Diet and Anxiety: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
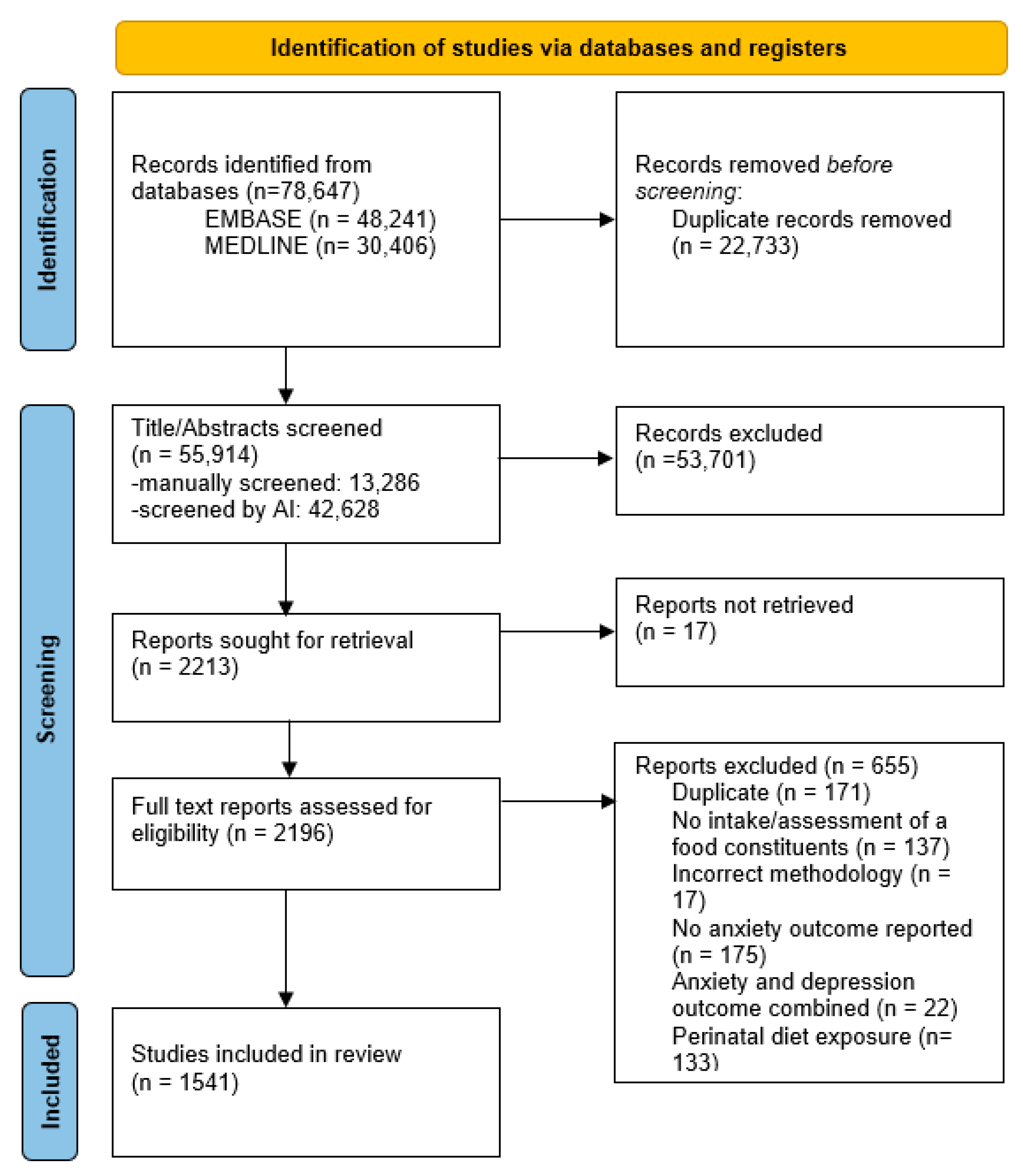
3.1. Search Results
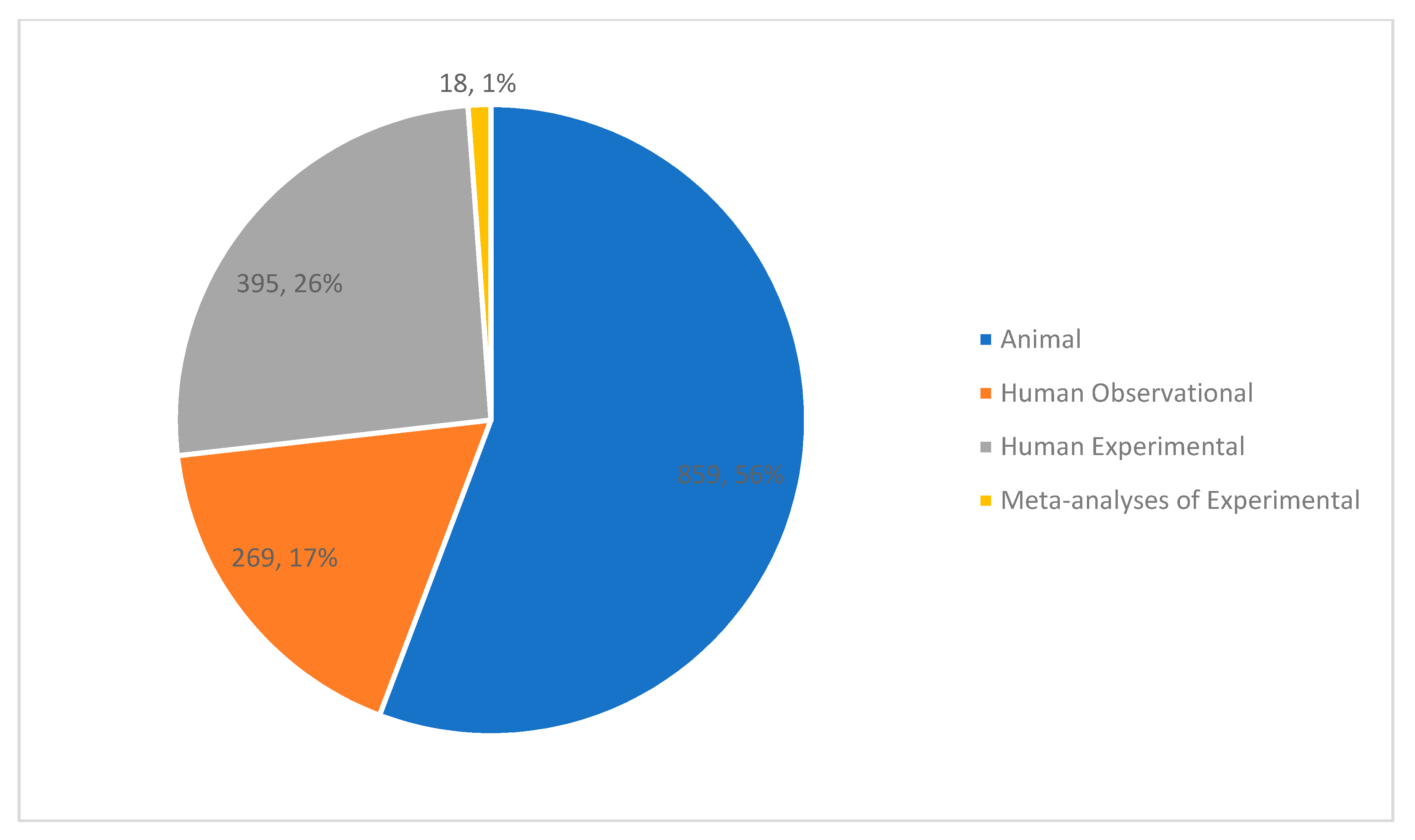
