Rhus chinensis Mill. Fruits Ameliorate Hepatic Glycolipid Metabolism Disorder in Rats Induced by High Fat/High Sugar Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical, Reagents and Sample
2.2. Extract Preparation and Analysis
2.3. Animals and Treatment
2.4. Biochemical Parameters
2.5. Magnetic Resonance Imaging
2.6. Histopathological and Immunohistochemical Analyses
2.7. Western Blot Analysis
2.8. Statistical Analysis and Evaluation of the Protein Expression via Image Pro Plus
3. Results and Discussion
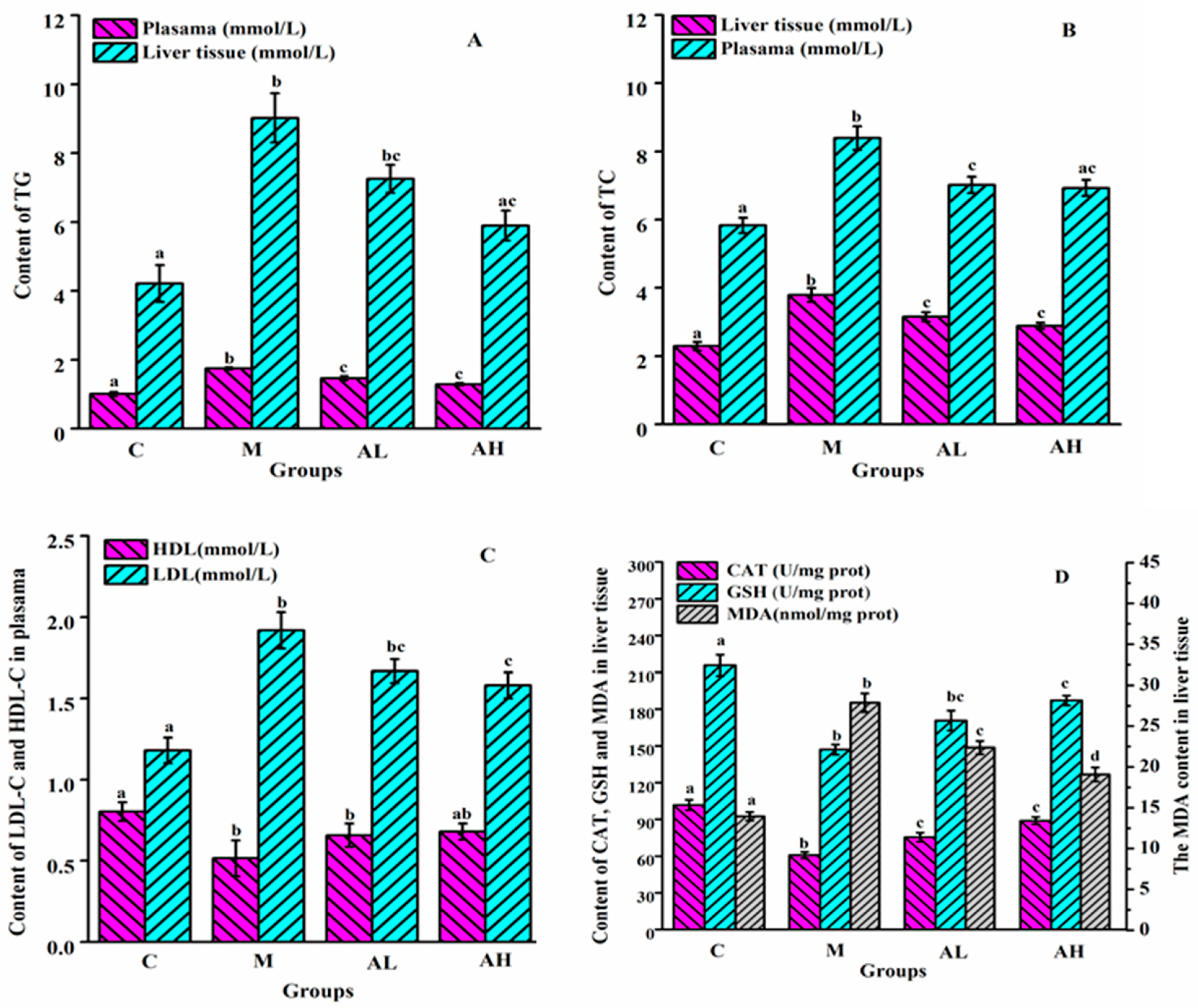
3.1. Analysis of Biochemical Indicators
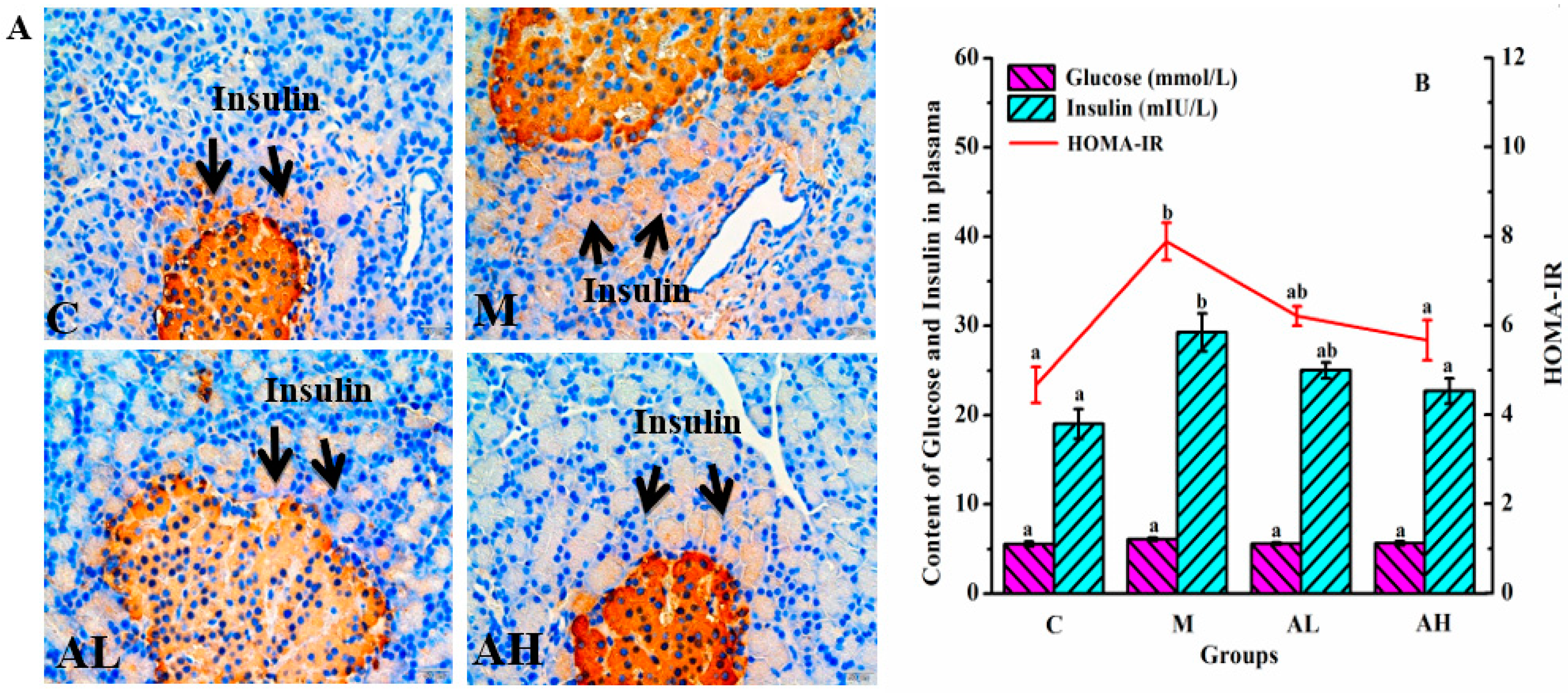
3.2. Fat Distribution and Insulin Resistance
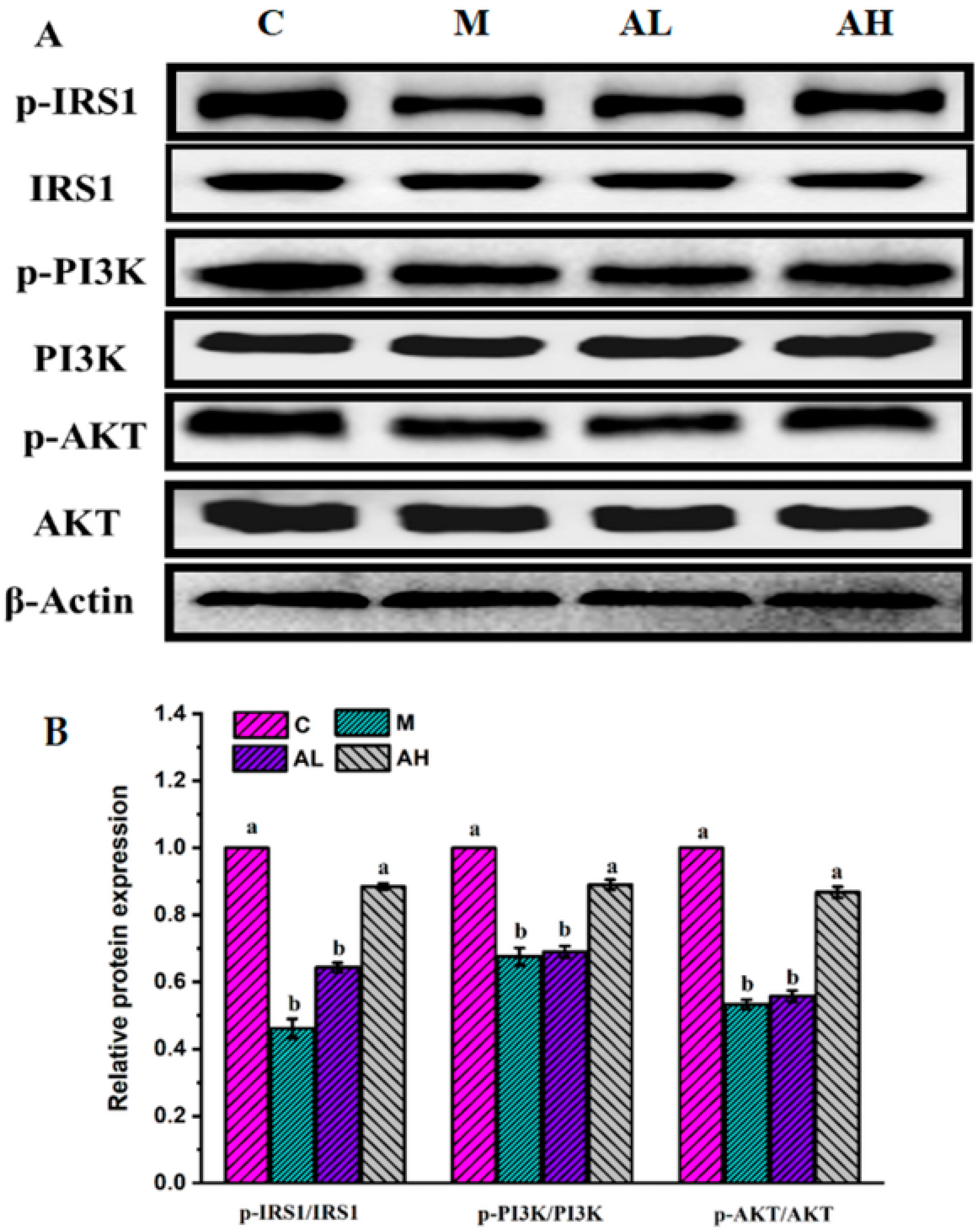
3.3. Immunohistochemistry and Protein Expression Analysis
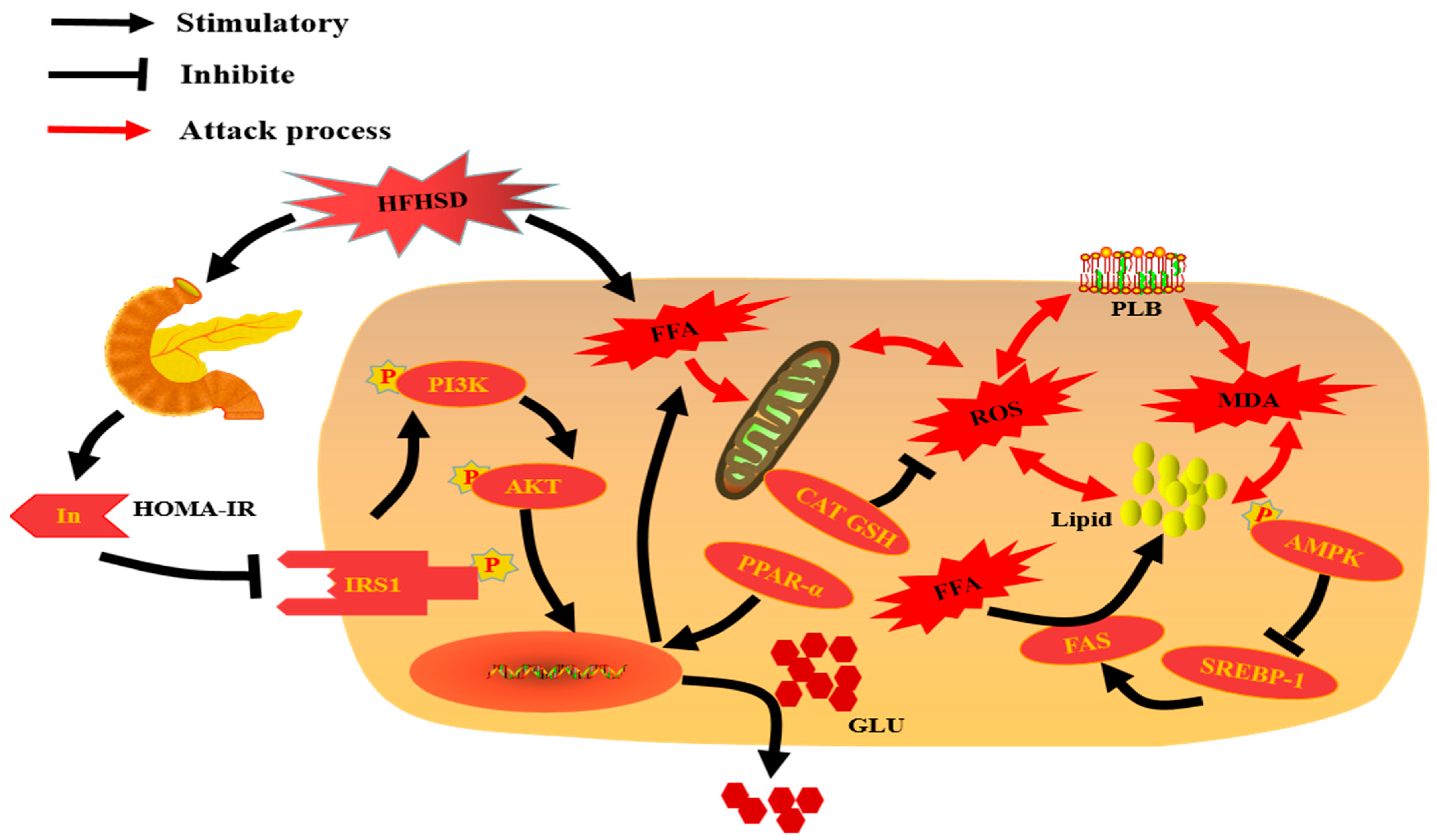
4. Conclusions
5. Limitations of Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, H.; Zhu, C.; Deng, J.; Fan, D. Ginsenoside Rg5 relieves type 2 diabetes by improving hepatic insulin resistance in db/db mice. J. Funct. Foods 2020, 71, 104014. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Gong, X.; Cheng, G.; Pu, S.; Cai, S. The preventive effect of phenolic-rich extracts from Chinese sumac fruits against nonalcoholic fatty liver disease in rats induced by a high-fat diet. Food Funct. 2020, 11, 799–812. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhao, Y.; Hong, Y.; Cai, S.; Pang, M. Phenolic composition, antioxidant and pancreatic lipase inhibitory activities of Chinese sumac (Rhus chinensis Mill.) fruits extracted by different solvents and interaction between myricetin-3-O-rhamnoside and quercetin-3-o-rhamnoside. Int. J. Food Sci. Tech. 2018, 53, 1045–1053. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, K.; Horikawa, Y. ChREBP: A glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr. J. 2008, 55, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Moynihan, K.A.; Grimm, A.A.; Plueger, M.M.; Bernal-Mizrachi, E.; Ford, E.; Cras-Méneur, C.; Permutt, M.A.; Imai, S.-i. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005, 2, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, D.G.; Nehra, V.; Lindor, K.D.; Buchman, A.L. Metabolic and nutritional considerations in nonalcoholic fatty liver. Hepatology 2000, 32, 3–10. [Google Scholar] [CrossRef]
- Nagle, C.A.; Klett, E.L.; Coleman, R.A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009, 50, S74–S79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppälä-Lindroos, A.; Vehkavaara, S.; Häkkinen, A.-M.; Goto, T.; Westerbacka, J.; Sovijärvi, A.; Halavaara, J.; Yki-Järvinen, H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocr. Metab. 2002, 87, 3023–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-L.; Chen, Y.-L.; Chiang, Y.-M.; Wang, S.-G.; Lee, H.-M. Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARα/AMPK/FoxO1/ATGL pathway. Biochem. Pharmacol. 2012, 84, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Hu, M.; Tao, J.; Yang, H.; Yan, P.; An, G.; Wang, H. The protective effects of Chinese yam polysaccharide against obesity-induced insulin resistance. J. Funct. Foods 2019, 55, 238–247. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Zhao, L.; Cai, S.; Cheng, G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. 2018, 119, 14–23. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis–folklore to modern evidence. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Taylor, E.; Huang, N.; Bodde, J.; Ellison, A.; Killiany, R.; Bachschmid, M.M.; Hamilton, J. MRI of atherosclerosis and fatty liver disease in cholesterol fed rabbits. J. Transl. Med. 2018, 16, 215. [Google Scholar] [CrossRef]
- Hedderich, D.M.; Hasenberg, T.; Haneder, S.; Schoenberg, S.O.; Kücükoglu, Ö.; Canbay, A.; Otto, M. Effects of bariatric surgery on non-alcoholic fatty liver disease: Magnetic resonance imaging is an effective, non-invasive method to evaluate changes in the liver fat fraction. Obes. Surg. 2017, 27, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Saitoh, S.; Takahashi, J.; Tsuji, Y.; Ikeda, K.; Kobayashi, M.; Kawamura, Y.; Fujii, T.; Inoue, M.; Miyati, T. Hepatic fat quantification using the two-point Dixon method and fat color maps based on non-alcoholic fatty liver disease activity score. Hepatol. Res. 2017, 47, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agulnik, J.; Kasymjanova, G.; Wang, A.; Jiménez, P.; Cohen, V.; Small, D.; Pepe, C.; Sakr, L.; Fiset, P. Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann. Oncol. 2018, 29, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cui, Y.; Zhou, H.; Li, Q. Function of mitochondrial pyruvate carriers in hepatocellular carcinoma patients. Oncol. Lett. 2018, 15, 9110–9116. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-J.; Zhou, Z.-G.; Holmqvist, A.; Zhang, H.; Li, Y.; Adell, G.; Sun, X.-F. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 530–535. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Li, Y.; Guo, L.; Cui, Y.; Zhang, X.; Li, E. Metabolomic analysis of serum from obese adults with hyperlipemia by UHPLC-Q-TOF MS/MS. Biomed. Chromatogr. 2016, 30, 48–54. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Z.; Lin, Y.; Ge, S.; Hamzah, S.S.; Hu, J. Bound phenolics from fresh lotus seeds exert anti-obesity effects in 3T3-L1 adipocytes and high-fat diet-fed mice by activation of AMPK. J. Funct. Foods 2019, 58, 74–84. [Google Scholar] [CrossRef]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C.S. Green tea polyphenol EGCG alleviates metabolic abnormality and fatty liver by decreasing bile acid and lipid absorption in mice. Mol. Nutr. Food Res. 2018, 62, 1700696. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Lin, Y.-J.; Ho, C.-T.; Yen, G.-C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Nosadini, R.; Tonolo, G. Role of oxidized low-density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr. Metab. Cardiovas. 2011, 21, 79–85. [Google Scholar] [CrossRef]
- Boyce, G.; Button, E.; Soo, S.; Wellington, C. The pleiotropic vasoprotective functions of high-density lipoproteins (HDL). J. Biomed. Res. 2018, 32, 164. [Google Scholar]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef]
- Xiang, J.; Li, W.; Ndolo, V.U.; Beta, T. A comparative study of the phenolic compounds and in vitro antioxidant capacity of finger millets from different growing regions in Malawi. J. Cereal Sci. 2019, 87, 143–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur. Food Res. Technol. 2010, 231, 151–158. [Google Scholar] [CrossRef]
