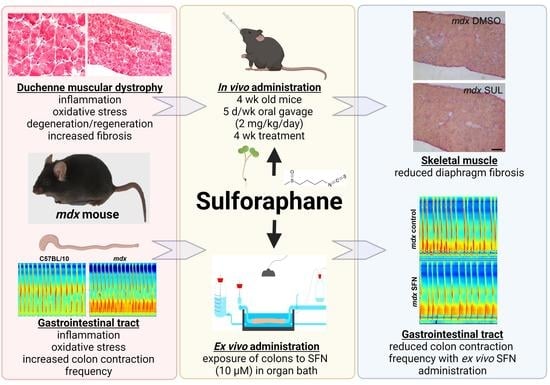
Investigating the Potential for Sulforaphane to Attenuate Gastrointestinal Dysfunction in mdx Dystrophic Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
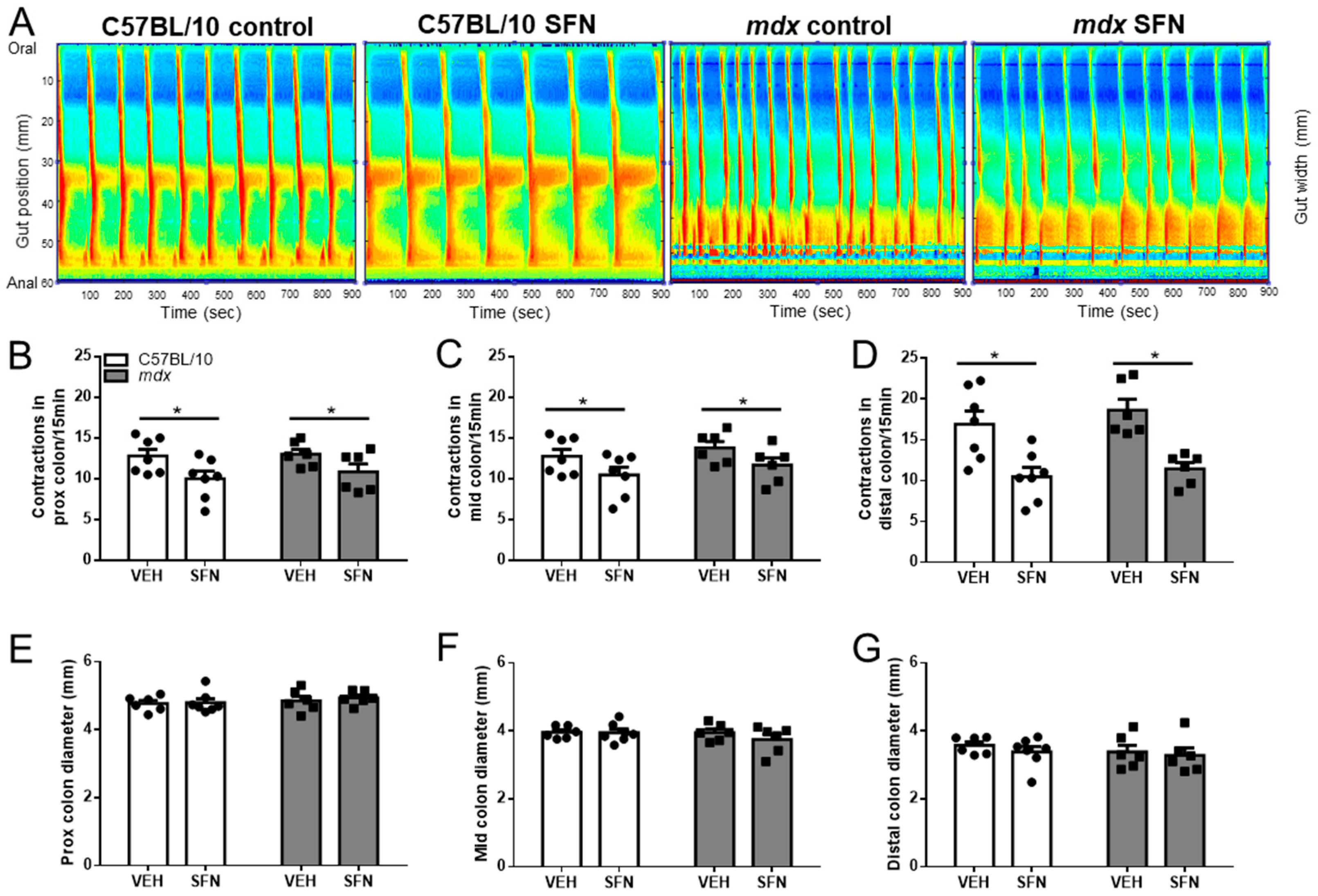
3.1. Sulforaphane Reduces Number of Contractions in Excised Colon Preparations Ex Vivo
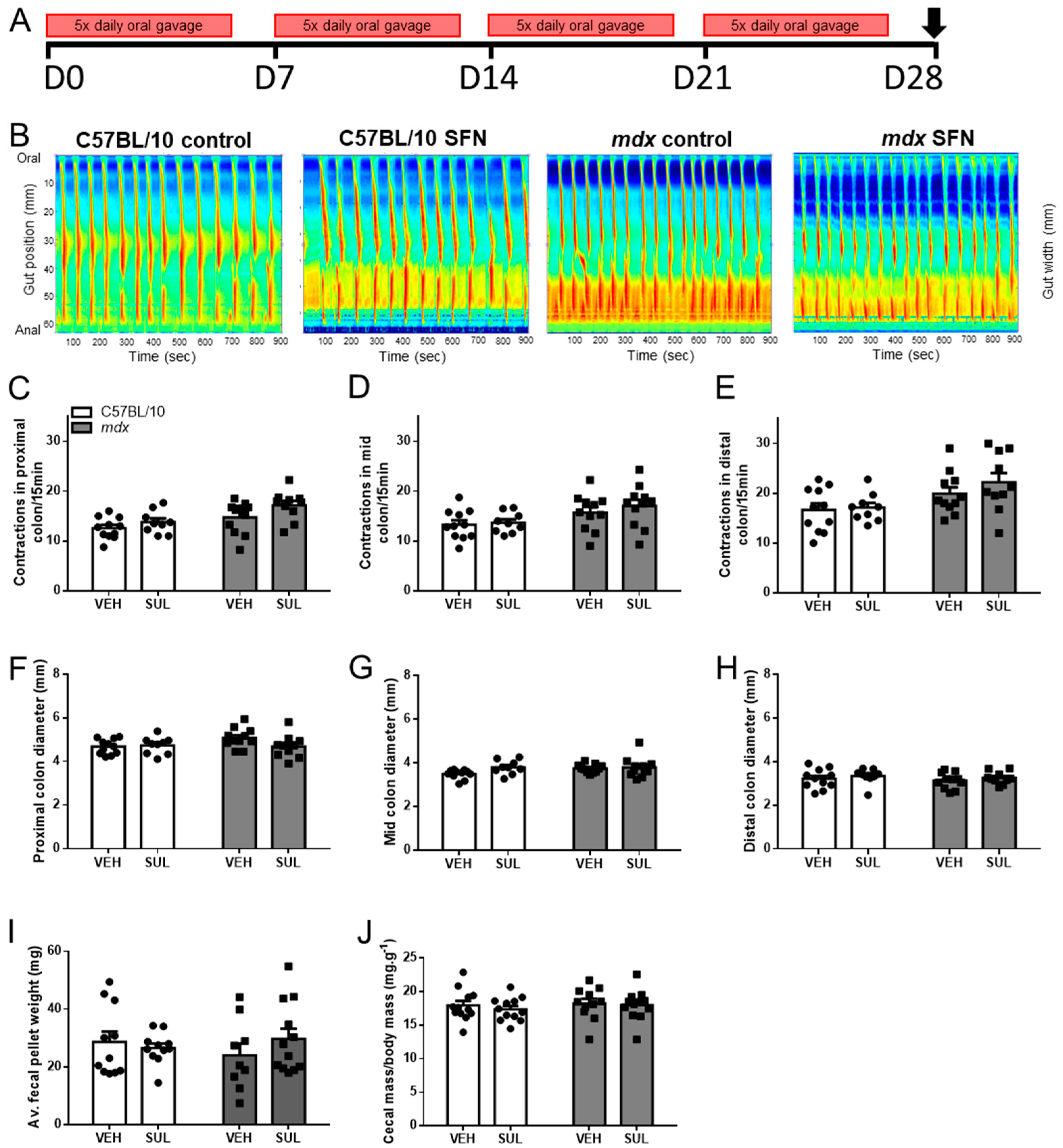
3.2. Oral Sulforaphane Supplementation Does Not Ameliorate Gastrointestinal Dysfunction in mdx Mice
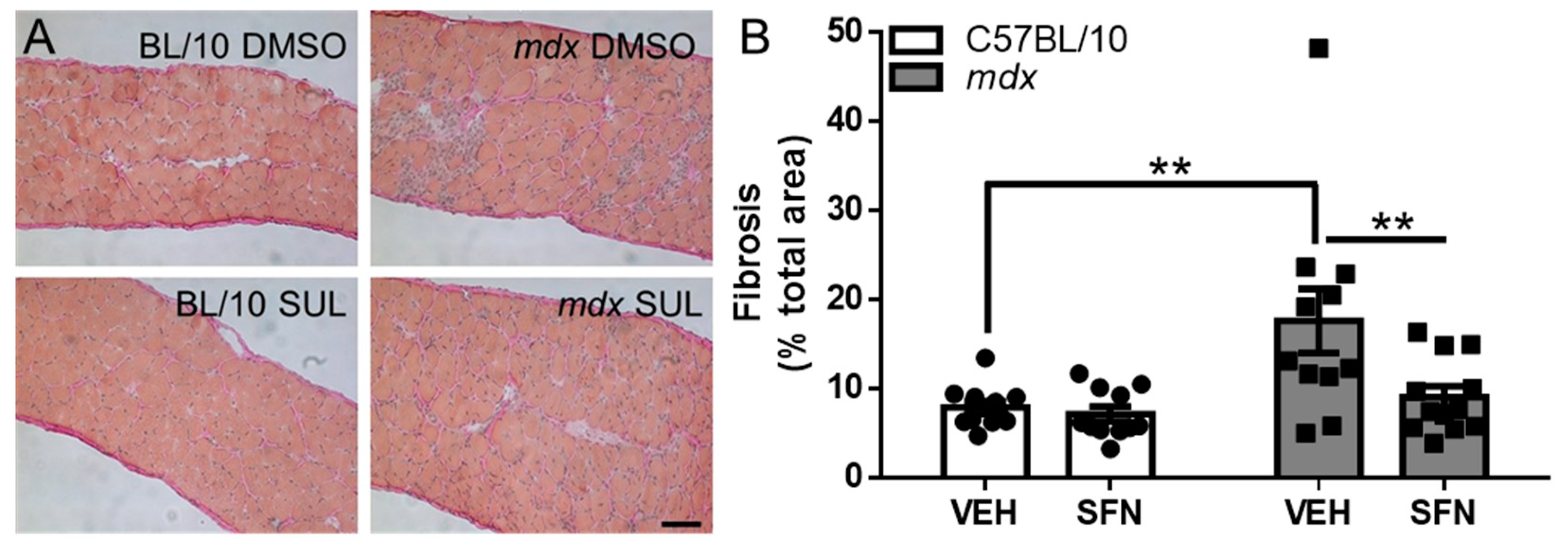
3.3. Oral Sulforaphane Supplementation Reduces Diaphragm Fibrosis but Not Other Markers of Dystrophic Pathology in mdx Mice
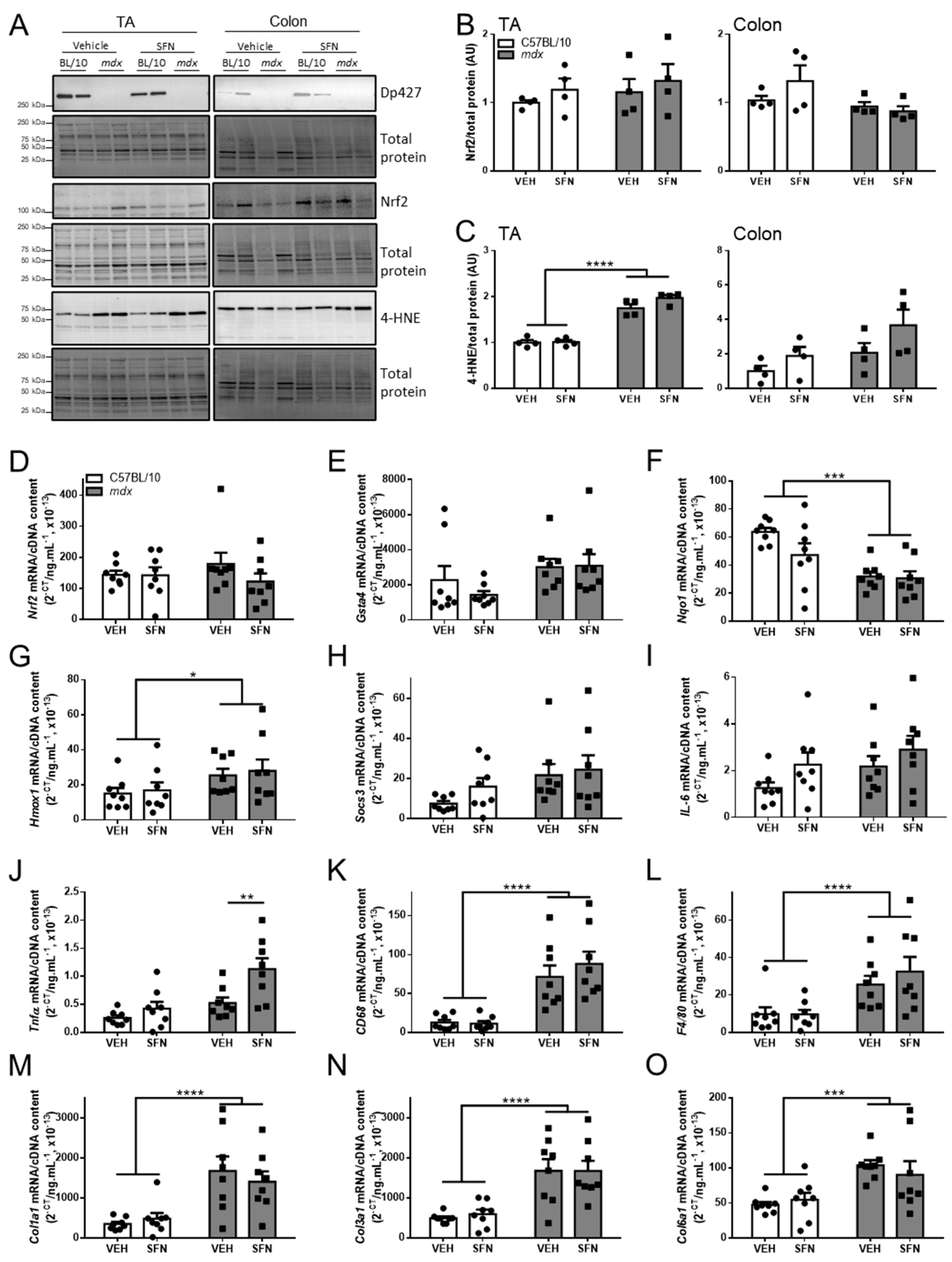
3.4. Oral Sulforaphane Supplementation Did Not Activate Nrf2 Signalling in C57BL/10 or mdx Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barohn, R.J.; Levine, E.J.; Olson, J.O.; Mendell, J.R. Gastric hypomotility in duchenne’s muscular dystrophy. N. Engl. J. Med. 1988, 319, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bensen, E.S.; Jaffe, K.M.; Tarr, P.I. Acute gastric dilatation in duchenne muscular dystrophy: A case report and review of the literature. Arch. Phys. Med. Rehabil. 1996, 77, 512–514. [Google Scholar] [CrossRef]
- Borrelli, O.; Salvia, G.; Mancini, V.; Santoro, L.; Tagliente, F.; Romeo, E.F.; Cucchiara, S. Evolution of gastric electrical features and gastric emptying in children with duchenne and becker muscular dystrophy. Am. J. Gastroenterol. 2005, 100, 695–702. [Google Scholar] [CrossRef]
- Dinan, D.; Levine, M.S.; Gordon, A.R.; Rubesin, S.E.; Rombeau, J.L. Gastric wall weakening resulting in separate perforations in a patient with duchenne’s muscular dystrophy. AJR Am. J. Roentgenol. 2003, 181, 807–808. [Google Scholar] [CrossRef]