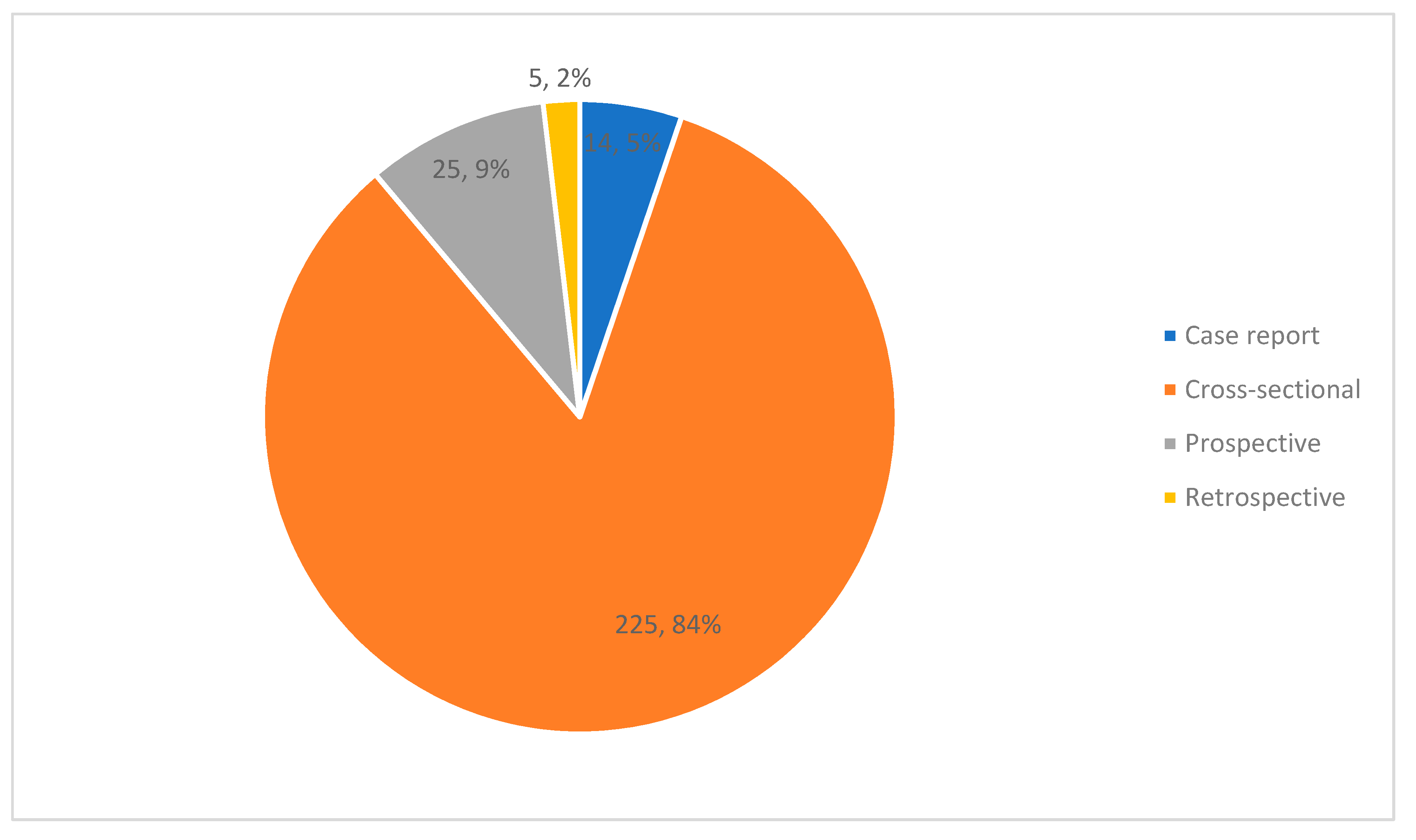
