A New Peracetylated Oleuropein Derivative Ameliorates Joint Inflammation and Destruction in a Murine Collagen-Induced Arthritis Model via Activation of the Nrf-2/Ho-1 Antioxidant Pathway and Suppression of MAPKs and NF-κB Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
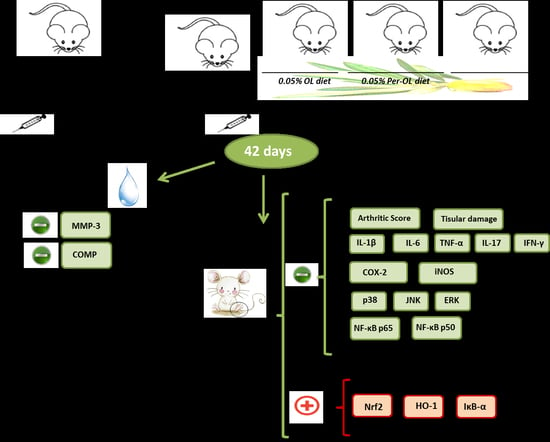
2.3. Induction of CIA
2.4. Histological and Immunohistochemical Analyses
2.5. Study of Inflammatory Markers
2.6. Isolation of Cytoplasmic and Nuclear Proteins and Immunoblotting Detection
2.7. Statistical Evaluation
3. Results
3.1. Dietary OL and Per-OL Treatments Alleviated CIA-Related Symptoms and RA-Induced Infiltration of Inflammatory Cells
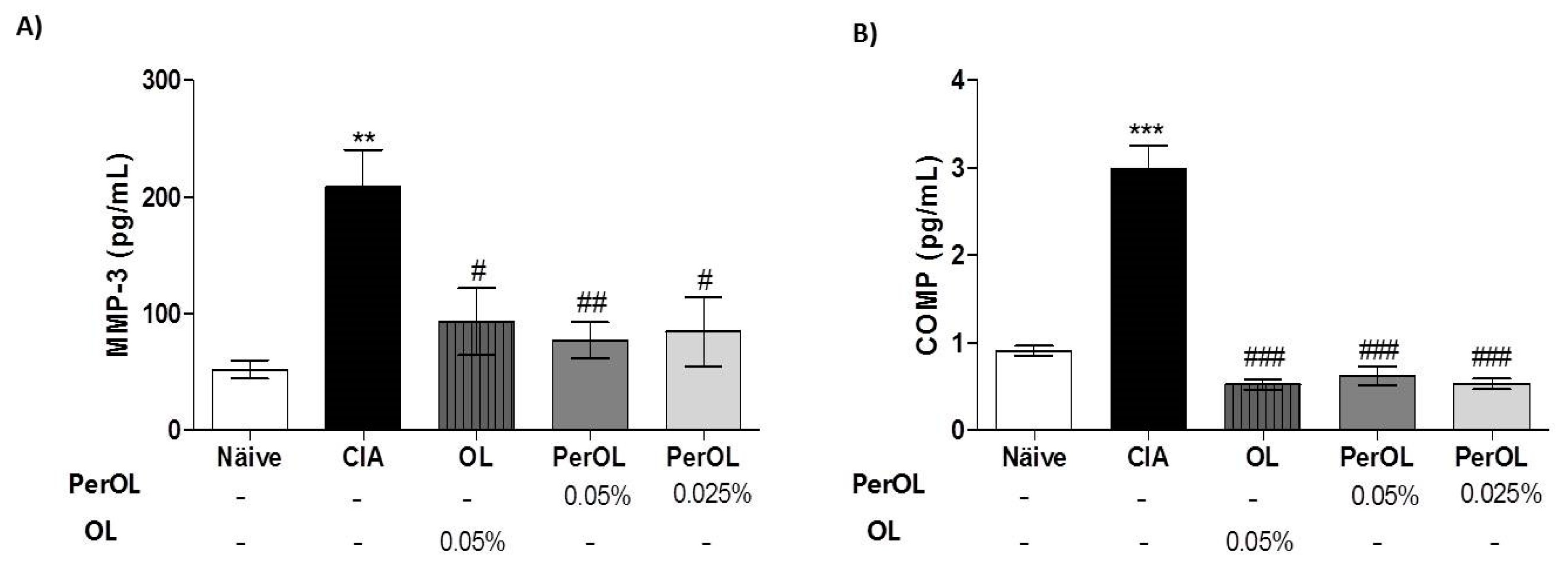
3.2. Effects of Dietary OL and Per-OL Treatments on Serum Pro-Inflammatory Biomarkers Levels in CIA Model
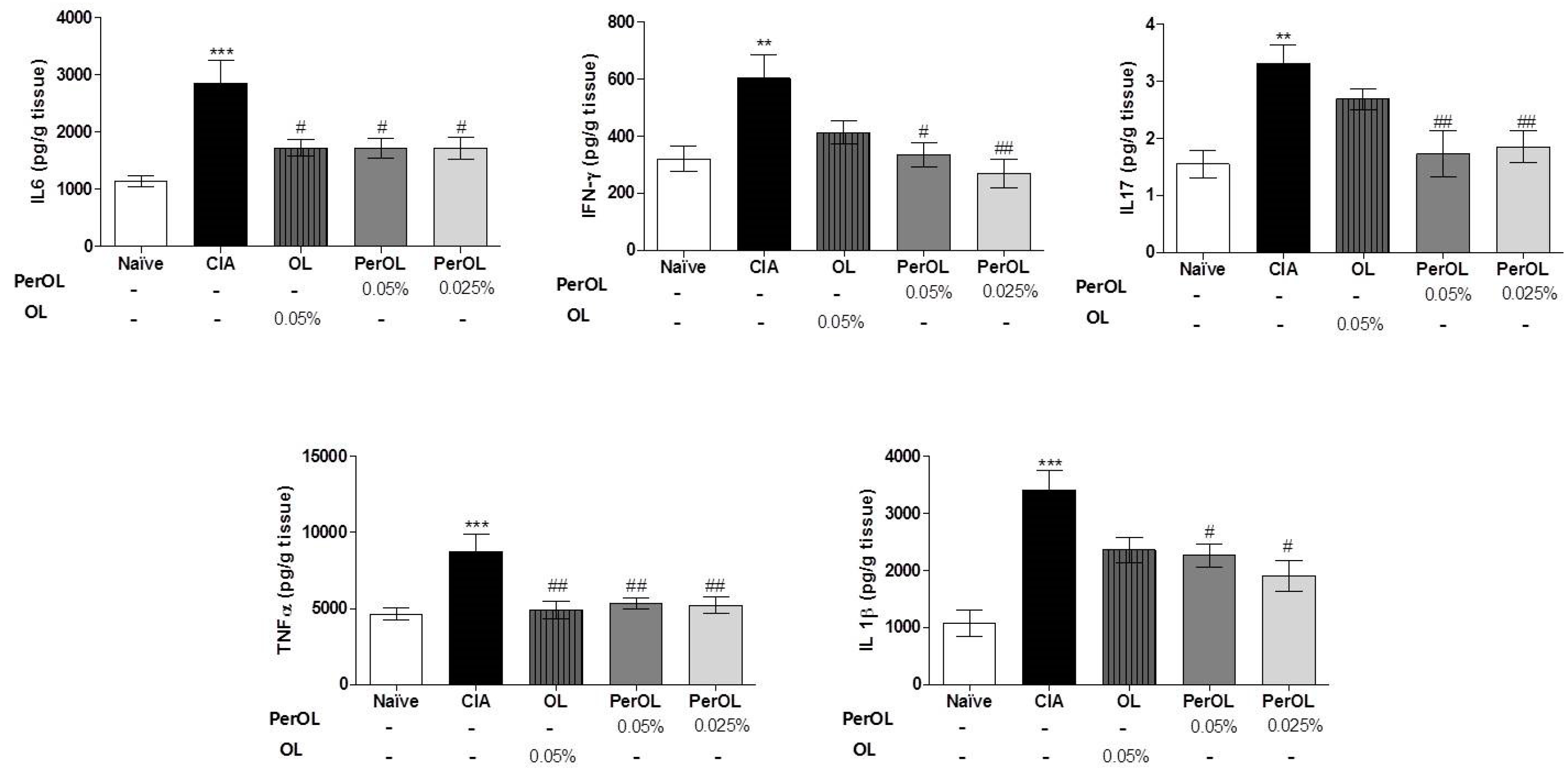
3.3. Dietary OL and Per-OL Treatments Suppressed the Production of Pro-Inflammatory Cytokines in CIA Model
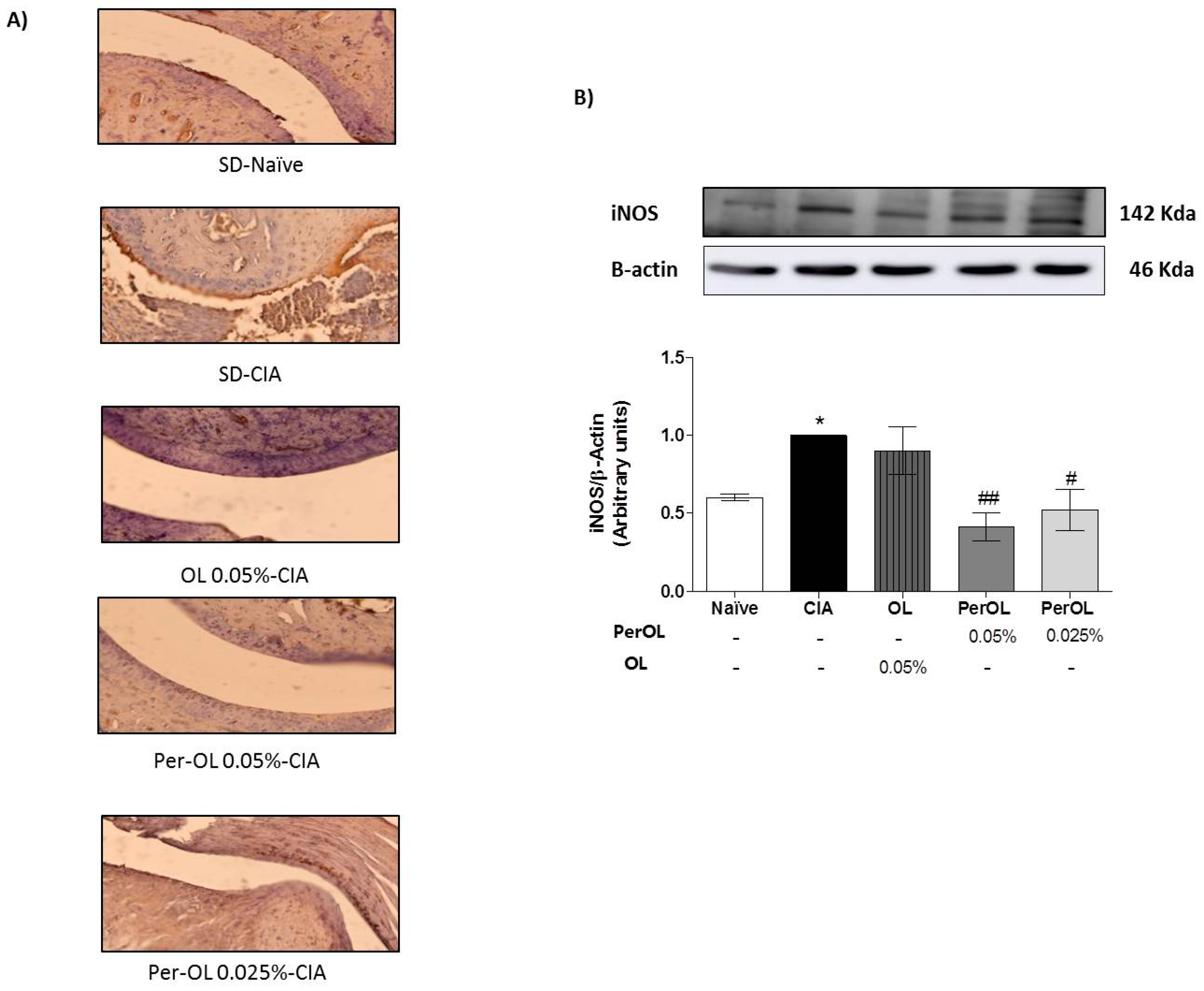
3.4. OL and Per-OL Experimental Diets Reduced COX-2 and iNOS Overexpressions
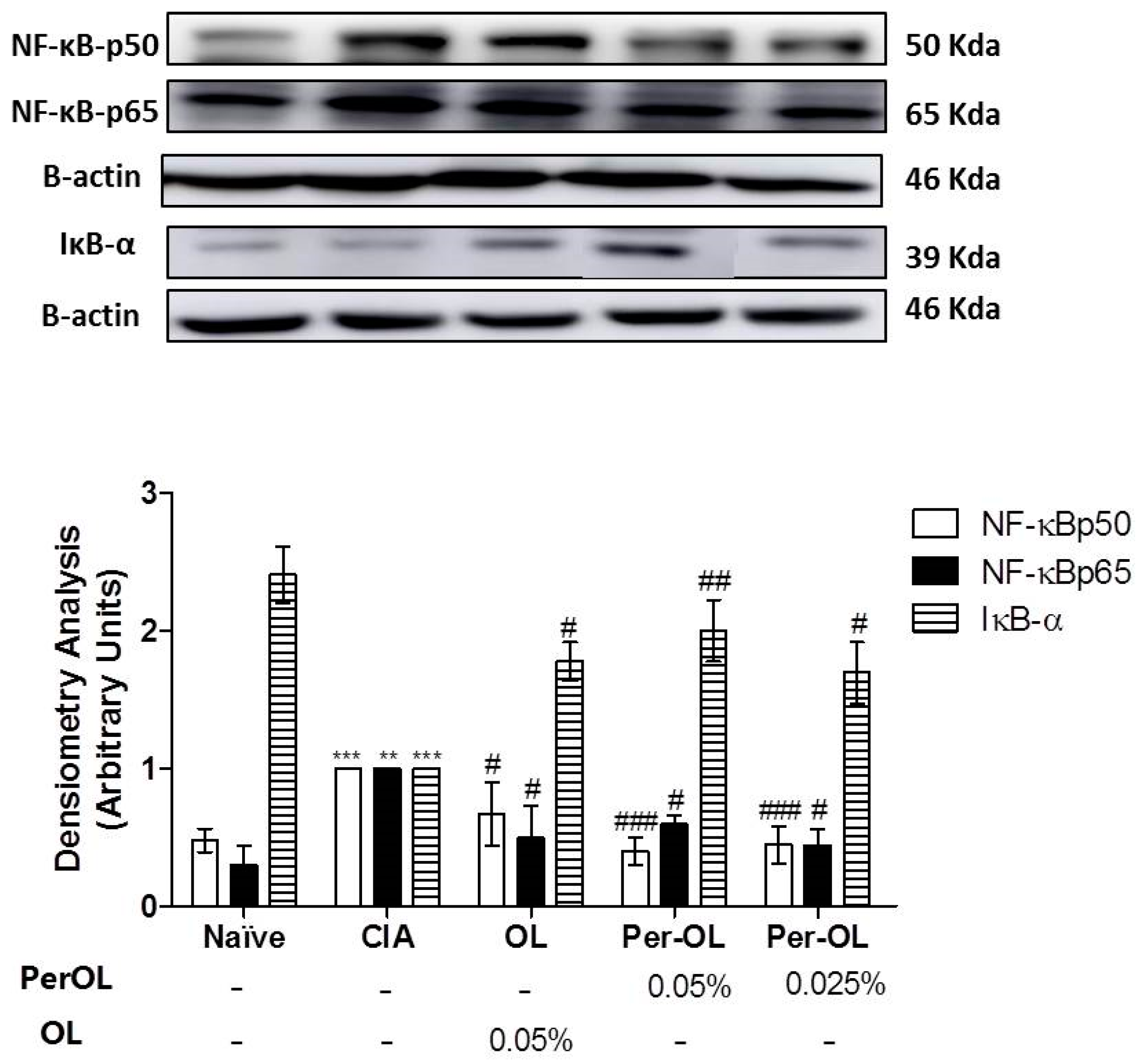
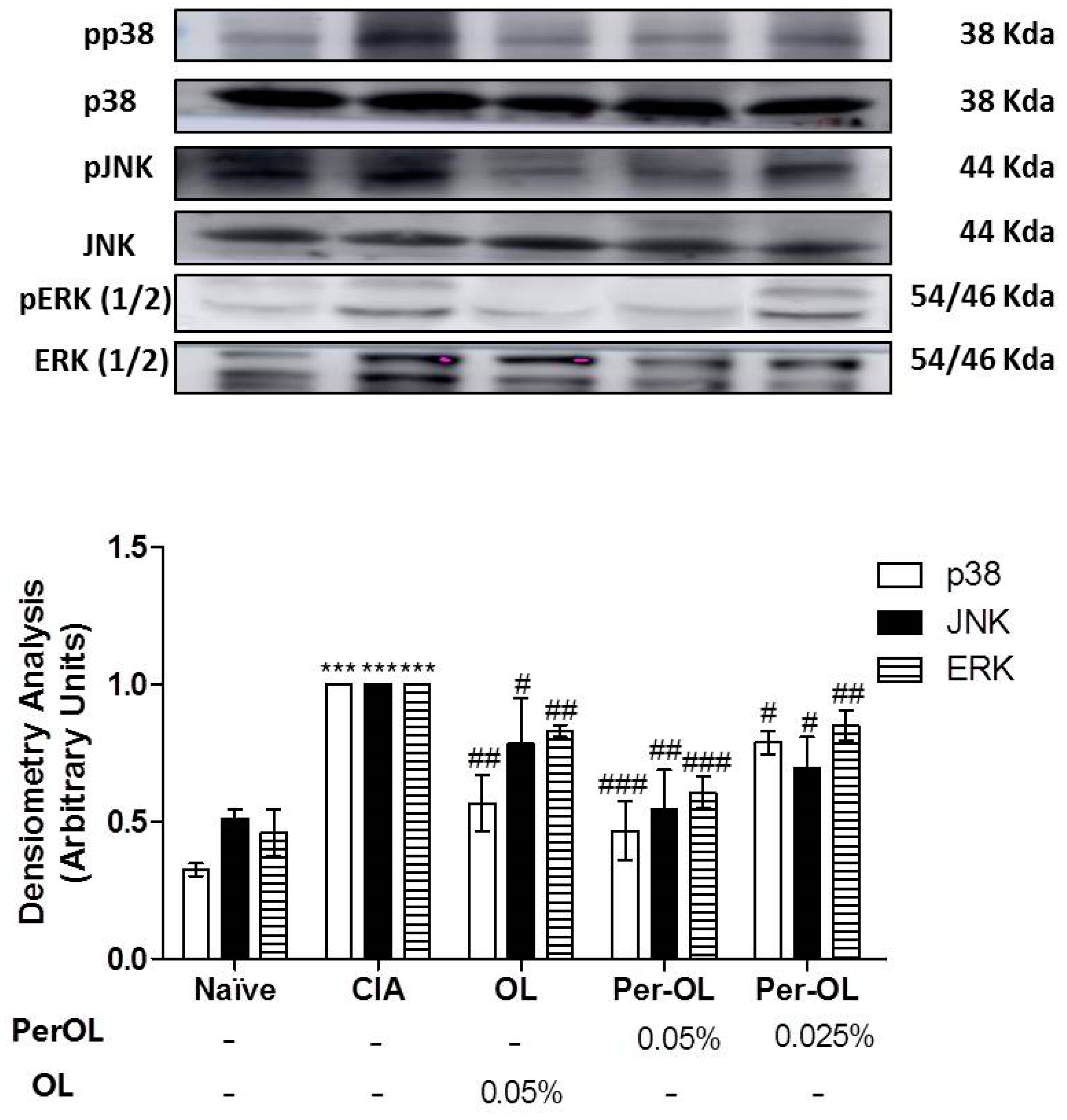
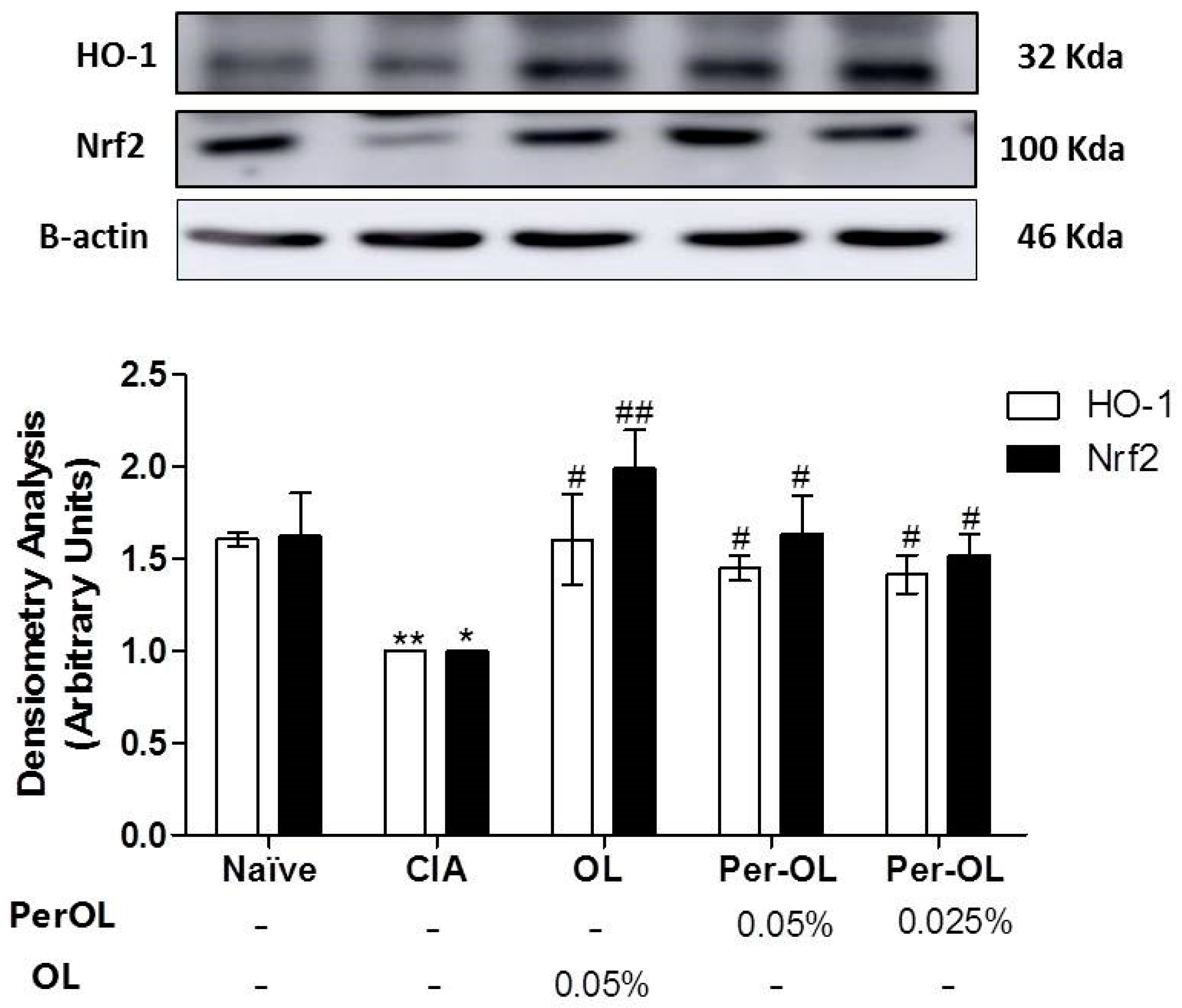
