Ergogenic Effects of Green Tea Combined with Isolated Soy Protein on Increasing Muscle Mass and Exercise Performance in Resistance-Trained Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition of ISP with Green Tea
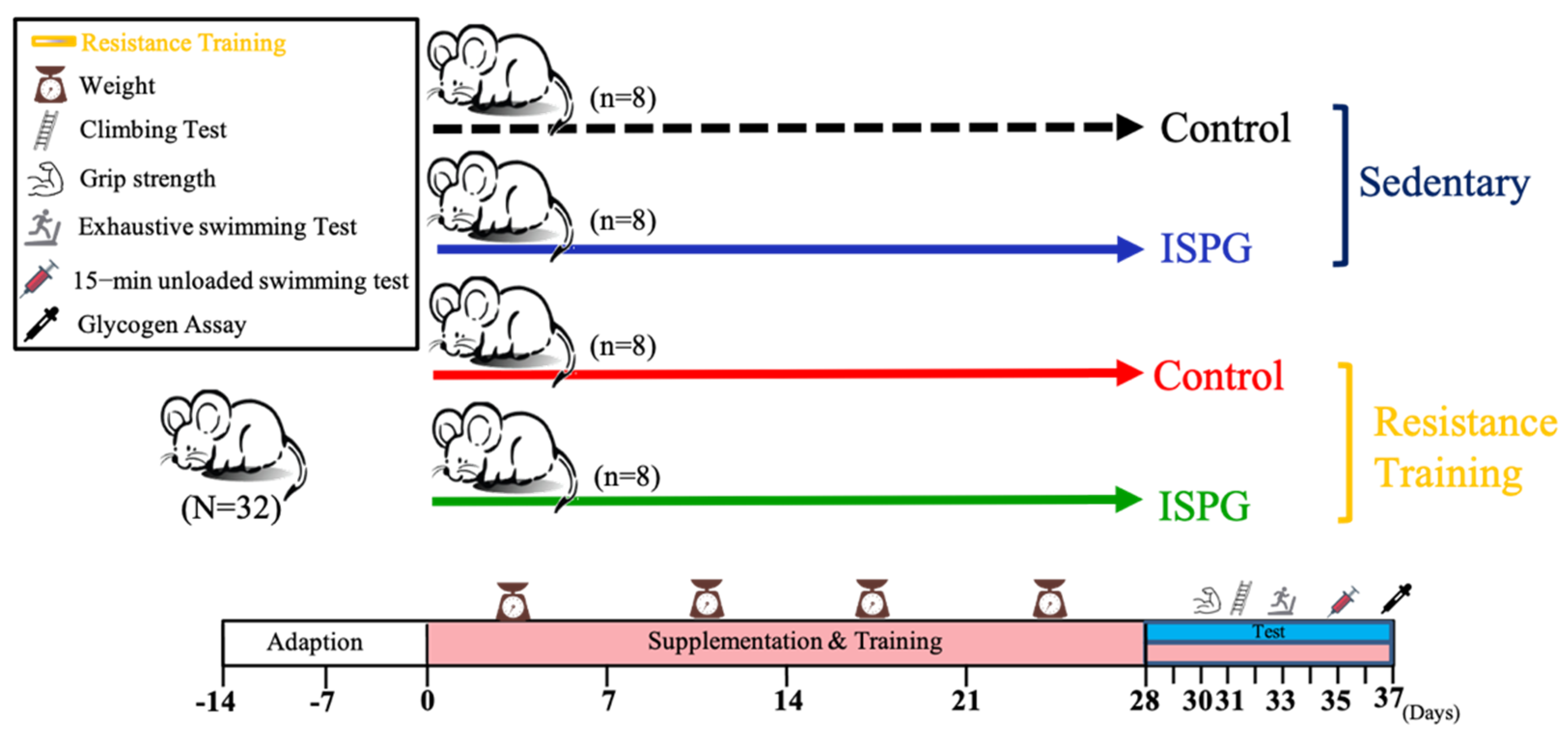
2.2. Animals and Experimental Design
2.3. Resistance Training Intervention and Test
2.4. Forelimb Grip Strength
2.5. Exercise Endurance Test
2.6. Determination of Fatigue-Associated Biochemical Variables
2.7. Body Composition, Glycogen Content and Histological Staining of Tissue
2.8. Haematological Assessment
2.9. Statistical Analysis
3. Results
3.1. Body Weight Dietary and Water Record
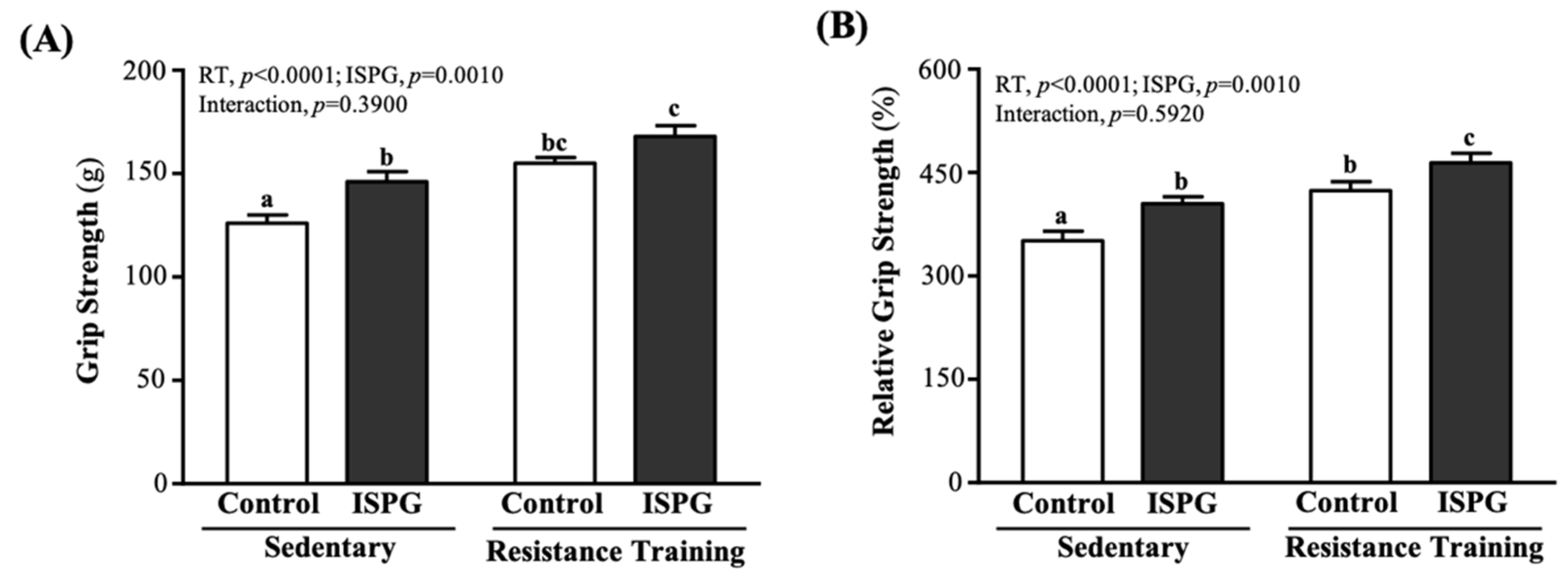
3.2. Effect of ISPG and RT on Grip Strength
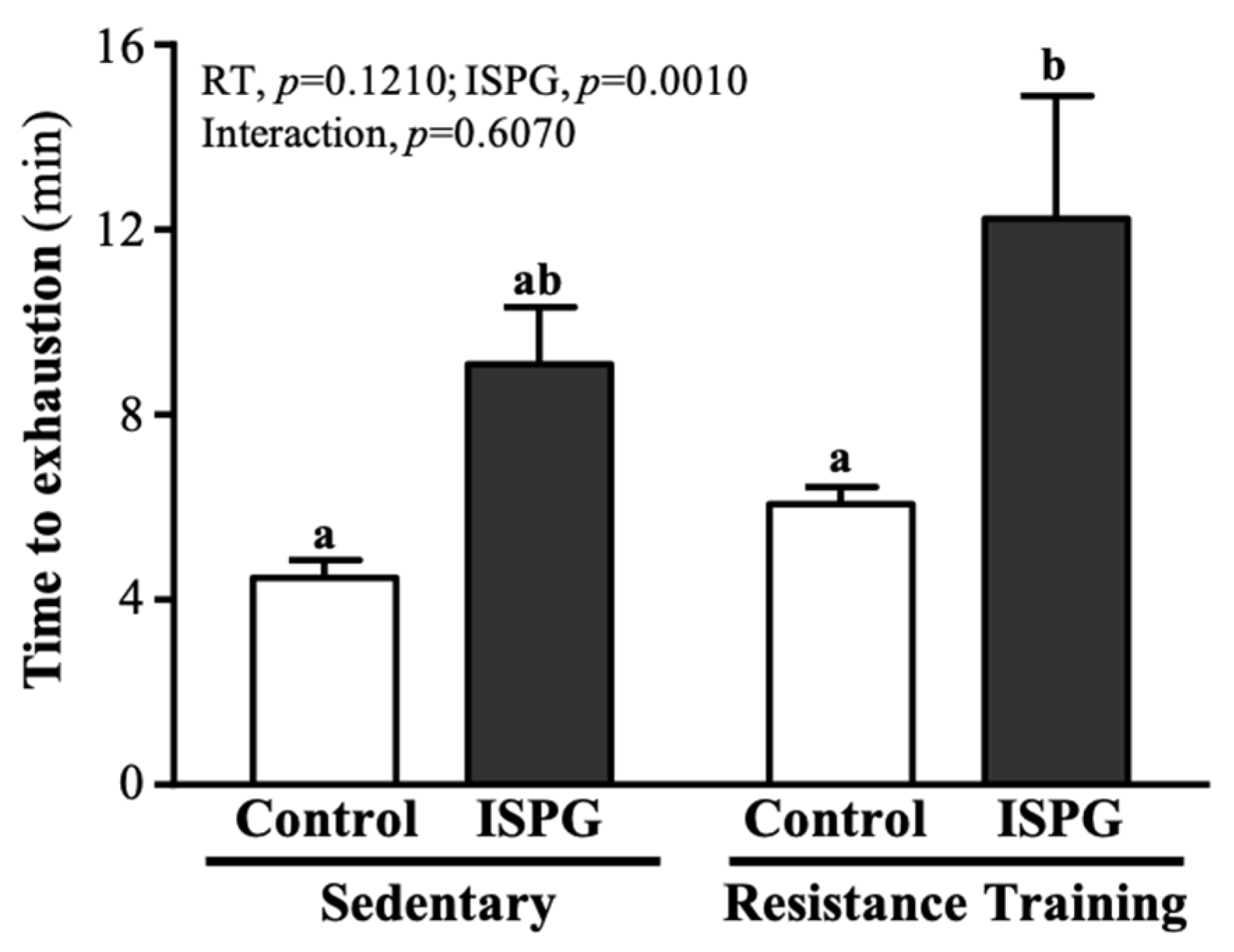
3.3. Effect of ISPG and RT on Swimming Endurance Test
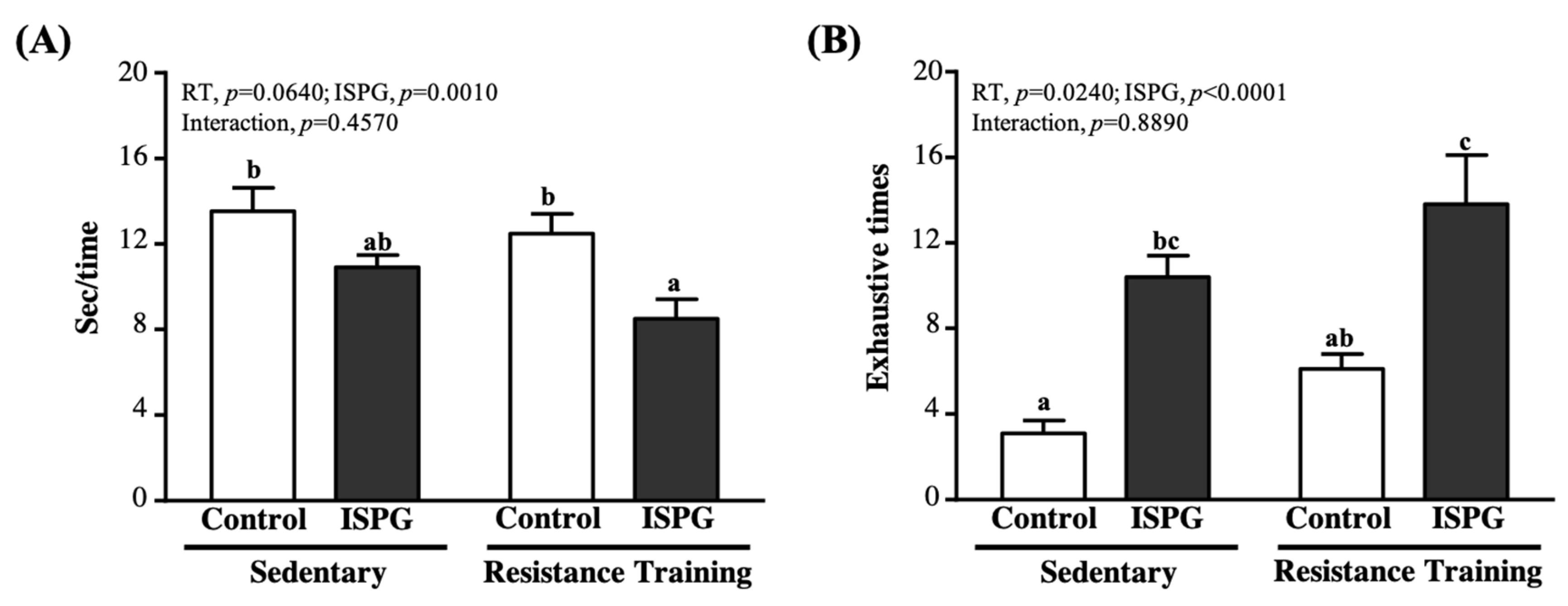
3.4. Effect of ISPG and RT on Muscle Strength and Muscular Endurance Test
3.5. Effect of ISPG and RT on Fatigue Indexes by 15 Min Exercise Challenge
3.6. Effects of ISPG and RT on Body Composition
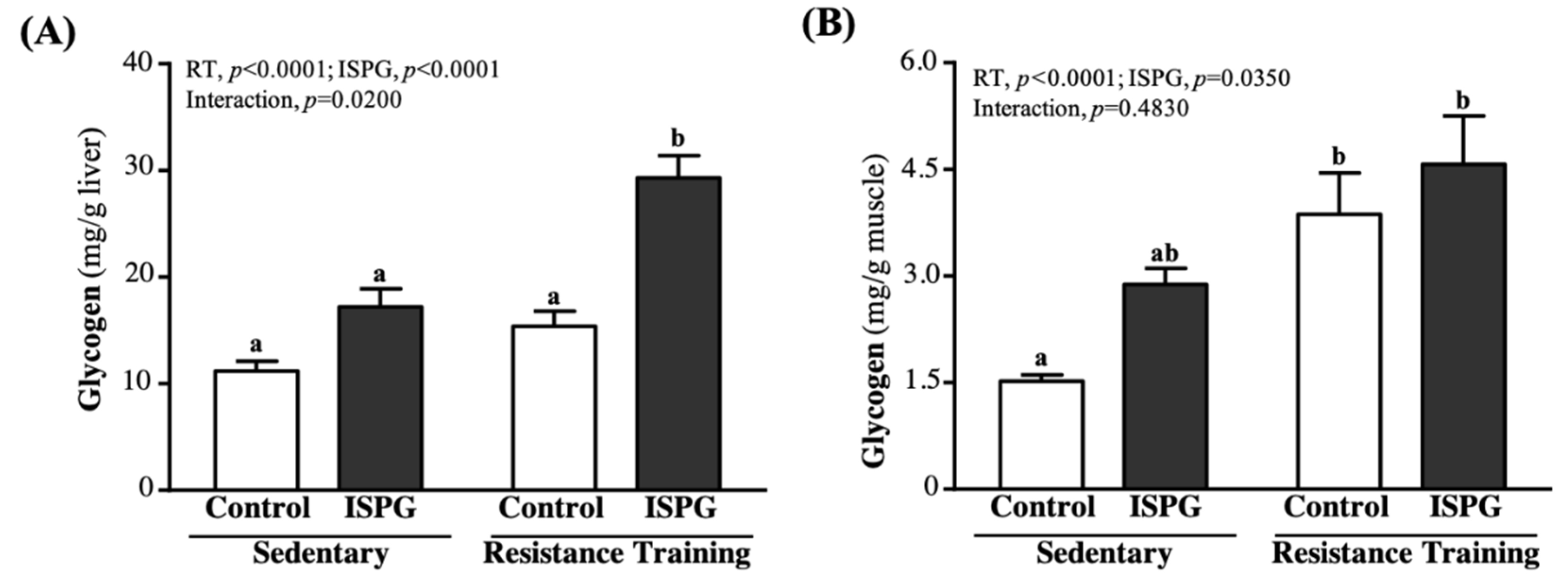
3.7. Effects of ISPG and RT on Liver and Muscular Storage
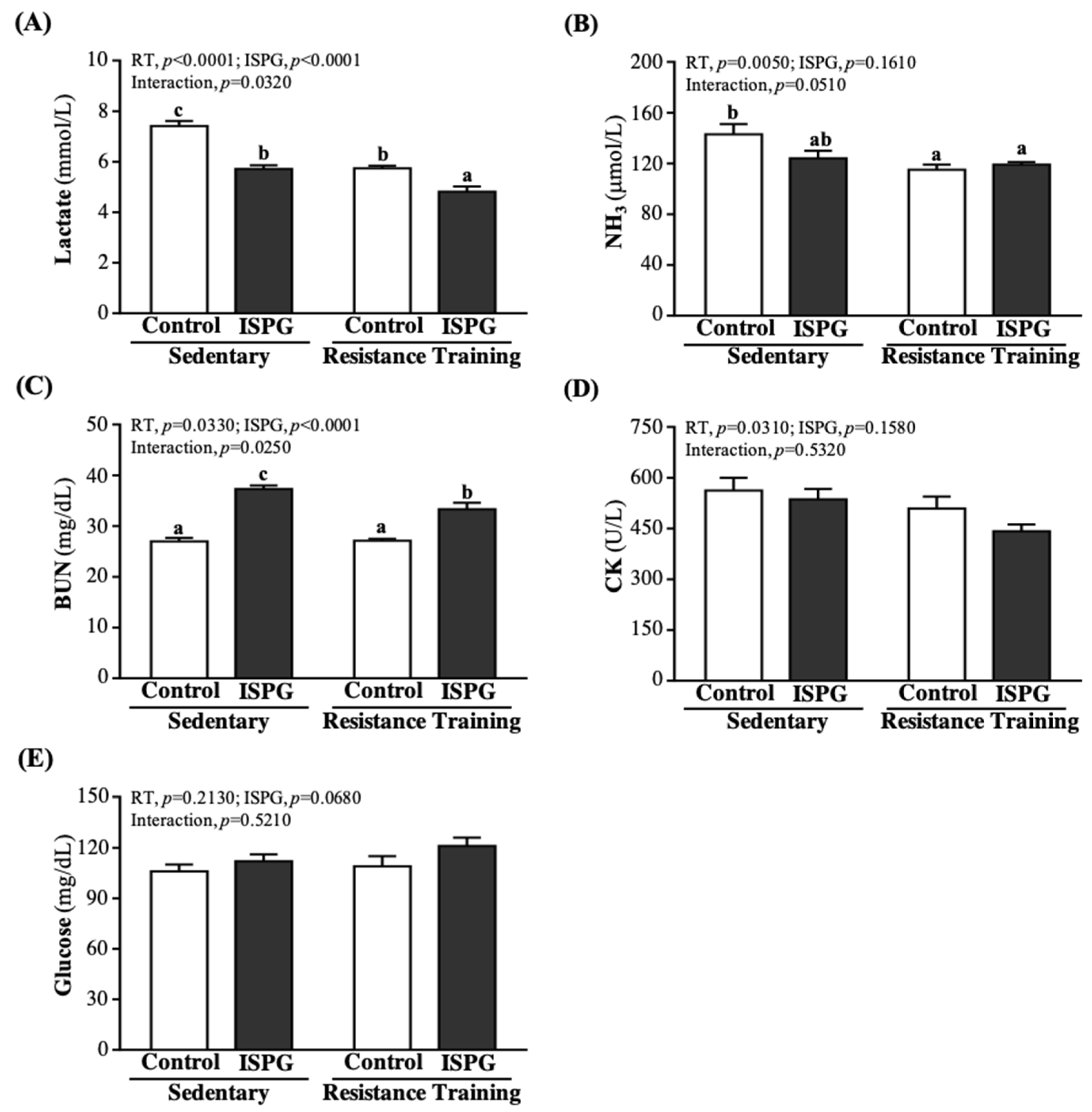
3.8. Effects of ISPG and RT on Clinical Biochemistries
3.9. Effects of ISPG and RT on Histological Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Butteiger, D.N.; Cope, M.; Liu, P.; Mukherjea, R.; Volpi, E.; Rasmussen, B.B.; Krul, E.S. