 (19.5, 31.9) | 27.2 ± 10.5 | 25.4 (20.4, 32.5) | −3.0 | 0.690 |
| MUFA (g/day) | 23.3 ± 8.8 | 21.2 (17.0, 29.3) | 25.3 ± 9.6 | 23.6 (19.4, 29.2) | −8.6 | 0.100 |
| PUFA (g/day) | 8.6 ± 3.3 | 8.1 (6.2, 11.1) | 9.6 ± 3.8 | 8.7 (7.1, 11.6) | −11.6 | 0.030 |
| TFA (g/day) | 0.15 ± 0.10 | 0.14 (0.08, 0.20) | 0.16 ± 0.08 | 0.15 (0.10, 0.19) | −6.7 | 0.420 |
| P/S ratio | 0.34 ± 0.11 | 0.33 (0.28, 0.38) | 0.36 ± 0.11 | 0.34 (0.31, 0.38) | 15.0 | 0.003 |
| ω-3 (g/day) | 0.50 ± 0.35 | 0.41 (0.26, 0.70) | 0.63 ± 0.38 | 0.52 (0.39, 0.81) | −26.0 | 0.002 |
| ω-6 (g/day) | 8.1 ± 3.1 | 7.5 (5.8, 10.3) | 9.0 ± 3.5 | 8.1 (6.6, 10.9) | −11.1 | 0.063 |
| Nutrients | r * | p-Value |
|---|---|---|
| FFQ versus DR | ||
| Total Fat (g/day) | 0.357 | <0.001 |
| SFA (g/day) | 0.346 | <0.001 |
| MUFA (g/day) | 0.346 | <0.001 |
| PUFA (g/day) | 0.234 | 0.015 |
| TFA (g/day) | 0.391 | <0.001 |
| P/S ratio | 0.225 | 0.019 |
| ω-3 FAs (g/day) | 0.095 | 0.329 |
| ω-6 FAs (g/day) | 0.245 | 0.011 |
| FFQ versus plasma TG | ||
| SFA (% fat) | 0.184 | 0.057 |
| MUFA (% fat) | −0.175 | 0.069 |
| PUFA (% fat) | 0.192 | 0.047 |
| TFA (% fat) | −0.004 | 0.964 |
| P/S ratio | 0.245 | 0.011 |
| ω-3 FAs (% fat) | 0.098 | 0.315 |
| ω-6 FAs (% fat) | 0.295 | 0.007 |
| DR versus plasma TG | ||
| SFA (% fat) | 0.197 | 0.041 |
| MUFA (% fat) | −0.043 | 0.657 |
| PUFA (% fat) | 0.223 | 0.02 |
| TFA (% fat) | 0.174 | 0.072 |
| P/S ratio | 0.205 | 0.034 |
| ω-3 FAs (% fat) | 0.272 | 0.004 |
| ω-6 FAs (% fat) | 0.241 | 0.012 |
| Nutrients | Same Quartile (%) | Adjacent Quartile (%) | Grossly Misclassified (%) |
|---|---|---|---|
| FFQ versus DR | |||
| Total Fat (g/day) | 33.3 | 40.7 | 5.6 |
| SFA (g/day) | 34.3 | 38.9 | 5.6 |
| MUFA (g/day) | 28.7 | 43.5 | 3.7 |
| PUFA (g/day) | 25.0 | 43.5 | 5.6 |
| TFA (g/day) | 38.0 | 34.3 | 8.3 |
| P/S ratio | 33.3 | 38.9 | 9.3 |
| ω-3 FAs (g/day) | 21.3 | 70.4 | 8.3 |
| ω-6 FAs (g/day) | 23.1 | 71.3 | 5.6 |
| FFQ versus Plasma TG | |||
| SFA (% fat) | 24.1 | 39.8 | 4.6 |
| MUFA (% fat) | 15.7 | 40.7 | 16.7 |
| PUFA (% fat) | 25.0 | 39.8 | 8.3 |
| TFA (% fat) | 30.6 | 34.3 | 12.0 |
| P/S ratio | 30.6 | 38.9 | 11.1 |
| ω-3 FAs (% fat) | 28.7 | 59.3 | 12.0 |
| ω-6 FAs (% fat) | 32.4 | 60.2 | 7.4 |
| DR versus Plasma TG | |||
| SFA (% fat) | 19.4 | 65.8 | 14.8 |
| MUFA (% fat) | 23.2 | 64.8 | 12.0 |
| PUFA (% fat) | 36.1 | 58.3 | 5.6 |
