Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Variables
2.2.1. Body Composition Variables
2.2.2. Muscle Strength
2.2.3. Dietary Calcium Intake
2.2.4. Family Socioeconomic Status
2.3. Statistical Analyses
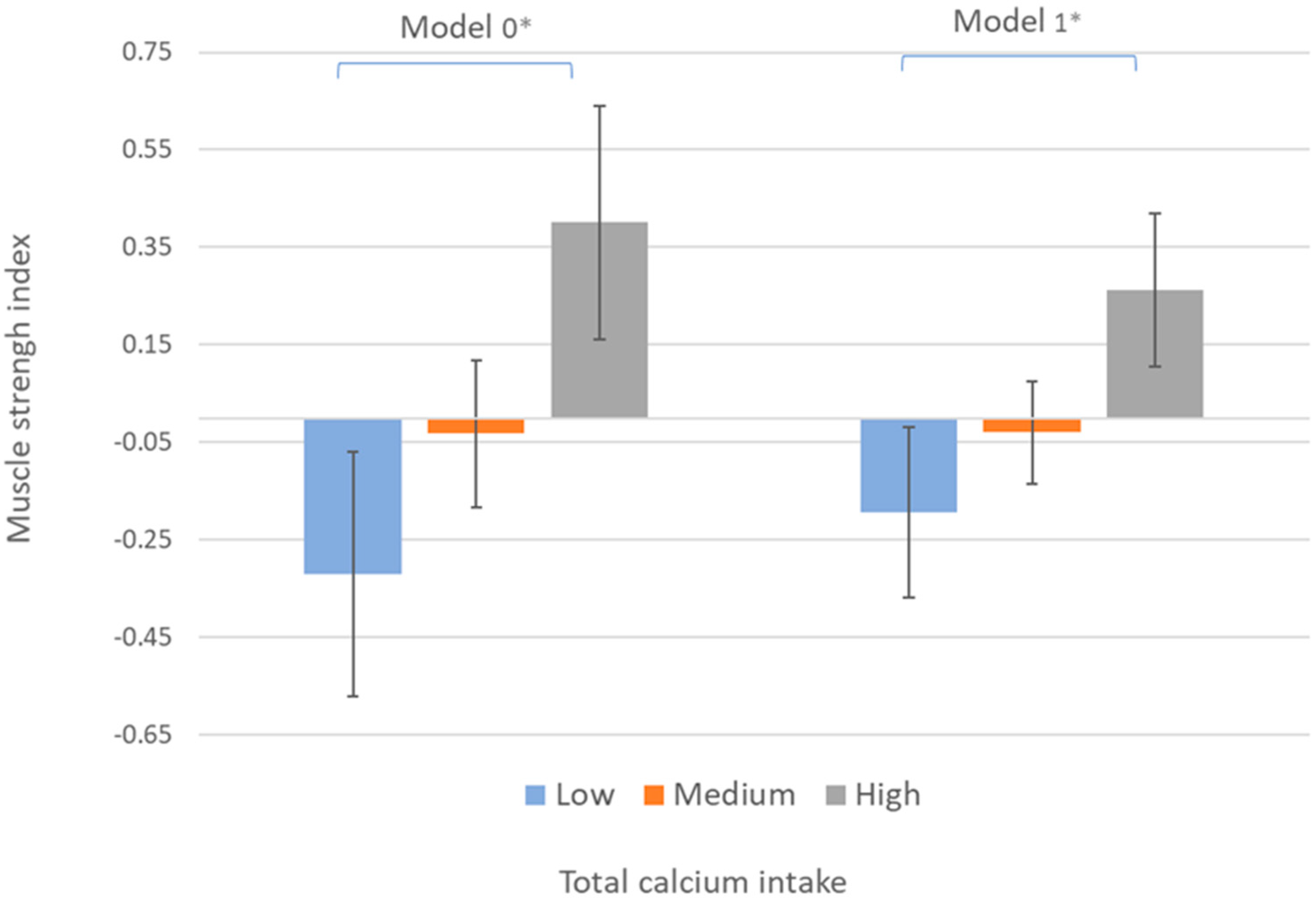
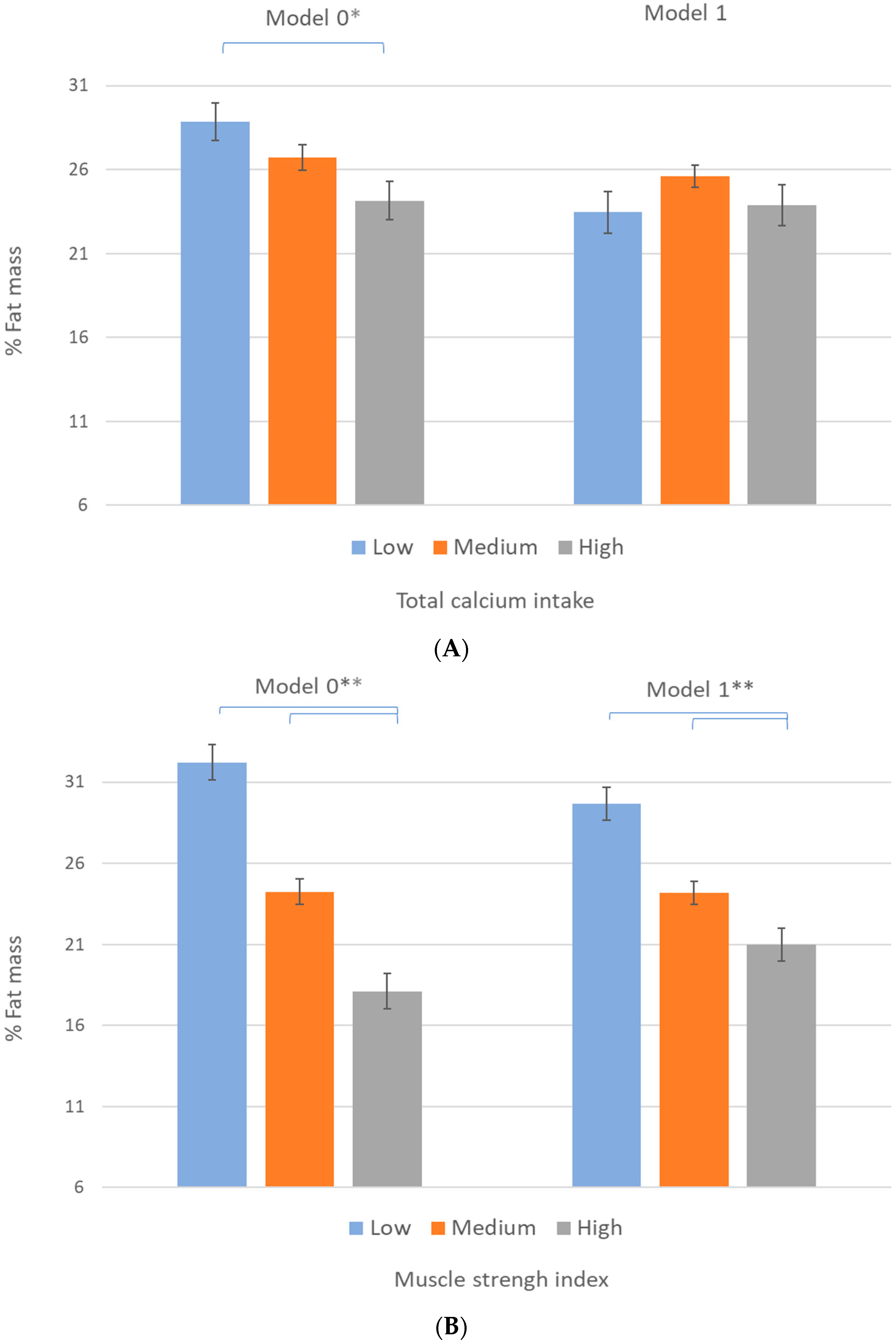
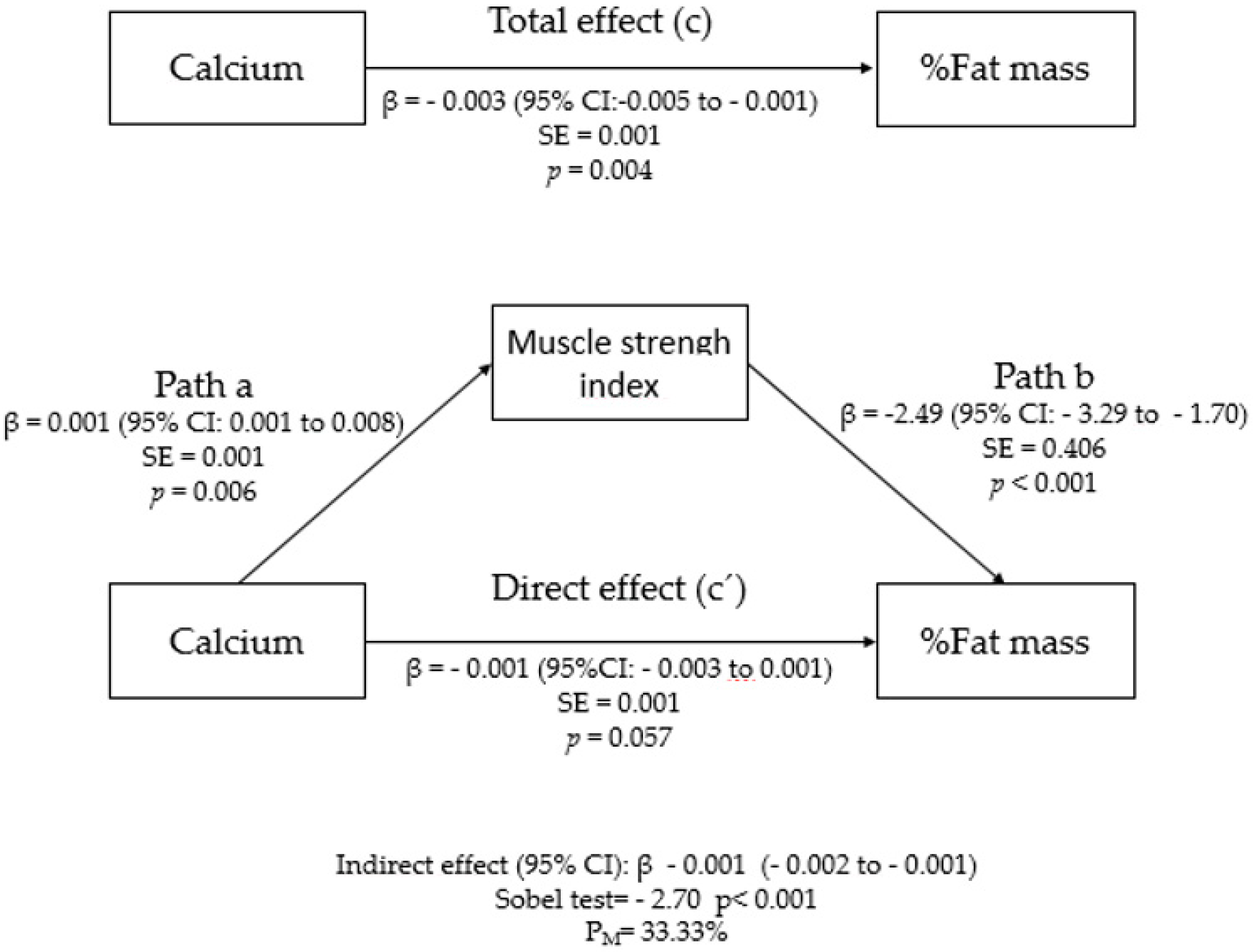
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Rodrigo, C.; Bárbara, G.H.; Citores, M.G.; Aranceta-Bartrina, J. Prevalence of obesity and associated cardiovascular risk factors in the Spanish population: The ENPE study. Rev. Española De Cardiol. (Engl. Ed.) 2021. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Olsen, L.W.; Sørensen, T.I. Childhood Body-Mass Index and the Risk of Coronary Heart Disease in Adulthood. N. Engl. J. Med. 2007, 357, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; Hanson, R.L.; Knowler, W.C.; Sievers, M.L.; Bennett, P.H.; Looker, H.C. Childhood Obesity, Other Cardiovascular Risk Factors, and Premature Death. N. Engl. J. Med. 2010, 362, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Poobalan, A.S.; Aucott, L.S.; Precious, E.; Crombie, I.K.; Smith, W.C.S. Weight loss interventions in young people (18 to 25 year olds): A systematic review. Obes. Rev. 2009, 11, 580–592. [Google Scholar] [CrossRef]
- Smith, K.J.; McNaughton, S.A.; Gall, S.L.; Blizzard, L.; Dwyer, T.; Venn, A.J. Takeaway food consumption and its associations with diet quality and abdominal obesity: A cross-sectional study of young adults. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Atkin, A.J.; Sharp, S.J.; Corder, K.; van Sluijs, E.M.; International Children’s Accelerometry Database (ICAD) Collaborators. Prevalence and correlates of screen time in youth: An international perspective. Am. J. Prev. Med. 2014, 47, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Lobelo, F.; Izquierdo, M.; Alonso-Martínez, A.M.; Rodriguez, F.; Cristi-Montero, C. High muscular fitness has a powerful protective cardiometabolic effect in adults: Influence of weight status. BMC Public Health 2016, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-R.; Jung, S.M.; Kim, H.S.; Kim, Y.B. Association of muscle strength with cardiovascular risk in Korean adults: Findings from the Korea National Health and Nutrition Examination Survey (KNHANES) VI to VII (2014–2016). Medicine 2018, 97, e13240. [Google Scholar] [CrossRef]