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin. Nutr. 2013, 32, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. 1), A8–A15. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ogawa, S.; Egusa, S.; Ono, Y. Soymilk Improves Muscle Weakness in Young Ovariectomized Female Mice. Nutrients 2017, 9, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, S.; Dhawan, M.; Singh Sandhu, J. Four Weeks of Supplementation With Isolated Soy Protein Attenuates Exercise-Induced Muscle Damage and Enhances Muscle Recovery in Well Trained Athletes: A Randomized Trial. Asian J. Sports Med. 2016, 7, e33528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, Y.T.; Lee, M.C.; Ho, H.M.; Huang, C.C.; Hsu, Y.J. Effects of isolated soy protein and strength exercise training on exercise performance and biochemical profile in postpartum mice. Metabolism 2019, 94, 18–27. [Google Scholar] [CrossRef]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Huang, H.Y.; Hsu, Y.J.; Su, W.H.; Shen, S.Y.; Lee, M.C.; Lin, C.L.; Huang, C.C. The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement. Nutrients 2018, 10, 851. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Hsu, Y.J.; Chuang, H.L.; Hsieh, P.S.; Ho, H.H.; Chen, W.L.; Chiu, Y.S.; Huang, C.C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidy, P.T.; Borack, M.S.; Markofski, M.M.; Dickinson, J.M.; Deer, R.R.; Husaini, S.H.; Walker, D.K.; Igbinigie, S.; Robertson, S.M.; Cope, M.B.; et al. Protein Supplementation Has Minimal Effects on Muscle Adaptations during Resistance Exercise