- Okan, M.; Alper, E.; Cil, E.; Eralp, O.; Agir, H. Gastric emptying time in children with progressive muscular dystrophy. Turk. J. Pediatr. 1997, 39, 69–74. [Google Scholar]
- Robin, G.C.; de Falewski, G.L. Acute gastric dilatation in progressive muscular dystrophy. Lancet 1963, 2, 171–172. [Google Scholar] [CrossRef]
- Ronnblom, A.; Andersson, S.; Hellstrom, P.M.; Danielsson, A. Gastric emptying in myotonic dystrophy. Eur. J. Clin. Investig. 2002, 32, 570–574. [Google Scholar] [CrossRef]
- Stark, P.; Maves, C.; Wertz, R.A. Acute gastric dilatation as a manifestation of duchenne’s muscular dystrophy. Rofo 1988, 149, 554. [Google Scholar] [CrossRef]
- Davis, J.; Samuels, E.; Mullins, L. Nutrition considerations in duchenne muscular dystrophy. Nutr. Clin. Pract. 2015, 30, 511–521. [Google Scholar] [CrossRef]
- Pane, M.; Vasta, I.; Messina, S.; Sorleti, D.; Aloysius, A.; Sciarra, F.; Mangiola, F.; Kinali, M.; Ricci, E.; Mercuri, E. Feeding problems and weight gain in duchenne muscular dystrophy. Eur. J. Paediatr. Neurol. 2006, 10, 231–236. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Zardo, C.; Corsani, L.; Faussone-Pellegrini, M.S. Interstitial cells of cajal, enteric neurons, and smooth muscle and myoid cells of the murine gastrointestinal tract express full-length dystrophin. Histochem. Cell Biol. 2002, 118, 449–457. [Google Scholar] [CrossRef]
- Mule, F.; Amato, A.; Serio, R. Gastric emptying, small intestinal transit and fecal output in dystrophic (mdx) mice. J. Physiol. Sci. 2010, 60, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Mule, F.; D’Angelo, S.; Tabacchi, G.; Amato, A.; Serio, R. Mechanical activity of small and large intestine in normal and mdx mice: A comparative analysis. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 1999, 11, 133–139. [Google Scholar] [CrossRef]