- Chong, H.; Choi, Y.E.; Kong, J.Y.; Park, J.H.; Yoo, H.J.; Byeon, J.H.; Lee, H.J.; Lee, S.H. Association of Hand Grip Strength and Cardiometabolic Markers in Korean Adult Population: The Korea National Health and Nutrition Examination Survey 2015–2016. Korean J. Fam. Med. 2020, 41, 291–298. [Google Scholar] [CrossRef]
- Garcia-Vicencio, S.; Coudeyre, E.; Kluka, V.; Cardenoux, C.; Jegu, A.-G.; Fourot, A.-V.; Ratel, S.; Martin, V. The bigger, the stronger? Insights from muscle architecture and nervous characteristics in obese adolescent girls. Int. J. Obes. 2015, 40, 245–251. [Google Scholar] [CrossRef]
- Funai, K.; Song, H.; Yin, L.; Lodhi, I.; Wei, X.; Yoshino, J.; Coleman, T.; Semenkovich, C. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J. Clin. Investig. 2013, 123, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Tallis, J.; Hill, C.; James, R.S.; Cox, V.M.; Seebacher, F. The effect of obesity on the contractile performance of isolated mouse soleus, EDL, and diaphragm muscles. J. Appl. Physiol. 2017, 122, 170–181. [Google Scholar] [CrossRef]
- Saha, S.; Goswami, R.; Ramakrishnan, L.; Vishnubhatla, S.; Mahtab, S.; Kar, P.; Srinivasan, S.; Singh, N.; Singh, U. Vitamin D and calcium supplementation, skeletal muscle strength and serum testosterone in young healthy adult males: Randomized control trial. Clin. Endocrinol. 2018, 88, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Theobald, H.E. Dietary calcium and health. Nutr. Bull. 2005, 30, 237–277. [Google Scholar] [CrossRef]
- Annweiler, C.; Montero-Odasso, M.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Fall prevention and vitamin D in the elderly: An overview of the key role of the non-bone effects. J. Neuroeng. Rehabil. 2010, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. [Google Scholar] [CrossRef] [Green Version]
- Teegarden, D. Calcium intake and reduction in weight or fat mass. J. Nutr. 2003, 133, 249S–251S. [Google Scholar] [CrossRef]