Training in Young Men: A Double-Blind Randomized Clinical Trial. J. Nutr. 2016, 146, 1660–1669. [Google Scholar] [CrossRef] [Green Version]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Tipton, K.D.; Hamilton, D.L.; Gallagher, I.J. Assessing the Role of Muscle Protein Breakdown in Response to Nutrition and Exercise in Humans. Sports Med. 2018, 48 (Suppl. 1), 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of Protein Supplementation on Performance and Recovery in Resistance and Endurance Training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; McLellan, T.M.; Lieberman, H.R. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: A systematic review. Sports Med. 2015, 45, 111–131. [Google Scholar] [CrossRef]
- Wu, M.; Wei, Y.X.; Yu, C.Q.; Du, H.D.; Lyu, J.; Guo, Y.; Bian, Z.; Tan, Y.L.; Pei, P.; Chen, J.S.; et al. China Kadoorie Biobank Collaborative Group. [Levels of skeletal muscle mass and handgrip strength in adults from 10 regions of China]. Zhonghua Liu Xing Bing Xue Za Zhi 2019, 40, 376–381. [Google Scholar]
- Hakkinen, K.; Myllyla, E. Acute effects of muscle fatigue and recovery on force production and relaxation in endurance, power and strength athletes. J. Sports Med. Phys. Fit. 1990, 30, 5–12. [Google Scholar]
- Rennie, M.J.; Tipton, K.D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu. Rev. Nutr. 2000, 20, 457–483. [Google Scholar] [CrossRef]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, 533s–537s. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. (1985) 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Pourmasoumi, M.; Kafeshani, M.; Karimian, J.; Maracy, M.R.; Entezari, M.H. The Effect of Green Tea and Sour Tea (Hibiscus sabdariffa L.) Supplementation on Oxidative Stress and Muscle Damage in Athletes. J. Diet. Suppl. 2017, 14, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K.; Tonkonogi, M.; Soderlund, K. Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 1998, 162, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Kurland, I.J.; Pilkis, S.J. Indirect versus direct routes of hepatic glycogen synthesis. Faseb. J. 1989, 3, 2277–2281. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of Muscle Glycogen Metabolism during Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, M. Interactions between muscle glycogen and blood glucose during exercise. Exerc. Sport Sci. Rev. 1997, 25, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, C.R.; Laursen, P.B. Models to explain fatigue during prolonged endurance cycling. Sports Med. 2005, 35, 865–898. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.L.; Wang, K.M.; Liao, P.I.; Kao, Y.H.; Huang, Y.C. Effects of an 8-Week Outdoor Brisk Walking Program on Fatigue in Hi-Tech Industry Employees: A Randomized Control Trial. Workplace Health Saf. 2015, 63, 436–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, H.; Bazgir, B.; Daryanoosh, F.; Koushki, M.; Sobhani, V. Effect of aerobic exercise and fish oil supplements on plasma levels of inflammatory indexes in mice. Med. J. Islam Repub. Iran. 2014, 28, 6. [Google Scholar]