- Mule, F.; Serio, R. Spontaneous mechanical activity and evoked responses in isolated gastric preparations from normal and dystrophic (mdx) mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2002, 14, 667–675. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Zizzo, M.G.; Zardo, C.; Pieri, L.; Serio, R.; Mule, F.; Faussone-Pellegrini, M.S. Ultrastructural changes in the interstitial cells of cajal and gastric dysrhythmias in mice lacking full-length dystrophin (mdx mice). J. Cell Physiol. 2004, 199, 293–309. [Google Scholar] [CrossRef]
- Manning, J.; Buckley, M.M.; O’Halloran, K.D.; O’Malley, D. In vivo neutralization of il-6 receptors ameliorates gastrointestinal dysfunction in dystrophin-deficient mdx mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2016, 28, 1016–1026. [Google Scholar] [CrossRef]
- Mule, F.; Vannucchi, M.G.; Corsani, L.; Serio, R.; Faussone-Pellegrini, M.S. Myogenic nos and endogenous no production are defective in colon from dystrophic (mdx) mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1264–G1270. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Tonali, P.; Servidei, S.; Azzena, G.B. Analysis of peristaltic reflex in young mdx dystrophic mice. NeuroSci. Lett. 1995, 192, 57–60. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Garella, R.; Cipriani, G.; Baccari, M.C. Relaxin counteracts the altered gastric motility of dystrophic (mdx) mice: Functional and immunohistochemical evidence for the involvement of nitric oxide. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E380–E391. [Google Scholar] [CrossRef][Green Version]
- Azzena, G.B.; Mancinelli, R. Nitric oxide regenerates the normal colonic peristaltic activity in mdx dystrophic mouse. NeuroSci. Lett. 1999, 261, 9–12. [Google Scholar] [CrossRef]
