Cow’s Milk Allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants?
Abstract
1. Introduction
2. CMA and GERD: A Pathogenic Twist
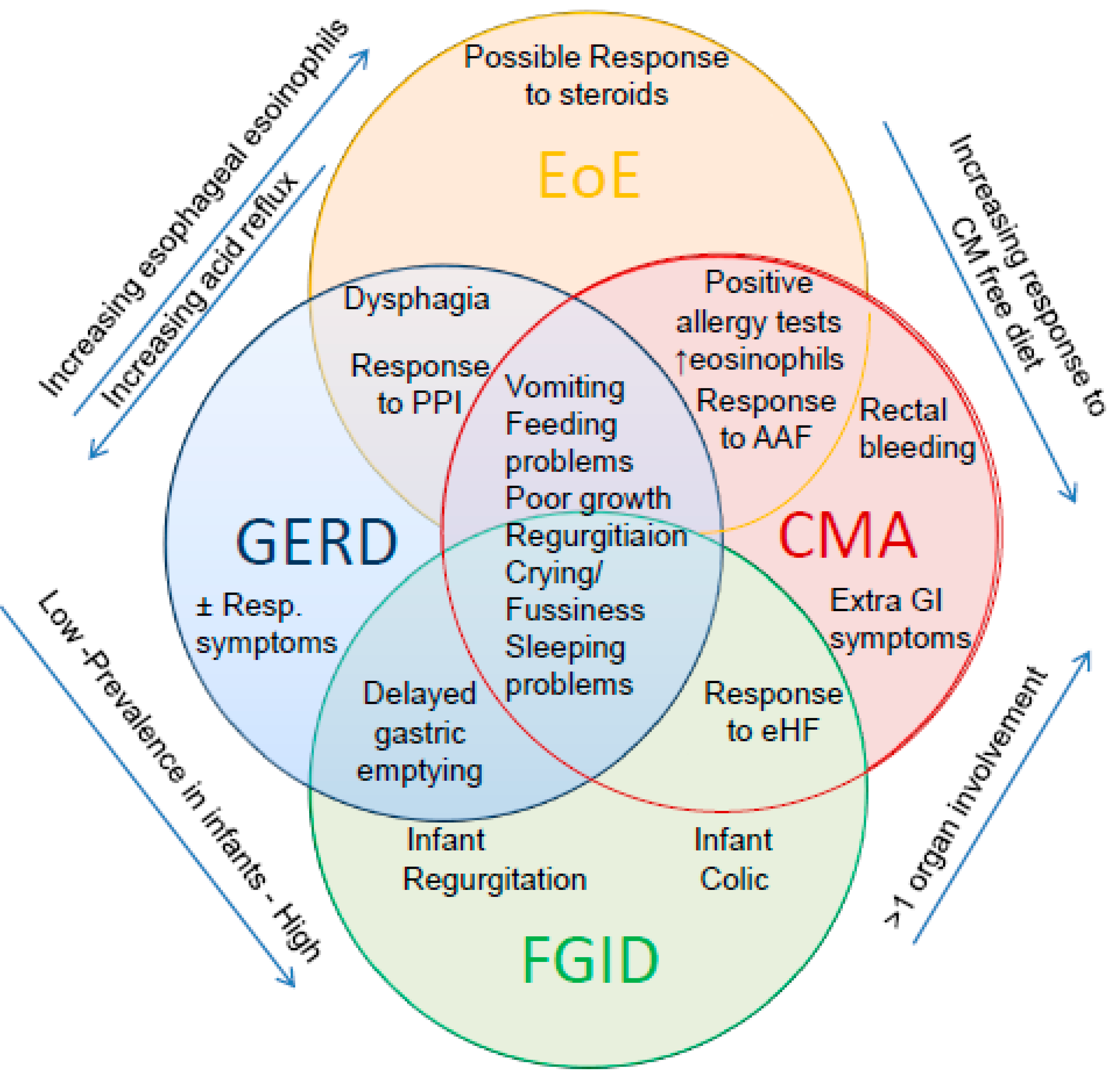
3. Functional Disorder, CMA or GERD: The Clinical Enigma
3.1. Definition and Epidemiological Data of Infant Regurgitation and Colic
3.2. Symptoms and Prevalence of GERD in Infants
3.3. Symptoms and Prevalence of CMA in Infants
3.4. Literature Data on the Association of CMA and GERD
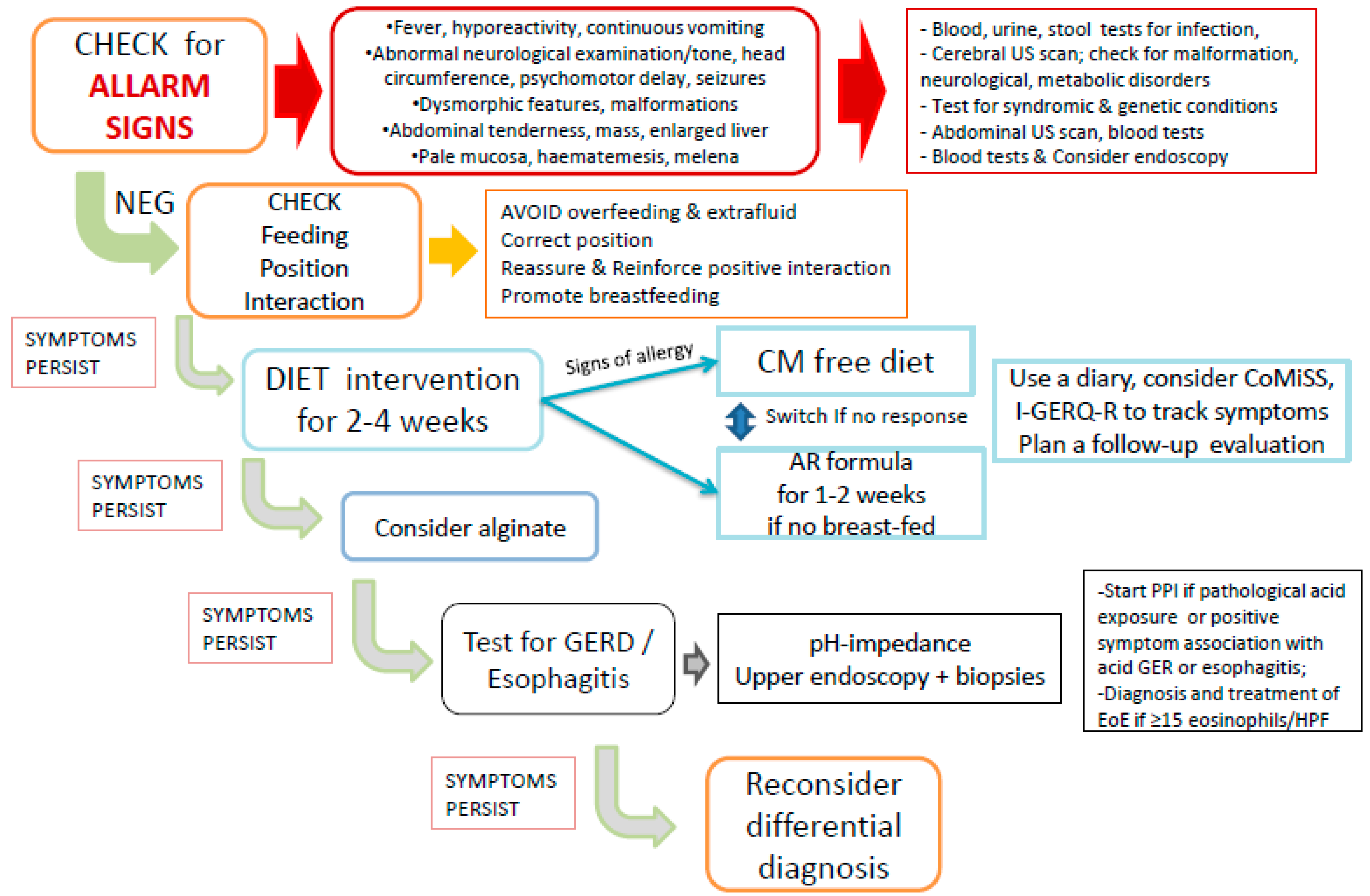
4. The Stepwise Approach to Infants with Regurgitation, Vomiting and Crying
4.1. Management of CMA and GER in Infants
4.2. Nutrition, Dietary Modification and Diagnosis of CMA in Infants
4.3. Diagnosis and Treatment of GER and GERD
5. The Third Wheel: Eosinophilic Esophagitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AAF | Amino acid-based formula |
| CM | cow’s milk |
| CMA | cow’s milk protein allergy |
| CoMiSS | cow’s milk related symptom score |
| EoE | eosinophilic esophagitis |
| FGIDs | functional gastrointestinal disorders |
| GER | gastroesophageal reflux |
| GERD | gastroesophageal reflux disease |
| I-GERQ-R | revised infant GER questionnaire |
| PPI | proton pump inhibitors |
References
- Salvatore, S.; Vandenplas, Y. Gastroesophageal reflux and cow milk allergy: Is there a link? Pediatrics 2002, 110, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. EAACI Food Allergy and Anaphylaxis Guidelines Group Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.A.; Chouraqui, J.P.; Çokuðraþ, F.; Harb, T.; Hegar, B.; Lifschitz, C.H.; Ludwig, T.; et al. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Benninga, M.; Broekaert, I.; Falconer, J.; Gottrand, F.; Guarino, A.; Lifschitz, C.; Lionetti, P.; Orel, R.; Papadopoulou, A.; et al. Functional gastro-intestinal disorder algorithms focus on early recognition, parental reassurance and nutritional strategies. Acta Paediatr. 2015, 105, 244–252. [Google Scholar] [CrossRef]
- Forget, P.; Arends, J.W. Cow’s milk protein allergy and gastro-oesophageal reflux. Eur. J. Pediatr. 1985, 144, 298–300. [Google Scholar] [CrossRef]
- McLain, B.I.; Cameron, D.J.S.; Barnes, G.L. Is cow’s milk protein intolerance a cause of gastro-oesophageal reflux in infancy? J. Paediatr. Child. Health 1994, 30, 316–318. [Google Scholar] [CrossRef]
- Cavataio, F.; Iacono, G.; Montalto, G.; Soresi, M.; Tumminello, M.; Campagna, P.; Notarbartolo, A.; Carroccio, A. Gastroesophageal reflux associated with cow’s milk allergy in infants, which diagnostic examinations are useful? Am. J. Gastroenterol. 1996, 91, 1215–1220. [Google Scholar]
- Cavataio, F.; Iacono, G.; Montalto, G.; Soresi, M.; Tumminello, M.; Carroccio, A. Clinical and pH-metric characteristics of gastro-oesophageal reflux secondary to cows’ milk protein allergy. Arch. Dis. Child. 1996, 75, 51–56. [Google Scholar] [CrossRef]
- Iacono, G.; Carroccio, A.; Cavataio, F.; Montalto, G.; Kazmierska, I.; Lorello, D.; Soresi, M.; Notarbartolo, A. Gastroesophageal reflux and cow’s milk allergy in infants, a prospective study. J. Allergy Clin. Immunol. 1996, 97, 822–827. [Google Scholar]