3.5. Effects of Dietary OL and Per-OL Treatments on MAPKs, NF-κB and Nrf2/HO Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Malm, K.; Bergman, S.; Andersson, M.L.E.; Bremander, A.; Larsson, I. Quality of life in patients with established rheumatoid arthritis: A phenomenographic study. SAGE Open Med. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Moon, S.J.; Park, J.S.; Woo, Y.J.; Lim, M.A.; Kim, S.M.; Lee, S.Y.; Kim, E.K.; Lee, H.J.; Lee, W.S.; Park, S.H.; et al. Rebamipide suppresses collagen-induced arthritis through reciprocal regulation of Th17/Treg cell differentiation and heme oxygenase 1 induction. Arthritis Rheumatol. 2014, 66, 874–885. [Google Scholar] [CrossRef]
- Yap, H.-Y.; Tee, S.; Wong, M.; Chow, S.-K.; Peh, S.-C.; Teow, S.-Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Paolino, S.; Pacini, G.; Patanè, M.; Alessandri, E.; Cattelan, F.; Goegan, F.; Pizzorni, C.; Gotelli, E.; Cutolo, M. Interactions between microbiota, diet/nutrients and immune/ inflammatory response in rheumatic diseases: Focus on rheumatoid arthritis. Reumatologia 2019, 57, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Feist, E.; Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Law, S.T.; Taylor, P.C. Role of Biological Agents in Treatment of Rheumatoid Arthritis. Pharmacol. Res. 2019, 150, 104497. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. An update on dietary phenolic compounds in the prevention and management of rheumatoid arthritis. Food Funct. 2016, 7, 2943–2969. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Molecular Sciences Olive Tree Biophenols in Inflammatory Bowel Disease: When Bitter is Better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Alarcón-de-la-Lastra, C. An Up-date of Olive Oil Phenols in Inflammation and Cancer: Molecular Mechanisms and Clinical Implications. Curr. Med. Chem. 2013, 20, 4758–4776. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Qabaha, K.; Al-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- Castejón, M.L.; Rosillo, M.Á.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.M.; Alarcón-De-La-Lastra, C. Oleuropein down-regulated IL-1β-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef]
- Horcajada, M.-N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.-F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. Oleuropein Aglycone, an Olive Oil Compound, Ameliorates Development of Arthritis Caused by Injection of Collagen Type II in Mice. J. Pharmacol. Exp. Ther. 2011, 339, 859–869. [Google Scholar] [CrossRef]
- Castejon, M.L.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Olive secoiridoid oleuropein and its semisynthetic acetyl-derivatives reduce LPS-induced inflammatory response in murine peritoneal macrophages via JAK-STAT and MAPKs signaling pathways. J. Funct. Foods 2019, 58, 95–104. [Google Scholar] [CrossRef]
- Castejon, M.L.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Montoya, T.; Martín-LaCave, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary oleuropein and its new acyl-derivate, attenuate murine lupus nephritis through HO-1/Nrf2 activation and suppressing JAK/STAT, NF-κB, MAPK and NLRP3 inflammasome signaling pathways. J. Nutr. Biochem. 2019, 74, 108229. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2013, 3, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Sánchez-Hidalgo, M.; Sánchez-Fidalgo, S.; Aparicio-Soto, M.; Villegas, I.; Alarcón-de-la-Lastra, C. Dietary extra-virgin olive oil prevents inflammatory response and cartilage matrix degradation in murine collagen-induced arthritis. Eur. J. Nutr. 2015, 55, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz, M.L.; Maicas, N.; Garcia-Arnandis, I.; Terencio, M.C.; Motterlini, R.; Devesa, I.; Joosten, L.A.B.; van den Berg, W.B.; Alcaraz, M.J. Treatment with a CO-releasing molecule (CORM-3) reduces joint inflammation and erosion in murine collagen-induced arthritis. Ann. Rheum. Dis. 2007, 67, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Alcaraz, M.J.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Ferrándiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcón-De-La-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Lubberts, E.; Alarcón-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef]