- Rosenberg, A.S.; Puig, M.; Nagaraju, K.; Hoffman, E.P.; Villalta, S.A.; Rao, V.A.; Wakefield, L.M.; Woodcock, J. Immune-mediated pathology in duchenne muscular dystrophy. Sci. Transl. Med. 2015, 7, 299rv4. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.M.; O’Halloran, K.D.; Rae, M.G.; Dinan, T.G.; O’Malley, D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J. Physiol. 2014, 592, 5235–5250. [Google Scholar] [CrossRef]
- O’Malley, D.; Cryan, J.F.; Dinan, T.G. Crosstalk between interleukin-6 and corticotropin-releasing factor modulate submucosal plexus activity and colonic secretion. Brain. Behav. Immun. 2013, 30, 115–124. [Google Scholar] [CrossRef]
- Lernoux, M.; Schnekenburger, M.; Dicato, M.; Diederich, M. Anti-cancer effects of naturally derived compounds targeting histone deacetylase 6-related pathways. Pharmacol. Res. 2018, 129, 337–356. [Google Scholar] [CrossRef]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.G.; Chen, T.Y.; Fahey, J.W.; Talalay, P. Keap1-nrf2 signaling: A target for cancer prevention by sulforaphane. Nat. Prod. Cancer Prev. Ther. 2013, 329, 163–177. [Google Scholar]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by nrf2 through binding to the amino-terminal neh2 domain. Genes. Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A. Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress in vitro, and demonstrates anti-inflammatory effects on helicobacter pylori-infected gastric mucosae in mice and human subjects. Curr. Pharm. Des. 2011, 17, 1532–1540. [Google Scholar] [CrossRef]
- Yanaka, A. Role of sulforaphane in protection of gastrointestinal tract against h. Pylori and nsaid-induced oxidative stress. Curr. Pharm. Des. 2017, 23, 4066–4075. [Google Scholar] [CrossRef]
- Yanaka, A. Role of nrf2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A. Contribution of nrf2 in gastrointestinal protection from oxidative injury. Curr. Pharm. Des. 2018, 24, 2023–2033. [Google Scholar] [CrossRef]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef]