- Milocco, C.; Torre, G.; Ventura, A. Gastro-oesophageal reflux and cows’ milk protein allergy. Arch. Dis. Child. 1997, 77, 183. [Google Scholar] [CrossRef]
- Staiano, A.; Troncone, R.; Simeone, D.; Mayer, M.; Finelli, E.; Cella, A.; Auricchio, S. Differentiation of cows’ milk intolerance and gastro-oesophageal reflux. Arch. Dis. Child. 1995, 73, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Garzi, A.; Messina, M.; Frati, F.; Carfagna, L.; Zagordo, L.; Belcastro, M.; Parmiani, S.; Sensi, L.; Marcucci, F. An extensively hydrolysed cow’s milk formula im-proves clinical symptoms of gastroesophageal reflux and reduces the gastric emptying time in infants. Allergol. Immunopathol. 2002, 30, 36–41. [Google Scholar]
- Farahmand, F.; Najafi, M.; Ataee, P.; Modarresi, V.; Shahraki, T.; Rezaei, N. Cow’s milk allergy among children with gastroesopha-geal reflux disease. Gut Liver. 2011, 5, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Heine, R.G.; Cameron, D.J.; Cairo-Smith, A.G.; Chow, C.W.; Francis, D.E.; Hosking, C.S. Role of food protein intolerance in infants with persistent distress attributed to reflux esophagitis. J. Pediatr. 2000, 136, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G.; Jordan, B.; Lubitz, L.; Meehan, M.; Catto-Smith, A.G. Clinical predictors of pathological gastro-oesophageal reflux in infants with persistent distress. J. Paediatr. Child. Health 2006, 42, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.G.; Bindslev-Jensen, C.; Kruse-Andersen, S.; Husby, S. Severe Gastroesophageal Reflux Disease and Cow Milk Hypersensitivity in Infants and Children: Disease Association and Evaluation of a New Challenge Procedure. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 383–391. [Google Scholar] [CrossRef]
- Nielsen, R.G.; Fenger, C.; Bindslev-Jensen, C.; Husby, S. Eosinophilia in the upper gastrointestinal tract is not a characteristic fea-ture in cow’s milk sensitive gastro-oesophageal reflux disease Measurement by two methodologies. J. Clin. Pathol. 2006, 59, 89–94. [Google Scholar] [CrossRef]
- Semeniuk, J.; Kaczmarski, M. 24-hour esophageal pH monitoring in children with pathological acid gastroesophageal reflux: Primary and secondary to food allergy. Part, I. Intraesophageal pH values in distal channel; preliminary study and control studies--after 1, 2, 4 and 9 years of clinical observation as well as dietary and pharmacological treatment. Adv. Med. Sci. 2007, 52, 199–205. [Google Scholar]
- Semeniuk, J.; Kaczmarski, M.; Uścinowicz, M. Manometric study of lower esophageal sphincter in children with primary acid gastroesophageal reflux and acid gastroesophageal reflux secondary to food allergy. Adv. Med. Sci. 2008, 53. [Google Scholar] [CrossRef][Green Version]