- Matsui, Y.; Takayanagi, S.; Ohira, T.; Watanabe, M.; Murano, H.; Furuhata, Y.; Miyakawa, S. Effect of a leucine-enriched essential amino acids mixture on muscle recovery. J. Phys. Ther. Sci. 2019, 31, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chen, K.C.; Hsieh, C.L.; Peng, R.Y. Swimming exercise prevents fibrogenesis in chronic kidney disease by inhibiting the myofibroblast transdifferentiation. PLoS ONE 2012, 7, e37388. [Google Scholar] [CrossRef] [Green Version]


| Nutrients | Content/100 g ISPG |
|---|---|
| Protein | 60.4 g |
| Fat | 2.0 g |
| Total amount of saturated fatty acids | 0.5 g |
| Carbohydrate | 33.5 g |
| Total dietary fiber | 3.0 g |
| Sodium | 545 mg |
| Total calories | 383.4 kcal |
| BCAA | g/100 g ISPG |
| Leucine | 2.7 |
| Isoleucine | 2.9 |
| Valine | 3.0 |
| 0.46 g total catechin/100 g ISPG | mg/100 g ISPG |
| Catechin Gallate (CG) | - |
| Catechin (CT) | - |
| Epicatechin gallate (ECG) | 1.79 |
| Epicatechin (EC) | 1.47 |
| Epigallocatechin gallate | 21.39 |
| Epigallocatechin (EGC) | 16.70 |
| Gallocatechin (GCG) | - |
| Gallocatechin (GC) | 1.01 |
| Isoflavones | mg/100 g ISPG |
| Daidzin | 3.75 |
| Daidzein | 5.00 |
| Genistin | 11.25 |
| Genistein | 10.00 |
| p Values for Two-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Control +Sed | ISPG +Sed | Control +RT | ISPG +RT | Main Effect of RT | Main Effect of ISPG | Interaction (RT × ISPG) |