- Yanaka, A.; Sato, J.; Ohmori, S. Sulforaphane protects small intestinal mucosa from aspirin/nsaid-induced injury by enhancing host defense systems against oxidative stress and by inhibiting mucosal invasion of anaerobic enterobacteria. Curr. Pharm. Des. 2013, 19, 157–162. [Google Scholar] [PubMed]
- Sun, C.; Li, S.; Li, D. Sulforaphane mitigates muscle fibrosis in mdx mice via nrf2-mediated inhibition of tgf-beta/smad signaling. J. Appl. Physiol. 2016, 120, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yang, C.; Xue, R.; Li, S.; Zhang, T.; Pan, L.; Ma, X.; Wang, L.; Li, D. Sulforaphane alleviates muscular dystrophy in mdx mice by activation of nrf2. J. Appl. Physiol. 2015, 118, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Li, S.J.; Yang, C.L.; Xue, R.L.; Xi, Y.Y.; Wang, L.; Zhao, Q.L.; Li, D.J. Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via nf-e2-related factor 2 (nrf2)-mediated inhibition of nf-kappab signaling pathway. J. Biol. Chem. 2015, 290, 17784–17795. [Google Scholar] [CrossRef]
- Swiderski, K.; Bindon, R.; Trieu, J.; Naim, T.; Schokman, S.; Swaminathan, M.; Leembruggen, A.J.L.; Hill-Yardin, E.L.; Koopman, R.; Bornstein, J.C.; et al. Spatiotemporal mapping reveals regional gastrointestinal dysfunction in mdx dystrophic mice ameliorated by oral l-arginine supplementation. J. Neurogastroenterol. Motil. 2020, 26, 133–146. [Google Scholar] [CrossRef]
- Murphy, K.T.; Ryall, J.G.; Snell, S.M.; Nair, L.; Koopman, R.; Krasney, P.A.; Ibebunjo, C.; Holden, K.S.; Loria, P.M.; Salatto, C.T.; et al. Antibody-directed myostatin inhibition improves diaphragm pathology in young but not adult dystrophic mdx mice. Am. J. Pathol. 2010, 176, 2425–2434. [Google Scholar] [CrossRef]