- Steneberg, P.; Rubins, N.; Bartoov-Shifman, R.; Walker, M.D.; Edlund, H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005, 1, 245–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Maduko, C.; Park, Y.; Akoh, C. Characterization and oxidative stability of structured lipids: Infant milk fat analog. J. Am. Oil Chem. Soc. 2008, 85, 197–204. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, H.; Liu, Y.; Shui, W.; Wang, J.; Cao, P.; Wang, H.; You, R.; Zhang, Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Després, J.-P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Mahabadi, A.A.; Massaro, J.M.; Rosito, G.A.; Levy, D.; Murabito, J.M.; Wolf, P.A.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur. Heart J. 2009, 30, 850–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Han, X.; Dong, P.; Li, Z.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea cucumber saponin liposomes ameliorate obesity-induced inflammation and insulin resistance in high-fat-diet-fed mice. Food Funct. 2018, 9, 861–870. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.; Lynch, L.; Cawood, T.J.; Kwasnik, A.; Nolan, N.; Geoghegan, J.; McCormick, A.; O’Farrelly, C.; O’Shea, D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS ONE 2010, 5, e9997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Chen, T.-H.; Lee, B.-H.; Hsu, Y.-W.; Pan, T.-M. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 2014, 64, 94–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Zhang, Y.; Li, L.; Fang, R.; Li, Y.; Liu, Q.; Zhang, W.; Qiu, L.; Liu, F. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Res. 2016, 76, 4696–4707. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.; Gao, Y.; Zheng, D.; Liu, Y.; Shan, S.; Fang, B.; Zhao, Y.; Song, D.; Zhang, Y.; Li, Q. Alisol A attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J. Cell. Mol. Med. 2019, 23, 5108–5118. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Liang, Y.; Tian, W.; Ji, M.; Ma, X. Regulating effect of β-ketoacyl synthase domain of fatty acid synthase on fatty acyl chain length in de novo fatty acid synthesis. BBA Mol. Cell Biol. Lipids 2016, 1861, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.; Kumazoe, M.; Fujimura, Y.; Tachibana, H. Diallyl disulfide potentiates anti-obesity effect of green tea in high-fat/high-sucrose diet-induced obesity. J. Nutr. Biochem. 2019, 64, 152–161. [Google Scholar] [CrossRef]
- Bai, F.; Liu, Y.; Tu, T.; Li, B.; Xiao, Y.; Ma, Y.; Qin, F.; Xie, J.; Zhou, S.; Liu, Q. Metformin regulates lipid metabolism in a canine model of atrial fibrillation through AMPK/PPAR-α/VLCAD pathway. Lipids Health Dis. 2019, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q.; Li, Y.-W.; Yang, C.-F.; Zhong, Y.-J.; He, H.; Zhu, F.-C.; Li, L. Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 pathways in rats and L-02 cells. Chem. Biol. Interact. 2018, 291, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Nihei, N.; Okamoto, H.; Furune, T.; Ikuta, N.; Sasaki, K.; Rimbach, G.; Yoshikawa, Y.; Terao, K. Dietary α-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. Biofactors 2018, 44, 336–347. [Google Scholar] [CrossRef]
- Laleh, P.; Yaser, K.; Abolfazl, B.; Shahriar, A.; Mohammad, A.J.; Nazila, F.; Alireza, O. Oleoylethanolamide increases the expression of PPAR-A and reduces appetite and body weight in obese people: A clinical trial. Appetite 2018, 128, 44–49. [Google Scholar] [CrossRef]