- Bao, C.; Liu, Y.; Sun, X.; Xing, C.; Zeng, L.; Sun, G. Periploca forrestii saponin ameliorates CIA via suppressing proinflammatory cytokines and nuclear factor kappa-B pathways. PLoS ONE 2017, 12, e0176672. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, E.; Billiau, A.; Matthys, P. Collagen-Induced Arthritis as an Animal Model for Rheumatoid Arthritis: Focus on Interferon-γ. J. Interf. Cytokine Res. 2011, 31, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Takayanagi, H. Inflammation and Bone Destruction in Arthritis: Synergistic Activity of Immune and Mesenchymal Cells in Joints. Front. Immunol. 2012, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bagchi, A.; De, S.; Mitra, S.; Mukherjee, S.; Ghosh, P.; Ghosh, A.; Chatterjee, M. Role of redox imbalance and cytokines in mediating oxidative damage and disease progression of patients with rheumatoid arthritis. Free Radic. Res. 2019, 53, 768–779. [Google Scholar] [CrossRef]
- Lai, Y.; Yu, X.-P.; Zhang, Y.; Tian, Q.; Song, H.; Mucignat, M.T.; Perris, R.; Samuels, J.; Krasnokutsky, S.; Attur, M.; et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthr. Cartil. 2012, 20, 854–862. [Google Scholar] [CrossRef][Green Version]
- Haikal, S.M.; Abdeltawab, N.F.; Rashed, L.A.; Abd El-Galil, T.I.; Elmalt, H.A.; Amin, M.A. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019, 8, 823. [Google Scholar] [CrossRef]