- Swiderski, K.; Todorov, M.; Gehrig, S.M.; Naim, T.; Chee, A.; Stapleton, D.I.; Koopman, R.; Lynch, G.S. Tranilast administration reduces fibrosis and improves fatigue resistance in muscles of mdx dystrophic mice. Fibrogenesis Tissue Repair 2014, 7, 1. [Google Scholar] [CrossRef]
- Swaminathan, M.; Hill-Yardin, E.; Ellis, M.; Zygorodimos, M.; Johnston, L.A.; Gwynne, R.M.; Bornstein, J.C. Video imaging and spatiotemporal maps to analyze gastrointestinal motility in mice. J. Vis. Exp. 2016, 108, e53828. [Google Scholar] [CrossRef]
- Swiderski, K.; Thakur, S.S.; Naim, T.; Trieu, J.; Chee, A.; Stapleton, D.I.; Koopman, R.; Lynch, G.S. Muscle-specific deletion of socs3 increases the early inflammatory response but does not affect regeneration after myotoxic injury. Skelet. Muscle 2016, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, C.; Gosselin, L.E.; Schertzer, J.D.; Ryall, J.G.; Swiderski, K.; Wondemaghen, M.; Lynch, G.S. Ageing prolongs inflammatory marker expression in regenerating rat skeletal muscles after injury. J. Inflamm. 2011, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, O.L.; Bhattacharya, A.; Marshall, J.R.; Zhang, Y. Organ-specific exposure and response to sulforaphane, a key chemopreventive ingredient in broccoli: Implications for cancer prevention. Br. J. Nutr. 2013, 109, 25–32. [Google Scholar] [CrossRef]
- Langston-Cox, A.; Anderson, D.; Creek, D.J.; Palmer, K.; Wallace, E.M.; Marshall, S.A. Measuring sulforaphane and its metabolites in human plasma: A high throughput method. Molecules 2020, 25, 829. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Li, X.; Zou, P.; Schwartz, S.J.; Sun, D. Kinetics of sulforaphane in mice after consumption of sulforaphane-enriched broccoli sprout preparation. Mol. Nutr. Food Res. 2013, 57, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Hsu, A.; Williams, D.E.; Dashwood, R.H.; Stevens, J.F.; Yamamoto, M.; Ho, E. Metabolism