- Ravelli, A.M.; Tobanelli, P.; Volpi, S.; Ugazio, A.G. Vomiting and gastric motility in infants with cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, O.; Mancini, V.; Thapar, N.; Giorgio, V.; Elawad, M.; Hill, S.; Shah, N.; Lindley, K.J. Cow’s Milk Challenge Increases Weakly Acidic Reflux in Children with Cow’s Milk Allergy and Gastroesophageal Reflux Disease. J. Pediatr. 2012, 161, 476–481.e1. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.T.; de Carvalho, E.; Sdepanian, V.L.; Morais, M.B.; Vieira, M.C.; Silva, L.R. Gastroesophageal reflux disease, exaggerations, evidence and clinical practice. J. Pediatr. 2014, 90, 105–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vandenplas, Y.; De Greef, E.; ALLAR Study Group. Extensive protein hydrolysate formula effectively reduces regurgitation in infants with positive and negative challenge tests for cow’s milk allergy. Acta Paediatr. 2014, 103, e243–e250. [Google Scholar] [CrossRef]
- Nocerino, R.; Pezzella, V.; Cosenza, L.; Amoroso, A.; Di Scala, C.; Amato, F.; Iacono, G.; Canani, R.B. The Controversial Role of Food Allergy in Infantile Colic: Evidence and Clinical Management. Nutrients 2015, 7, 2015–2025. [Google Scholar] [CrossRef]
- Yukselen, A.; Celtik, C. Food allergy in children with refractory gastroesophageal reflux disease. Pediatr. Int. 2015, 58, 254–258. [Google Scholar] [CrossRef]
- Pensabene, L.; Salvatore, S.; D’Auria, E.; Parisi, F.; Concolino, D.; Borrelli, O.; Thapar, N.; Staiano, A.; Vandenplas, Y.; Saps, M. Cow’s Milk Protein Allergy in Infancy: A Risk Factor for Functional Gastrointestinal Disorders in Children? Nutrients 2018, 10, 1716. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G.V. Cow’s Milk Allergy: Immunomodulation by Dietary Intervention. Nutrients 2019, 11, 1399. [Google Scholar] [CrossRef]
- Omari, T.; Tobin, J.M.; McCall, L. Characterization of Upper Gastrointestinal Motility in Infants with Persistent Distress and Non-IgE-mediated Cow’s Milk Protein Allergy. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 489–496. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children, ESPGHAN gastrointestinal committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.A.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.G.; Dahda, L.; Dupont, C.; Campoy, C.; Fierro, V.; Nieto, A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ. J. 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Vandenplas, Y.; Singendonk, M.; Cabana, M.; Di Lorenzo, C.; Gottrand, F.; Gupta, S.; Langendam, M.; Staiano, A.; Thapar, N.; et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines, Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2018, 66, 516–554. [Google Scholar] [PubMed]
- Quitadamo, P.; Tambucci, R.; Mancini, V.; Cristofori, F.; Baldassarre, M.; Pensabene, L.; Francavilla, R.; Di Nardo, G.; Caldaro, T.; Rossi, P.; et al. Esophageal pH-impedance monitoring in children: Position paper on indications, methodology and interpretation by the SIGENP working group. Dig. Liver Dis. 2019, 51, 1522–1536. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy, A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Schäppi, M.G.; Borrelli, O.; Knafelz, D.; Williams, S.; Smith, V.V.; Milla, P.J.; Lindley, K.J. Mast Cell–Nerve Interactions in Children with Functional Dyspepsia. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 472–480. [Google Scholar] [CrossRef]
- Shamir, R.; St James-Roberts, I.; Di Lorenzo, C.; Burns, A.J.; Thapar, N.; Indrio, F.; Riezzo, G.; Raimondi, F.; Di Mauro, A.; Francavilla, R.; et al. Infant crying, colic, and gastrointestinal discomfort in early childhood, a review of the evidence and most plausible mechanisms. J. Pediatr. Gastroenterol. Nutr. 2013, 57, S1. [Google Scholar]