- Altomonte, L.; Zoli, A.; Mirone, L.; Scolieri, P.; Magaró, M. Serum levels of interleukin-1b, tumour necrosis factor-a and interleukin-2 in rheumatoid arthritis. Correlation with disease activity. Clin. Rheumatol. 1992, 11, 202–205. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Anaparti, V.; Hitchon, C.; Mookherjee, N. Buprenorphine Alters Inflammatory and Oxidative Stress Molecular Markers in Arthritis. Mediat. Inflamm. 2017, 2017, 2515408. [Google Scholar] [CrossRef]
- Jing, R.; Ban, Y.; Xu, W.; Nian, H.; Guo, Y.; Geng, Y.; Zang, Y.; Zheng, C. Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine 2019, 58, 152825. [Google Scholar] [CrossRef]
- Smolen, J.S.; Steiner, G. Therapeutic strategies for rheumatoid arthritis. Nat. Rev. Drug Discov. 2003, 2, 473–488. [Google Scholar] [CrossRef]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gupta, D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018, 108, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, Y.; Yao, C.; Liu, X.; Liu, J.; Yu, B. DC32, a Dihydroartemisinin Derivative, Ameliorates Collagen-Induced Arthritis Through an Nrf2-p62-Keap1 Feedback Loop. Front. Immunol. 2018, 9, 2762. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zeng, S.; Huang, M.; Qiu, Q.; Xiao, Y.; Shi, M.; Zhan, Z.; Liang, L.; Yang, X.; Xu, H. Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Br. J. Pharmacol. 2017, 174, 893–908. [Google Scholar] [CrossRef]