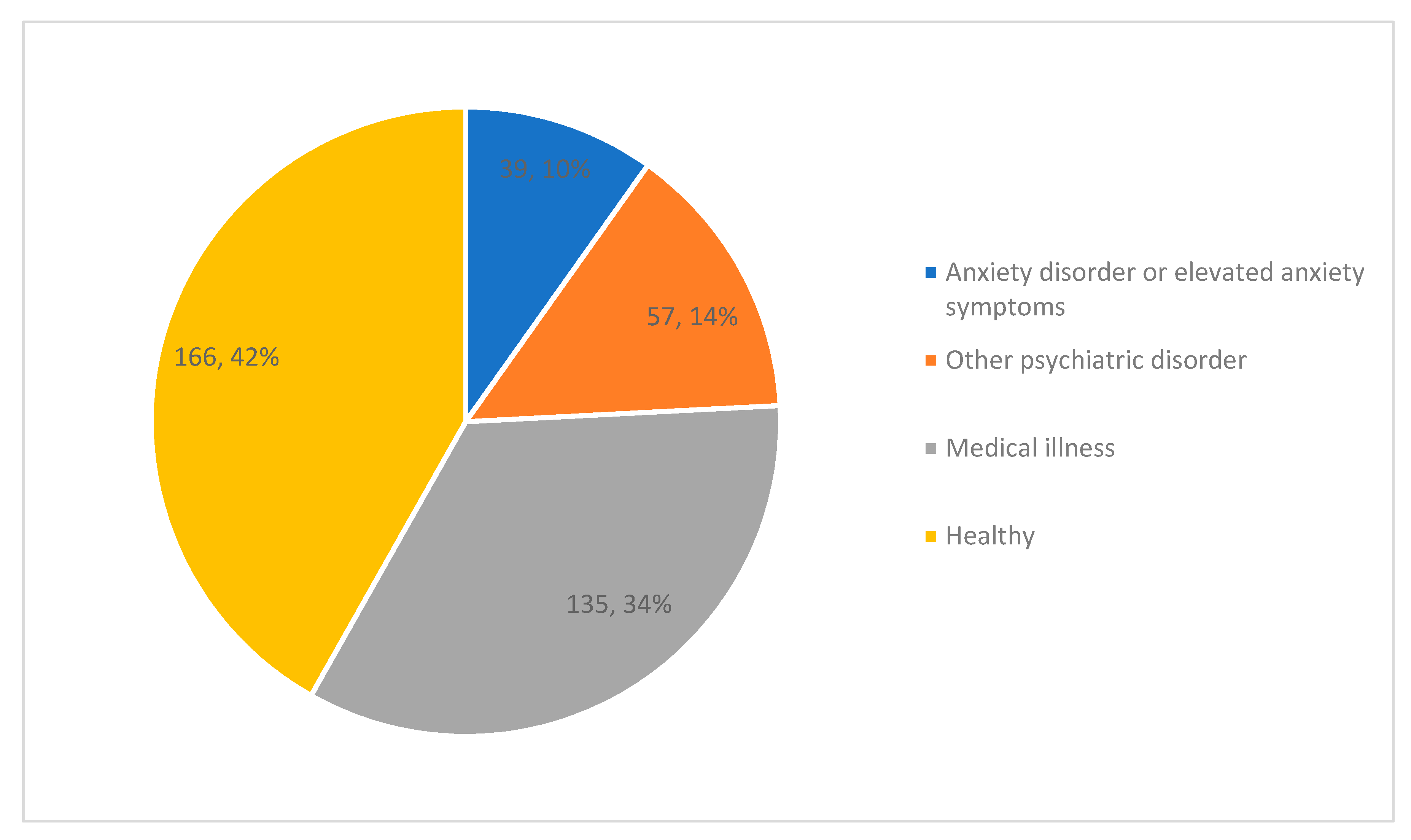
3.2. Study Characteristics
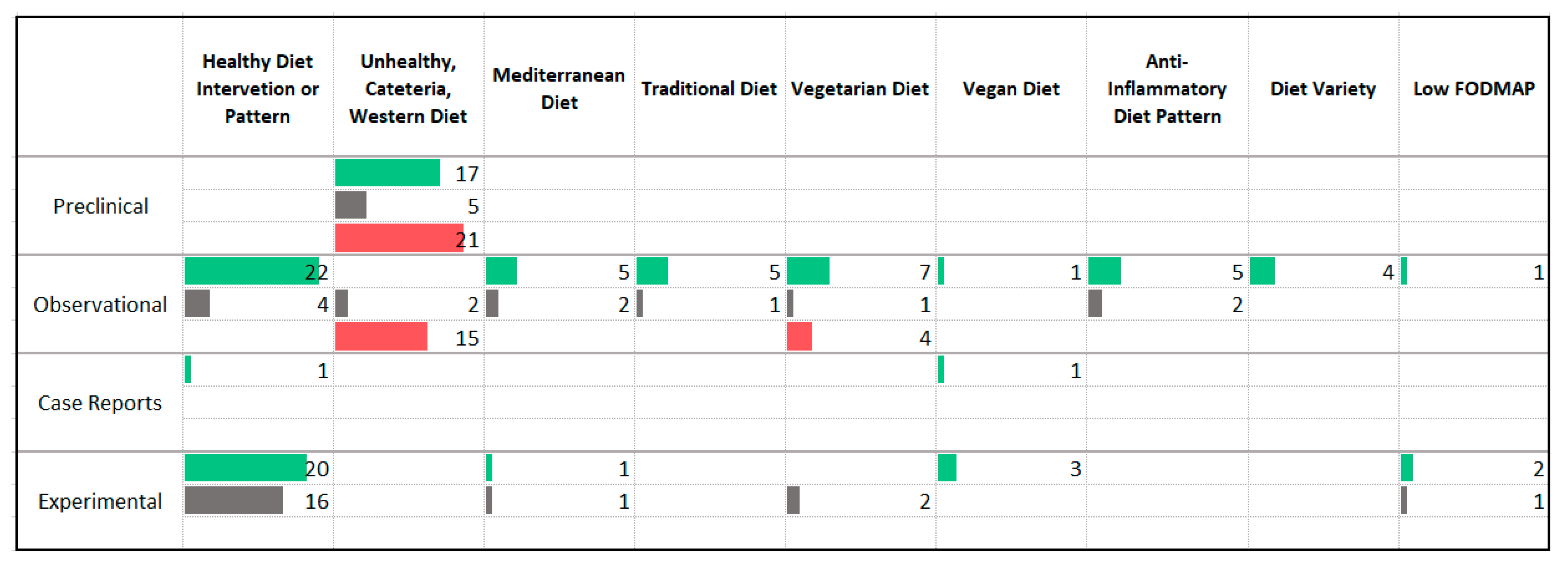