- Salvatore, S.; Abkari, A.; Cai, W.; Catto-Smith, A.; Cruchet, S.; Gottrand, F.; Hegar, B.; Lifschitz, C.; Ludwig, T.; Shah, N.; et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018, 107, 1512–1520. [Google Scholar] [CrossRef]
- Yadlapati, R.; Kahrilas, P.J. The “dangers” of chronic proton pump inhibitor use. J. Allergy Clin. Immunol. 2018, 141, 79–81. [Google Scholar] [CrossRef]
- Levy, E.J.; Vandenplas, Y. Proton pump inhibitors, microbiota and micronutrients. Acta Paediatr. 2020, 109, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Nurko, S.; Faure, C.; Hyman, P.E.; Roberts, I.S.J.; Schechter, N.L. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455.e2. [Google Scholar] [CrossRef]
- Wolke, D.; Bilgin, A.; Samara, M. Systematic review and meta-analysis, fussing and crying durations and prevalence of colic in infants. J. Pediatr. 2017, 185, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bellaiche, M.; Oozeer, R.; Gerardi-Temporel, G.; Faure, C.; Vandenplas, Y. Multiple functional gastrointestinal disorders are frequent in formula-fed infants and decrease their quality of life. Acta Paediatr. 2018, 107, 1276–1282. [Google Scholar] [CrossRef]
- Salvatore, S.; Baldassarre, M.E.; Di Mauro, A.; Laforgia, N.; Tafuri, S.; Bianchi, F.P.; Dattoli, E.; Morando, L.; Pensabene, L.; Meneghin, F.; et al. Neonatal Antibiotics and Prematurity Are Associated with an Increased Risk of Functional Gastrointestinal Disorders in the First Year of Life. J. Pediatr. 2019, 212, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Di Mauro, A.; Salvatore, S.; Tafuri, S.; Bianchi, F.P.; Dattoli, E.; Morando, L.; Pensabene, L.; Meneghin, F.; DiLillo, D.; et al. Birth Weight and the Development of Functional Gastrointestinal Disorders in Infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 366–376. [Google Scholar] [CrossRef]
- Salvatore, S.; Barberi, S.; Borrelli, O.; Castellazzi, A.; Di Mauro, D.; Di Mauro, G.; Doria, M.; Francavilla, R.; Landi, M.; Martelli, A.; et al. Pharmacological interventions on early functional gastrointestinal disorders. Ital. J. Pediatr. 2016, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.P.; Chen, E.H.; Syniar, G.M. Prevalence of symptoms of gastroesophageal reflux in infancy. Arch. Pediatr. Adolesc. Med. 1997, 151, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Hegar, B.; Dewanti, N.R.; Kadim, M.; Alatas, S.; Firmansyah, A.; Vandenplas, Y. Natural evolution of regurgitation in healthy infants. Acta Paediatr. 2009, 98, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Vandenplas, Y. Epidemiology. In Gastroesophageal Reflux in Children; Vandenplas, Y., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Salvatore, S.; Hauser, B.; Vandemaele, K.; Novario, R.; Vandenplas, Y. Gastroesophageal reflux disease in infants, how much is predictable with questionnaires, pH-metry, endoscopy and histology? J. Pediatr. Gastroenterol. Nutr. 2005, 40, 210–215. [Google Scholar] [CrossRef]