- Schrager, S. Dietary calcium intake and obesity. J. Am. Board Fam. Pract. 2005, 18, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Jürimäe, J.; Mäestu, E.; Mengel, E.; Remmel, L.; Purge, P.; Tillmann, V. Association between Dietary Calcium Intake and Adiposity in Male Adolescents. Nutrients 2019, 11, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappo, A.; Sparano, S.; Intemann, T.; Kourides, Y.A.; Lissner, L.; Molnar, D.; Moreno, L.A.; Pala, V.; Sioen, I.; Veidebaum, T.; et al. Dietary calcium intake and adiposity in children and adolescents: Cross-sectional and longitudinal results from IDEFICS/I. Family cohort. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos (Food Composition Tables); Ediciones Pirámide: Madrid, Spain, 2005. [Google Scholar]
- Chilet-Rosell, E.; Álvarez-Dardet, C.; Domingo-Salvany, A. Utilización de las propuestas españolas de medición de la clase social en salud. Gac. Sanit. 2012, 26, 566–569. [Google Scholar] [CrossRef]
- Özdil, S.Ö.; Kutlu, Ö. Investigation of the Mediator Variable Effect Using BK, Sobel and Bootstrap Methods (Mathematical Literacy Case). Int. J. Progress. Educ. 2019, 15, 30–43. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2017; pp. 120–141. [Google Scholar]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Hansen, M.; Jakobsen, J.; Kristensen, M.; Larsen, R.; Jørgensen, N.R.; Hansen, B.S.; Bügel, S. Vitamin D Status and Muscle Function Among Adolescent and Young Swimmers. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyere, O. The Effects of Vitamin D on Skeletal Muscle Strength, Muscle Mass, and Muscle Power: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.; Vatsa, M.; Sreenivas, V.; Singh, U.; Gupta, N.; Lakshmy, R.; Aggarwal, S.; Ganapathy, A.; Joshi, P.; Bhatia, H. Skeletal Muscle Strength in Young Asian Indian Females after Vitamin D and Calcium Supplementation: A Double-Blind Randomized Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2012, 97, 4709–4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onakpoya, I.J.; Perry, R.; Zhang, J.; Ernst, E. Efficacy of calcium supplementation for management of overweight and obesity: Systematic review of randomized clinical trials. Nutr. Rev. 2011, 69, 335–343. [Google Scholar] [CrossRef]