and tissue distribution of sulforaphane in nrf2 knockout and wild-type mice. Pharm. Res. 2011, 28, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Myzak, M.C.; Dashwood, W.M.; Orner, G.A.; Ho, E.; Dashwood, R.H. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in apc-minus mice. FASEB J. 2006, 20, 506–508. [Google Scholar] [CrossRef]
- Singh, K.; Randhwa, G.; Salloum, F.N.; Grider, J.R.; Murthy, K.S. Decreased smooth muscle function, peristaltic activity, and gastrointestinal transit in dystrophic (mdx) mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2021, 33, e13968. [Google Scholar] [CrossRef]
- Pilgram, G.S.; Potikanond, S.; Baines, R.A.; Fradkin, L.G.; Noordermeer, J.N. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol. Neurobiol. 2010, 41, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pu, D.; Zhao, Y.; Chen, J.; Zhu, S.; Lu, A.; Liao, Z.; Sun, Y.; Xiao, Q. Sulforaphane protects against skeletal muscle dysfunction in spontaneous type 2 diabetic db/db mice. Life Sci. 2020, 255, 117823. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.; Alves, I.; Singh, P.; Palade, P.T.; Carvalho, E.; Borsheim, E.; Jun, S.R.; Cheema, A.; Boerma, M.; Awasthi, S.; et al. Sulforaphane prevents age-associated cardiac and muscular dysfunction through nrf2 signaling. Aging Cell 2020, 19, e13261. [Google Scholar] [CrossRef]
- Malaguti, M.; Angeloni, C.; Garatachea, N.; Baldini, M.; Leoncini, E.; Collado, P.S.; Teti, G.; Falconi, M.; Gonzalez-Gallego, J.; Hrelia, S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009, 107, 1028–1036. [Google Scholar] [CrossRef]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y.; et al. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 12902. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Socs3 | GCTGGCCAAAGAAATAACCA | AGCTCACCAGCCTCATCTGT-3 |