| Initial BW (g) | 30.14 ± 0.38 | 30.24 ± 0.27 | 30.11 ± 0.42 | 30.29 ± 0.42 | 0.9740 | 0.7180 | 0.9220 |
| Final BW (g) | 37.28 ± 0.41 | 37.26 ± 0.81 | 37.65 ± 1.15 | 37.34 ± 0.25 | 0.7650 | 0.8290 | 0.8420 |
| Food intake (g) | 7.49 ± 0.31 | 6.95 ± 0.23 | 7.81 ± 0.30 | 6.99 ± 0.28 | 0.5220 | 0.0160 * | 0.6150 |
| Water intake (mL) | 7.72 ± 0.09 ab | 6.95 ± 0.05 a | 7.76 ± 0.08 ab | 7.91 ± 0.10 b | 0.0050* | 0.6180 | 0.0250 * |
| Tissue weight (g) | |||||||
| Liver | 2.06 ± 0.05 | 2.07 ± 0.09 | 2.17 ± 0.06 | 2.07 ± 0.07 | 0.4250 | 0.5430 | 0.4660 |
| Gastrocnemius muscle | 0.39 ± 0.02 | 0.40 ± 0.01 | 0.40 ± 0.02 | 0.41 ± 0.01 | 0.4730 | 0.5260 | 0.8990 |
| Quadriceps | 0.48 ± 0.02 a | 0.53 ± 0.01 a | 0.54 ± 0.02 a | 0.55 ± 0.01 b | 0.0220 * | 0.0360 * | 0.2890 |
| Heart | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.00 | 0.8440 | 0.5560 | 0.5560 |
| Lung | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.5030 | 0.9110 | 0.5770 |
| Kidney | 0.75 ± 0.04 | 0.79 ± 0.03 | 0.78 ± 0.03 | 0.75 ± 0.03 | 0.8930 | 0.8620 | 0.2940 |
| EFP | 0.51 ± 0.04 b | 0.34 ± 0.05 a | 0.31 ± 0.03 a | 0.33 ± 0.03 a | 0.0130 * | 0.0620 | 0.0190 * |
| BAT | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.4820 | 0.4820 | 0.4820 |
| Relative tissue weight (%) | |||||||
| Liver | 5.53 ± 0.14 | 5.54 ± 0.18 | 5.78 ± 0.17 | 5.55 ± 0.17 | 0.4160 | 0.5150 | 0.4690 |
| Gastrocnemius muscle | 1.04 ± 0.04 | 1.06 ± 0.03 | 1.05 ± 0.03 | 1.09 ± 0.04 | 0.4880 | 0.4070 | 0.8350 |