- Orenstein, S.R.; Hassall, E.; Furmaga-Jablonska, W.; Atkinson, S.; Raanan, M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J. Pediatr. 2009, 154, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Arrigo, S.; Luini, C.; Vandenplas, Y. Esophageal Impedance in Children: Symptom-Based Results. J. Pediatr. 2010, 157, 949–954.e2. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Goyvaerts, H.; Helven, R.; Sacre, L. Gastroesophageal reflux, as measured by 24-hour pH monitoring, in 509 healthy infants screened for risk of sudden infant death syndrome. Pediatrics 1991, 88, 834–840. [Google Scholar] [PubMed]
- Gilger, M.A.; El-Serag, H.B.; Gold, B.D.; Dietrich, C.L.; Tsou, V.; McDuffie, A.; Shub, M.D. Prevalence of Endoscopic Findings of Erosive Esophagitis in Children: A Population-based Study. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Volonaki, E.; Sebire, N.J.; Borrelli, O.; Lindley, K.J.; Elawad, M.; Thapar, N.; Shah, N. Gastrointestinal endoscopy and mucosal biopsy in the first year of life, indications and outcome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Ripepi, A.; Huysentruyt, K.; Van De Maele, K.; Nosetti, L.; Agosti, M.; Salvatoni, A.; Vandenplas, Y. The Effect of Alginate in Gastroesophageal Reflux in Infants. Pediatr. Drugs 2018, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Pagliarin, F.; Huysentruyt, K.; Bosco, A.; Fumagalli, L.; Van De Maele, K.; Agosti, M.; Vandenplas, Y. Distress in Infants and Young Children, Don’t Blame Acid Reflux. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 465–469. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, K.M.; Juntunen-Backman, K.; Järvenpää, A.-L.; Klemetti, P.; Kuitunen, P.; Lope, L.; Renlund, M.; Siivola, M.; Vaarala, O.; Savilahti, E. Breast-Feeding and the Development of Cows’ Milk Protein Allergy. Adv. Exp. Med. Biol. 2000, 478, 121–130. [Google Scholar] [CrossRef]
- Meyer, R.; Chebar Lozinsky, A.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants-An EAACI Position Paper. Allergy 2020, 75, 14–32. [Google Scholar] [CrossRef]
- Hait, E.J.; McDonald, D.R. Impact of Gastroesophageal Reflux Disease on Mucosal Immunity and Atopic Disorders. Clin. Rev. Allergy Immunol. 2019, 57, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome, executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126. [Google Scholar] [PubMed]
- Labrosse, R.; Graham, F.; Caubert, J.C. Non-IgE-mediated gastrointestinal food allergies in children, an update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Ayerbe, J.I.; Hauser, B.; Salvatore, S.; Vandenplas, Y. Diagnosis and Management of Gastroesophageal Reflux Disease in Infants and Children, from Guidelines to Clinical Practice. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Savino, F.; Singendonk, M.; Tabbers, M.; Benninga, M.A.; Staiano, A.; Vandenplas, Y. Thickened formula, what to know. Nutrition 2018, 49, 51–56. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Di Mauro, A.; Pignatelli, M.C.; Fanelli, M.; Salvatore, S.; Di Nardo, G.; Chiaro, A.; Pensabene, L.; Laforgia, N. Magnesium Alginate in Gastro-Esophageal Reflux: A Randomized Multicenter Cross-Over Study in Infants. Int. J. Environ. Res. Public Health 2019, 17, 83. [Google Scholar] [CrossRef]