- Zemel, M.B. Role of dietary calcium and dairy products in modulating adiposity. Lipids 2003, 38, 139–146. [Google Scholar] [CrossRef]
- Bendsen, N.T.; Hother, A.-L.; Jensen, S.K.; Lorenzen, J.K.; Astrup, A. Effect of dairy calcium on fecal fat excretion: A randomized crossover trial. Int. J. Obes. 2008, 32, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obes. Rev. 2021, e13256. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Noh, H.; Kim, S.Y. Weight loss effects of circuit training interventions: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1642–1650. [Google Scholar] [CrossRef]
- Andreato, L.V.; Esteves, J.V.; Coimbra, D.R.; Moraes, A.J.P.; De Carvalho, T. The influence of high-intensity interval training on anthropometric variables of adults with overweight or obesity: A systematic review and network meta-analysis. Obes. Rev. 2019, 20, 142–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef] [Green Version]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221, jeb163840. [Google Scholar] [CrossRef] [Green Version]
- Artero, E.G.; España-Romero, V.; Jiménez-Pavón, D.; Martinez-Gómez, D.; Warnberg, J.; Gómez-Martínez, S.; González-Gross, M.; Vanhelst, J.; Kafatos, A.; Molnar, D. Muscular fitness, fatness and inflammatory biomarkers in adolescents. Pediatric Obes. 2014, 9, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.B.; Sanchez-Lopez, M.; Solera-Martinez, M.; Fernandez-Sanchez, A.; Sjöström, M.; Martínez-Vizcaino, V. Self-reported and measured cardiorespiratory fitness similarly predict cardiovascular disease risk in young adults. Scand. J. Med. Sci. Sports 2013, 23, 749–757. [Google Scholar] [CrossRef]
- Baldini, M.; Pasqui, F.; Bordoni, A.; Maranesi, M. Is the Mediterranean lifestyle still a reality? Evaluation of food consumption and energy expenditure in Italian and Spanish university students. Public Health Nutr. 2009, 12, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Cobo-Cuenca, A.I.; Garrido-Miguel, M.; Soriano-Cano, A.; Ferri-Morales, A.; Martínez-Vizcaíno, V.; Espínosa, N.M.M. Adherence to the Mediterranean Diet and Its Association with Body Composition and Physical Fitness in Spanish University Students. Nutrients 2019, 11, 2830. [Google Scholar] [CrossRef] [Green Version]