| Quadriceps | 1.28 ± 0.06 a | 1.43 ± 0.02 b | 1.42 ± 0.03 b | 1.48 ± 0.04 b | 0.0150 * | 0.0130 * | 0.2780 |
| Heart | 0.50 ± 0.02 | 0.51 ± 0.02 | 0.52 ± 0.03 | 0.50 ± 0.01 | 0.9760 | 0.6140 | 0.5730 |
| Lung | 0.61 ± 0.04 | 0.63 ± 0.02 | 0.65 ± 0.03 | 0.63 ± 0.03 | 0.5770 | 0.9050 | 0.6610 |
| Kidney | 2.00 ± 0.07 | 2.11 ± 0.06 | 2.07 ± 0.08 | 2.01 ± 0.08 | 0.8230 | 0.7570 | 0.2350 |
| EFP | 1.37 ± 0.09 b | 0.89 ± 0.12 a | 0.83 ± 0.08 a | 0.89 ± 0.07 a | 0.0070 * | 0.0370 * | 0.0070 * |
| BAT | 0.29 ± 0.01 | 0.29 ± 0.02 | 0.29 ± 0.02 | 0.31 ± 0.01 | 0.4820 | 0.4820 | 0.5260 |
| p Values for Two-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Control +Sed | ISPG +Sed | Control +RT | ISPG +RT | Main Effect of RT | Main Effect of ISPG | Interaction (RT×ISPG) |
| AST (U/L) | 66 ± 4 | 68 ± 2 | 67 ± 3 | 67 ± 5 | 0.9210 | 0.7670 | 0.8690 |
| ALT (U/L) | 56 ± 3 | 55 ± 3 | 53.5 ± 3 | 52 ± 3 | 0.1980 | 0.5280 | 0.8660 |
| TC (mg/dL) | 132 ± 3 | 130 ± 3 | 132 ± 5 | 128 ± 6 | 0.8950 | 0.4720 | 0.8250 |
| TG (mg/dL) | 130 ± 4 | 128 ± 4 | 127 ± 4 | 117 ± 4 | 0.0900 | 0.1770 | 0.3190 |
| CK (U/L) | 80 ± 5 | 81 ± 7 | 82 ± 4 | 83 ± 5 | 0.7020 | 0.8110 | 0.9620 |
| LDH (U/L) | 427 ± 37 | 511 ± 45 | 390 ± 41 | 373 ± 43 | 0.0440 * | 0.4270 | 0.2299 |
| BUN (mg/dL) | 27.5 ± 0.6 a | 33.8 ± 1.5 b | 27.7 ± 0.8 a | 31.9 ± 0.9 b | 0.3980 | <0.0001 * | 0.2790 |
| CREA (mg/dL) | 0.45 ± 0.01 | 0.45 ± 0.01 | 0.43 ± 0.01 | 0.44 ± 0.01 | 0.1350 | 0.8420 | 0.6430 |
| UA (mg/dL) | 4.0 ± 0.2 | 4.2 ± 0.2 | 4.0 ± 0.3 | 4.1 ± 0.5 | 0.8300 | 0.6680 | 0.8580 |