- Vandenplas, Y. Prevention and Management of Cow’s Milk Allergy in Non-Exclusively Breastfed Infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plant-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef]
- Salvatore, S.; Vandenplas, Y. Hydrolyzed Proteins in Allergy. Nestle Nutr. Inst. Workshop Ser. 2016, 86, 11–27. [Google Scholar]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Steenhout, P.; Järvi, A.; Garreau, A.-S.; Mukherjee, R. Pooled Analysis of the Cow’s Milk-related-Symptom-Score (CoMiSSTM) as a Predictor for Cow’s Milk Related Symptoms. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Bertoni, E.; Bogni, F.; Bonaita, V.; Armano, C.; Moretti, A.; Baù, M.; Luini, C.; D’Auria, E.; Marinoni, M.; et al. Testing the Cow’s Milk-Related Symptom Score (CoMiSSTM) for the Response to a Cow’s Milk-Free Diet in Infants, A Prospective Study. Nutrients. 2019, 11, 2402. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Salvatore, S.; Ribes-Koninckx, C.; Carvajal, E.; Szajewska, H.; Huysentruyt, K. The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. PLoS ONE 2018, 13, e0200603. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P. Colic in infants. BMJ Clin. Evid. 2010, 2010, 309. [Google Scholar]
- Iacovou, M.; Ralston, R.A.; Muir, J.; Walker, K.Z.; Truby, H. Dietary management of infantile colic, a systematic review. Matern. Child Health J. 2012, 16, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Biagioli, E.; Sorrenti, M.; Lingua, C.; Moja, L.; Banks, S.S.; Ceratto, S.; Savino, F. Dietary modification for infantile colic. Cochrane Database Syst. Rev. 2018, 10, CD011029. [Google Scholar] [CrossRef] [PubMed]
- Taubman, B. Parental counseling compared with elimination of cow’s milk or soy milk protein for the treatment of infant colic syndrome, a randomized trial. Pediatrics 1988, 81, 756–761. [Google Scholar]
- Savino, F.; Palumeri, E.; Castagno, E.; Cresi, F.; Dalmasso, P.; Cavallo, F.; Oggero, R. Reduction of crying episodes owing to infantile colic: A randomized controlled study on the efficacy of a new infant formula. Eur. J. Clin. Nutr. 2006, 60, 1304–1310. [Google Scholar] [CrossRef]
- Orenstein, S.R. Symptoms and reflux in infants, Infant Gastroesophageal Reflux Questionnaire Revised (I-GERQ-R)—Utility for symptom tracking and diagnosis. Curr. Gastroenterol. Rep. 2010, 12, 431–436. [Google Scholar] [CrossRef]
- Gieruszczak-Białek, D.; Konarska, Z.; Skórka, A.; Vandenplas, Y.; Szajewska, H. No Effect of Proton Pump Inhibitors on Crying and Irritability in Infants: Systematic Review of Randomized Controlled Trials. J. Pediatr. 2015, 166, 767–770.e3. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, Á.; Molina-Infante, J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remis-sion in Patients With Symptomatic Esophageal Eosinophilia, A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 13–22. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Gastro-Oesophageal Reflux Disease, Recognition, Diagnosis and Management in Children and Young People. (Clinical Guideline 193). 2015. Available online: http,//www.nice.org.uk/guidance/NG1 (accessed on 30 April 2015).