- Escobar, P.C.; Lerma, J.C.; Marin, D.H.; Donat Aliaga, E.; Masip Simó, E.; Miquel, B.P.; Koninckx, C.R. Development and validation of two food frequency questionnaires to assess gluten intake in children up to 36 months of age. Nutr. Hosp. 2015, 32, 2080–2090. [Google Scholar]
- Vioque, J.; Navarrete-Muñoz, E.-M.; Gimenez-Monzó, D.; García-De-La-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr. J. 2013, 12, 26–26. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-T.; Chen, Y.-Y.; Wang, C.-W.; Chuang, C.-L.; Chiang, L.-M.; Lai, C.-L.; Lu, H.-K.; Dwyer, G.B.; Chao, S.-P.; Shih, M.-K.; et al. Comparison of Standing Posture Bioelectrical Impedance Analysis with DXA for Body Composition in a Large, Healthy Chinese Population. PLoS ONE 2016, 11, e0160105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achamrah, N.; Colange, G.; DeLay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | All (n = 355) | Men (n = 125) | Women (n = 230) | p * |
|---|---|---|---|---|
| Age (years) | 21.05 ± 3.11 | 21.19 ± 2.85 | 20.97 ± 3.25 | 0.534 |
| Weight (Kg) | 65.41 ± 12.34 | 72.60 ± 10.94 | 61.50 ± 11.27 | <0.001 |
| Height (cm) | 167.30 ± 8.66 | 175.39 ± 7.03 | 162.93 ± 5.86 | <0.001 |
| % Fat mass | 26.61 ± 10.01 | 18.85 ± 6.83 | 30.68 ± 8.95 | <0.001 |
| Low (%) & | 24.9 | 45.7 | 10.1 | <0.001 |
| Moderate (%) & | 50.2 | 37.8 | 53.4 | |
| High (%) & | 24.9 | 2.4 | 36.4 | |
| BMI (Kg/m2) | 23.28 ± 3.62 | 23.55 ± 3.03 | 23.14 ± 3.90 | 0.308 |
| Underweight (%) | 3.1 | 0.8 | 4.4 | |
| Normal weight (%) | 70.6 | 70.6 | 70.6 | 0.068 |
| Overweight (%) | 21.8 | 26.2 | 19.3 | |
| Obesity (%) | 4.5 | 2.4 | 5.7 | |
| Handgrip strength (Kg) | 30.41 ± 9.51 | 39.22 ± 7.75 | 24.43 ± 4.77 | <0.001 |
| Standing long jump (cm) | 161.21 ± 43.78 | 195.49 ± 31.91 | 136.82 ± 33.55 | <0.001 |
| Muscle strength index (z-score) a | 0.013 ± 1.7 | 1.52 ± 1.25 | −1.05 ± 1.21 | <0.001 |
| Calcium (mg/dL) | 1219.77 ± 555.30 | 1241.32 ± 562.64 | 1208.07 ± 542.65 | 0.693 |