- An, J.Y.; Jheng, H.F.; Nagai, H.; Sanada, K.; Takahashi, H.; Iwase, M.; Watanabe, N.; Kim, Y.I.; Teraminami, A.; Takahashi, N. A phytol-enriched diet activates PPAR-α in the liver and Brown adipose tissue to ameliorate obesity-induced metabolic abnormalities. Mol. Nutr. Food Res. 2018, 62, 1700688. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, J.; Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab. Res. Rev. 2001, 17, 250–272. [Google Scholar] [CrossRef]
- Sah, S.P.; Singh, B.; Choudhary, S.; Kumar, A. Animal models of insulin resistance: A review. Pharmacol. Rep. 2016, 68, 1165–1177. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Ma, Q.; Cai, S.; Sun, Y.; Zhang, Y.; Yi, J. Rhus chinensis Mill. Fruits Ameliorate Hepatic Glycolipid Metabolism Disorder in Rats Induced by High Fat/High Sugar Diet. Nutrients 2021, 13, 4480. https://doi.org/10.3390/nu13124480
Wu Z, Ma Q, Cai S, Sun Y, Zhang Y, Yi J. Rhus chinensis Mill. Fruits Ameliorate Hepatic Glycolipid Metabolism Disorder in Rats Induced by High Fat/High Sugar Diet. Nutrients. 2021; 13(12):4480. https://doi.org/10.3390/nu13124480
Chicago/Turabian StyleWu, Zihuan, Qingqing Ma, Shengbao Cai, Yilin Sun, Yuanyue Zhang, and Junjie Yi. 2021. "Rhus chinensis Mill. Fruits Ameliorate Hepatic Glycolipid Metabolism Disorder in Rats Induced by High Fat/High Sugar Diet" Nutrients 13, no. 12: 4480. https://doi.org/10.3390/nu13124480
APA StyleWu, Z., Ma, Q., Cai, S., Sun, Y., Zhang, Y., & Yi, J. (2021). Rhus chinensis Mill. Fruits Ameliorate Hepatic Glycolipid Metabolism Disorder in Rats Induced by High Fat/High Sugar Diet. Nutrients, 13(12), 4480. https://doi.org/10.3390/nu13124480