- Levy, E.I.; Salvatore, S.; Vandenplas, Y.; De Winter, J.P. Prescription of acid inhibitors in infants: An addiction hard to break. Eur. J. Pediatr. 2020, 179, 1957–1961. [Google Scholar] [CrossRef]
- Mantegazza, C.; Mallardo, S.; Rossano, M.; Meneghin, F.; Ricci, M.; Rossi, P.; Capra, G.; Latorre, P.; Schindler, A.; Isoldi, S.; et al. Laryngeal signs and pH-multichannel intraluminal impedance in infants and children, The missing ring, LPR and MII-pH in children. Dig. Liver Dis. 2020, 52, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.J.; Lazenby, A.J.; Rowe, P.C.; Yardley, J.H.; Perman, J.A.; Sampson, H.A. Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with an amino acid-based formula. Gastroenterology 1995, 109, 1503–1512. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Koletzko, S.; Heuschkel, R.; Dias, J.; Allen, K.; Murch, S.; Chong, S.; Gottrand, F.; Husby, S.; Lionetti, P.; et al. Management Guidelines of Eosinophilic Esophagitis in Childhood. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Fleischer, D.M. Diets for diagnosis and management of food allergy, The role of the dietitian in eosinophilic esophagitis in adults and children. Ann. Allergy Asthma Immunol. 2016, 117, 468–471. [Google Scholar] [CrossRef]
- Atwal, K.; Hubbard, G.P.; Venter, C.; Stratton, R.J. The use of amino acid-based nutritional feeds is effective in the dietary management of pediatric eosinophilic oesophagitis. Immunity Inflamm. Dis. 2019, 7, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.T.; Kuhl, J.T.; Martin, L.J.; Rothenberg, M.E.; Dellon, E.S. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J. Allergy Clin. Immunol. 2018, 141, 214–222. [Google Scholar] [CrossRef]
- Votto, M.; Marseglia, G.L.; De Filippo, M.; Brambilla, I.; Caimmi, S.M.E.; Licari, A. Early Life Risk Factors in Pediatric EoE: Could We Prevent This Modern Disease? Front. Pediatr. 2020, 8, 263. [Google Scholar] [CrossRef]
- Cheng, E. Translating New Developments in Eosinophilic Esophagitis Pathogenesis into Clinical Practice. Curr Treat Options Gastroenterol. 2015, 13, 30–46. [Google Scholar] [CrossRef]
| Author, Year | Population | Investigation | Main Results |
|---|---|---|---|
| Forget, 1985 [5] | 15 children with recurrent vomiting | Contrast X-ray, small bowel biopsy | All children showed GER on X-ray. 3/15 (20%) had enteropathy with IgE plasmatocytes, reported no improvement with GER treatment but disappearance on symptoms on CM free diet |
| McLain, 1994 [6] | 10 infants with GERD who failed to respond to reflux treatment | pH-monitoring | Symptoms improved in 2/10 (20%) infants on CM free diet. No infant showed significant improvement in pH monitoring indices |
| Staiano, 1995 [11] | 25 infants with recurrent vomiting | Endoscopy and small bowel biopsies, permeability test | Primary GERD in 16/25 (64%), GERD + CMA in 4/25 (16%), CMA alone in 4/25 (16%). Enteropathy in 19% GERD, 67% CMA. Abnormal permeability test in 6% GERD, 100% CMA |
| Iacono, 1996 [9] | 204 infants (median age, 6.3 months) with GERD | pH-monitoring, upper endoscopy, allergy tests, CM challenge | 93 (45%) had positive allergy tests, 85 (42%) improved with hydrolyzed formula and reappeared on challenge. GER + CMA significantly associated with the presence of diarrhea or atopic dermatitis |
| Cavataio, 1996 [8] | 96 infants with suspected GERD, CMA and controls | Serum specific IgE and IgG, blood eosinophils, pH-monitoring, endoscopy, CM challenge | 14 out of 47 (30%) infants with GERD had CMA These infants had similar symptoms to those with primary GERD but significantly higher concentrations of total IgE, circulating eosinophils and IgG anti-beta lactoglobulin. A specific phasic pH pattern, with progressive decrease in pH tracing, occurred in 24/25 infants with CMA, 12/14 GERD + CMA and 0 controls. CM free diet improved only in the ones with CMA |
| Milocco, 1997 [10] | 112 infants with GERD | pH-monitoring, CM challenge | 18 infants (16%) had CMA, 10/18 had failure to thrive. A phasic pH-pattern was present in 1/18 with CMA and in 3 with only GERD |
| Hill, 2000 [14] | 19 infants with persistent distress and GER symptoms with no response to eHF and GERD treatment | Endoscopy, pH-monitoring, CM challenge | Nine infants had histologic evidence of esophagitis and 9 had inflammatory changes in the stomach and/or duodenum. Symptoms remitted in all infants within 2 weeks of starting AAF. On double blind challenge, after a median period of 3 months of AAF, 12 infants were still intolerant to CM |
| Ravelli, 2001 [21] | 26 vomiting infants (7 CMA, 9, GER, 10 controls) | Electrogastrography electrical impedance tomography, CM challenge | Children with CMA showed more gastric dysrythmia (67% vs. 29.4% GER and 30.4% controls) and delayed gastric emptying (89 ± 26 min) compared to infants with GERD (54 ± 13 min) and controls (62 ± 13 min). 7/7 CMA patients had regurgitation and/or vomiting, colic and positive family history of allergy |