| Total EI (Kcal) | 2795.79 ± 1804.77 | 2865.92 ± 1287.02 | 2757.68 ± 2033.24 | 0.590 |
| Carbohydrate (% EI) | 43.01 ± 7.10 | 43.10 ± 6.63 | 42.95 ± 7.36 | 0.852 |
| Protein (% EI) | 17.47 ± 3.46 | 17.39 ± 3.23 | 17.51 ± 3.59 | 0.749 |
| Fat (% EI) | 38.18 ± 6.21 | 37.93 ± 5.98 | 38.32 ± 6.34 | 0.578 |
| Socioeconomic status | ||||
| Low (%) | 28.3 | 30.8 | 27.0 | 0.048 |
| Medium (%) | 46.6 | 52.1 | 43.7 | |
| High (%) | 25.1 | 17.1 | 29.3 |
| Calcium | %Fat Mass | BMI | Handgrip Strength | Standing Long Jump | Muscle Strength Index a | Total EI | |
|---|---|---|---|---|---|---|---|
| Calcium | - | −0.153 ** | −0.091 | 0.070 | 0.149 * | 0.188 ** | 0.883 ** |
| %fat mass | - | 0.493 ** | −0.390 ** | −0.531 ** | −0.632 ** | −0.166 ** | |
| BMI | - | 0.224 ** | −0.66 | −0.065 | −0.116* | ||
| Handgrip strength | - | 0.611 ** | 0.184 ** | 0.101 | |||
| Standing long jump | - | 0.783 ** | 0.206 ** | ||||
| Muscle strength index a | - | 0.243 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Costoso, A.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Sequí-Dominguez, I.; Reina-Gutiérrez, S.; Núñez de Arenas-Arroyo, S.; Garrido-Miguel, M. Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength. Nutrients 2021, 13, 4498. https://doi.org/10.3390/nu13124498
Torres-Costoso A, Martínez-Vizcaíno V, Fernández-Rodríguez R, Sequí-Dominguez I, Reina-Gutiérrez S, Núñez de Arenas-Arroyo S, Garrido-Miguel M. Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength. Nutrients. 2021; 13(12):4498. https://doi.org/10.3390/nu13124498
Chicago/Turabian StyleTorres-Costoso, Ana, Vicente Martínez-Vizcaíno, Rubén Fernández-Rodríguez, Irene Sequí-Dominguez, Sara Reina-Gutiérrez, Sergio Núñez de Arenas-Arroyo, and Miriam Garrido-Miguel. 2021. "Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength" Nutrients 13, no. 12: 4498. https://doi.org/10.3390/nu13124498
APA StyleTorres-Costoso, A., Martínez-Vizcaíno, V., Fernández-Rodríguez, R., Sequí-Dominguez, I., Reina-Gutiérrez, S., Núñez de Arenas-Arroyo, S., & Garrido-Miguel, M. (2021). Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength. Nutrients, 13(12), 4498. https://doi.org/10.3390/nu13124498