| IL-6 | CCGGAGAGGAGACTTCACAG | TCCACGATTTCCCAGAGAAC |
| TNFα | GGCCTTCCTACCTTCAGACC | AGCAAAAGAGGAGGCAACAA |
| CD68 | TCCAAGCCCAAATTCAAATC | ATTGTATTCCACCGCCATGT |
| F4/80 | CATCAGCCATGTGGGTACAG | CATCACTGCCTCCACTAGCA |
| Nfe2l2 | CGCTGGAAAAAGAAGTGGGC | AGTGACTGACTGATGGCAGC |
| Nqo1 | AGCCAATCAGCGTTCGGTAT | GCCTCCTTCATGGCGTAGTT |
| Hmox1 | GAACCCAGTCTATGCCCCAC | GCGTGCAAGGGATGATTTCC |
| Col1a1 | CACCCTCAAGAGCCTGAGTC | GTTCGGGCTGATGTACCAGT |
| Col3a1 | ACCAAAAGGTGATGCTGGAC | GACCTCGTGCTCCAGTTAGC |
| Col6a1 | CCCCATTGGACCTAAAGGAT | TCTCCCACTTCACCCTCATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiderski, K.; Read, S.J.; Chan, A.S.; Chung, J.D.; Trieu, J.; Naim, T.; Koopman, R.; Lynch, G.S. Investigating the Potential for Sulforaphane to Attenuate Gastrointestinal Dysfunction in mdx Dystrophic Mice. Nutrients 2021, 13, 4559. https://doi.org/10.3390/nu13124559
Swiderski K, Read SJ, Chan AS, Chung JD, Trieu J, Naim T, Koopman R, Lynch GS. Investigating the Potential for Sulforaphane to Attenuate Gastrointestinal Dysfunction in mdx Dystrophic Mice. Nutrients. 2021; 13(12):4559. https://doi.org/10.3390/nu13124559
Chicago/Turabian StyleSwiderski, Kristy, Suzannah J. Read, Audrey S. Chan, Jin D. Chung, Jennifer Trieu, Timur Naim, René Koopman, and Gordon S. Lynch. 2021. "Investigating the Potential for Sulforaphane to Attenuate Gastrointestinal Dysfunction in mdx Dystrophic Mice" Nutrients 13, no. 12: 4559. https://doi.org/10.3390/nu13124559
APA StyleSwiderski, K., Read, S. J., Chan, A. S., Chung, J. D., Trieu, J., Naim, T., Koopman, R., & Lynch, G. S. (2021). Investigating the Potential for Sulforaphane to Attenuate Gastrointestinal Dysfunction in mdx Dystrophic Mice. Nutrients, 13(12), 4559. https://doi.org/10.3390/nu13124559