- Xie, C.; Ma, L.; Liu, J.; Li, X.; Pei, H.; Xiang, M.; Chen, L. SKLB023 Blocks Joint Inflammation and Cartilage Destruction in Arthritis Models via Suppression of Nuclear Factor-Kappa B Activation in Macrophage. PLoS ONE 2013, 8, e56349. [Google Scholar] [CrossRef]
- Min, S.Y.; Yan, M.; Du, Y.; Wu, T.; Khobahy, E.; Kwon, S.R.; Taneja, V.; Bashmakov, A.; Nukala, S.; Ye, Y.; et al. Intra-articular nuclear factor-κB blockade ameliorates collagen-induced arthritis in mice by eliciting regulatory T cells and macrophages. Clin. Exp. Immunol. 2013, 172, 217–227. [Google Scholar] [CrossRef]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Wu, W.-J.; Jia, W.-W.; Liu, X.-H.; Pan, L.-L.; Zhang, Q.-Y.; Yang, D.; Shen, X.-Y.; Liu, L.; Zhu, Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016, 10, 157–167. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2007, 47, 409–414. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Harnett, W.; Liew, F.Y.; Harnett, M.M. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology 2003, 109, 415–425. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castejón, M.L.; Alarcón-de-la-Lastra, C.; Rosillo, M.Á.; Montoya, T.; Fernández-Bolaños, J.G.; González-Benjumea, A.; Sánchez-Hidalgo, M. A New Peracetylated Oleuropein Derivative Ameliorates Joint Inflammation and Destruction in a Murine Collagen-Induced Arthritis Model via Activation of the Nrf-2/Ho-1 Antioxidant Pathway and Suppression of MAPKs and NF-κB Activation. Nutrients 2021, 13, 311. https://doi.org/10.3390/nu13020311