| ALB (g/dL) | 3.1 ± 0.0 | 3.1 ± 0.0 | 3.1 ± 0.1 | 3.1 ± 0.0 | 0.6870 | 0.3510 | 0.8930 |
| TP (g/dL) | 5.1 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 | 5.2 ± 0.1 | 1.0000 | 0.1430 | 0.8030 |
| GLU | 235 ± 12 | 249 ± 12 | 243 ± 17 | 286 ± 28 | 0.2210 | 0.1370 | 0.4280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-C.; Hsu, Y.-J.; Yang, L.-H.; Huang, C.-C.; Ho, C.-S. Ergogenic Effects of Green Tea Combined with Isolated Soy Protein on Increasing Muscle Mass and Exercise Performance in Resistance-Trained Mice. Nutrients 2021, 13, 4547. https://doi.org/10.3390/nu13124547
Lee M-C, Hsu Y-J, Yang L-H, Huang C-C, Ho C-S. Ergogenic Effects of Green Tea Combined with Isolated Soy Protein on Increasing Muscle Mass and Exercise Performance in Resistance-Trained Mice. Nutrients. 2021; 13(12):4547. https://doi.org/10.3390/nu13124547
Chicago/Turabian StyleLee, Mon-Chien, Yi-Ju Hsu, Li-Hsuan Yang, Chi-Chang Huang, and Chun-Sheng Ho. 2021. "Ergogenic Effects of Green Tea Combined with Isolated Soy Protein on Increasing Muscle Mass and Exercise Performance in Resistance-Trained Mice" Nutrients 13, no. 12: 4547. https://doi.org/10.3390/nu13124547
APA StyleLee, M.-C., Hsu, Y.-J., Yang, L.-H., Huang, C.-C., & Ho, C.-S. (2021). Ergogenic Effects of Green Tea Combined with Isolated Soy Protein on Increasing Muscle Mass and Exercise Performance in Resistance-Trained Mice. Nutrients, 13(12), 4547. https://doi.org/10.3390/nu13124547