| Garzi, 2002 [12] | 10 infants with GER symptoms, 10 controls | Ultrasonography to measure gastric emptying time-with CM formula and protein hydrolysate | All infants with a clinical diagnosis for GER showed delayed gastric emptying vs. normal subjects (205 vs. 124 min, p = 0.000). With eHF there was a significant improvement in gastric emptying time and symptoms especially in infants with positive skin-test and RAST |
| Nielsen, 2004 [17] | 18 infants and children (median age 8.7 years; range 2 months to 14.8 years) with GERD | Endoscopy, 48-h pH-metry (Day 1-elimination diet, Day 2-challenge test), 2nd CM challenge | 10 (56%) infants had CMA + GERD (higher acid exposure time vs. primary GERD), responded to CM free diet and had a positive challenge which was not associated with a significant increase in the esophageal acid exposure in the simultaneous pH monitoring |
| Nielsen, 2006 [18] | 17 infants and children (aged 2–178 months) (mean age of 7.8 years) with GERD | Endoscopy and biopsies, pH-monitoring, allergy tests, CM challenge | 10/17 (59%) were classified as CMA-GERD. Two patients showed >15 eosinophils at biopsies (=EoE) No differences in the number of eosinophils, mast cells or T cells were found between children with CMA and those with primary GERD |
| Semeniuk, 2007 [19] and 2008 [20] | 264 children with suspected GERD (mean age 21 ± 17 months) or CMA | Esophageal manometry, pH-monitoring, allergy tests and CM challenge | 138 children with GERD: 76 only GERD, 62 (23.5%) GER + CMA/FA, 32 only CMA/FA. No differences between primary GERD and GERD+ CMA in reflux parameters, in the mean values of resting LES pressure and LES length at baseline and during 2 years of follow-up |
| Farahmand, 2011 [13] | 81 children (aged 1mo-2 yrs, median 12.5 mo) with supsected GERD. | Clinical study | 54 (66%) responded to PPI, 27 (33%) to CM elimination diet |
| Borrelli, 2012 [22] | 17 children (median age: 14 months) with proven f CMA and suspected GERD | 48-h pH-impedance. Day 1-amino acid formula Day 2-challenge with cow’s milk | The total reflux episodes and the number of weakly acidic episodes were higher during CM challenge compared with the amino acid-based formula period. No differences were found for either acid or weakly alkaline reflux |
| Vandenplas, 2014 [24] | 72 Infants with suspected CMA | Clinical study comparing a thickened and non-thickened eHF casein formula: results after one month. | Regurgitation was reduced in all infants (from 6.4 ± 3.2 to 2.8 ± 2.9, p < 0.001) but fell more with the thickened hydrolyzed formula (−4.2 ± 3.2 regurgitations/day) vs. non thickened formula, especially in infants with a negative challenge (−3.9 ± 4.0 vs. −1.9 ± 3.4, ns). In the group with positive challenge the two formulas showed a similar decrease (−4.4 ± 2.6 vs. 4.7 ± 5.6). The global reduction of a symptom-based score was −7.4 points and the non-thickened hydrolysate was more effective in the group with a positive challenge (−9.2 vs. −5.7 points) |
| Yukselen, 2016 [26] | 151 children (aged 3–60 mo) with GERD resistant to 8 wks PPI treatment | skin prick test, specific serum IgE, eosinophil count, atopy patch test and CM challenge | 58 children (38.4%) had positive CM challenge and 28 (48%) of them had positive skin prck tests or IgE, 16 (28%) had positive patch tests. Bloody stools, atopic dermatitis and recurrent wheezing episodes were significantly more common in these children Vomiting and diarrhea were more common in non-IgE children. Ten children who had positive challenge were finally diagnosed as EoE |
| Omari, 2020 [29] | 50 infants with persistent crying, vomiting and/or food refusal (suspected to be GERD and or CMA related) | 48 h cry-fuss chart, I-GERQ-R, allergy tests, blinded milk elimination-challenge sequence, pH-impedance before and after CM elimination, 13C-octanoate breath test for gastric emptying, dual-sugar intestinal permeability, fecal calprotectin | 14 (28%) were diagnosed as non-IgE-mediated CMA, 17 (34%) had negative challenge, 19 were excluded for equivocal findings or incomplete data. No baseline differences in any of the tests or GERD parameters between infants with and without CMA. In the CMA group, CM elimination significantly reduced reflux symptoms, esophageal acid exposure, acid clearance time and increased impedance baseline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, S.; Agosti, M.; Baldassarre, M.E.; D’Auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s Milk Allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants? Nutrients 2021, 13, 297. https://doi.org/10.3390/nu13020297
Salvatore S, Agosti M, Baldassarre ME, D’Auria E, Pensabene L, Nosetti L, Vandenplas Y. Cow’s Milk Allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants? Nutrients. 2021; 13(2):297. https://doi.org/10.3390/nu13020297
Chicago/Turabian StyleSalvatore, Silvia, Massimo Agosti, Maria Elisabetta Baldassarre, Enza D’Auria, Licia Pensabene, Luana Nosetti, and Yvan Vandenplas. 2021. "Cow’s Milk Allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants?" Nutrients 13, no. 2: 297. https://doi.org/10.3390/nu13020297
APA StyleSalvatore, S., Agosti, M., Baldassarre, M. E., D’Auria, E., Pensabene, L., Nosetti, L., & Vandenplas, Y. (2021). Cow’s Milk Allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants? Nutrients, 13(2), 297. https://doi.org/10.3390/nu13020297