Castejón ML, Alarcón-de-la-Lastra C, Rosillo MÁ, Montoya T, Fernández-Bolaños JG, González-Benjumea A, Sánchez-Hidalgo M. A New Peracetylated Oleuropein Derivative Ameliorates Joint Inflammation and Destruction in a Murine Collagen-Induced Arthritis Model via Activation of the Nrf-2/Ho-1 Antioxidant Pathway and Suppression of MAPKs and NF-κB Activation. Nutrients. 2021; 13(2):311. https://doi.org/10.3390/nu13020311
Chicago/Turabian StyleCastejón, María Luisa, Catalina Alarcón-de-la-Lastra, María Ángeles Rosillo, Tatiana Montoya, Jose G. Fernández-Bolaños, Alejandro González-Benjumea, and Marina Sánchez-Hidalgo. 2021. "A New Peracetylated Oleuropein Derivative Ameliorates Joint Inflammation and Destruction in a Murine Collagen-Induced Arthritis Model via Activation of the Nrf-2/Ho-1 Antioxidant Pathway and Suppression of MAPKs and NF-κB Activation" Nutrients 13, no. 2: 311. https://doi.org/10.3390/nu13020311
APA StyleCastejón, M. L., Alarcón-de-la-Lastra, C., Rosillo, M. Á., Montoya, T., Fernández-Bolaños, J. G., González-Benjumea, A., & Sánchez-Hidalgo, M. (2021). A New Peracetylated Oleuropein Derivative Ameliorates Joint Inflammation and Destruction in a Murine Collagen-Induced Arthritis Model via Activation of the Nrf-2/Ho-1 Antioxidant Pathway and Suppression of MAPKs and NF-κB Activation. Nutrients, 13(2), 311. https://doi.org/10.3390/nu13020311