| TFA (% fat) | 23.1 | 67.6 | 9.3 |
| P/S ratio | 23.2 | 57.4 | 13.4 |
| ω-3 FAs (% fat) | 32.4 | 61.1 | 6.5 |
| ω-6 FAs (% fat) | 29.6 | 62.1 | 8.3 |
| Fatty Acids Group | ra | rb | rc | z diff (p-Value) | ||
|---|---|---|---|---|---|---|
| ρ (DR vs. FFQ) | ρ (FFQ-TG) | ρ (DR-TG) | ra—rb | ra—rc | rb—rc | |
| SFA (% fat) | 0.49 | 0.52 | 0.46 | −0.03 (0.800) | 0.03 (0.772) | 0.06 (0.587) |
| MUFA (% fat) | 0.18 | 0.98 | 0.12 | −0.80 (<0.001) | 0.06 (0.654) | 0.80 (<0.001) |
| PUFA (% fat) | 0.37 | 0.48 | 0.56 | −0.11 (0.331) | −0.19 (0.066) | −0.08 (0.384) |
| TFA (% fat) | 0.93 | 0.38 | 0.10 | 0.55 (<0.001) | 0.83 (<0.001) | 0.28 (0.035) |
| P/S ratio | 0.37 | 0.53 | 0.51 | −0.16 (0.136) | −0.14 (0.199) | 0.02 (0.838) |
| ω-3 FAs (% fat) | 0.67 | 0.12 | 0.29 | 0.55 (<0.001) | 0.38 (<0.001) | −0.17 (0.207) |
| ω-6 FAs (% fat) | 0.44 | 0.48 | 0.53 | −0.04 (0.715) | −0.09 (0.393) | −0.05 (0.625) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeak, Z.-W.; Chuah, K.-A.; Tan, C.-H.; Ezhumalai, M.; Chinna, K.; Sundram, K.; Karupaiah, T. Providing Comprehensive Dietary Fatty Acid Profiling from Saturates to Polyunsaturates with the Malaysia Lipid Study-Food Frequency Questionnaire: Validation Using the Triads Approach. Nutrients 2021, 13, 120. https://doi.org/10.3390/nu13010120
Yeak Z-W, Chuah K-A, Tan C-H, Ezhumalai M, Chinna K, Sundram K, Karupaiah T. Providing Comprehensive Dietary Fatty Acid Profiling from Saturates to Polyunsaturates with the Malaysia Lipid Study-Food Frequency Questionnaire: Validation Using the Triads Approach. Nutrients. 2021; 13(1):120. https://doi.org/10.3390/nu13010120
Chicago/Turabian StyleYeak, Zu-Wei, Khun-Aik Chuah, Choon-Heen Tan, Menagah Ezhumalai, Karuthan Chinna, Kalyana Sundram, and Tilakavati Karupaiah. 2021. "Providing Comprehensive Dietary Fatty Acid Profiling from Saturates to Polyunsaturates with the Malaysia Lipid Study-Food Frequency Questionnaire: Validation Using the Triads Approach" Nutrients 13, no. 1: 120. https://doi.org/10.3390/nu13010120
APA StyleYeak, Z.-W., Chuah, K.-A., Tan, C.-H., Ezhumalai, M., Chinna, K., Sundram, K., & Karupaiah, T. (2021). Providing Comprehensive Dietary Fatty Acid Profiling from Saturates to Polyunsaturates with the Malaysia Lipid Study-Food Frequency Questionnaire: Validation Using the Triads Approach. Nutrients, 13(1), 120. https://doi.org/10.3390/nu13010120