3.3. Dietary Patterns
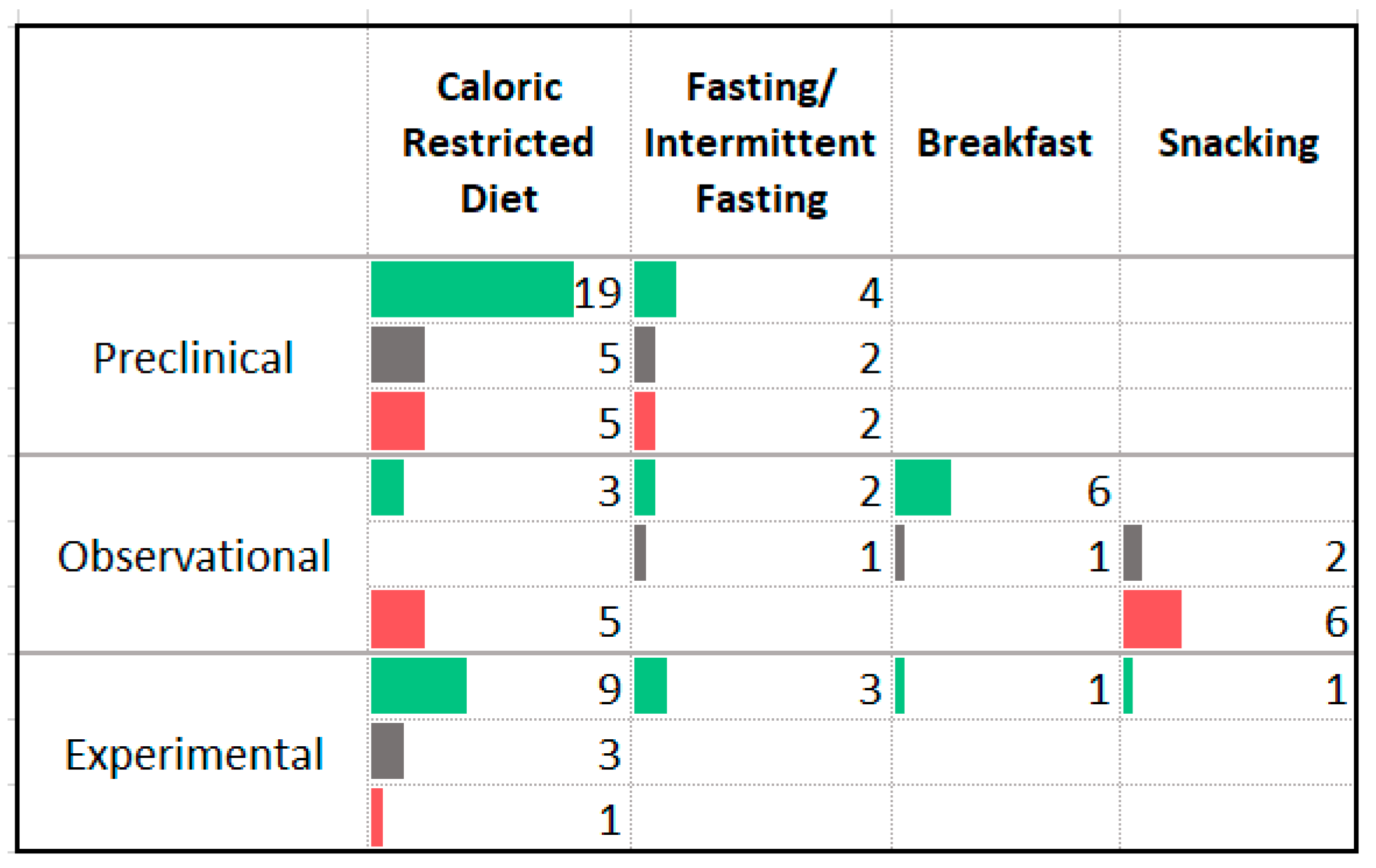
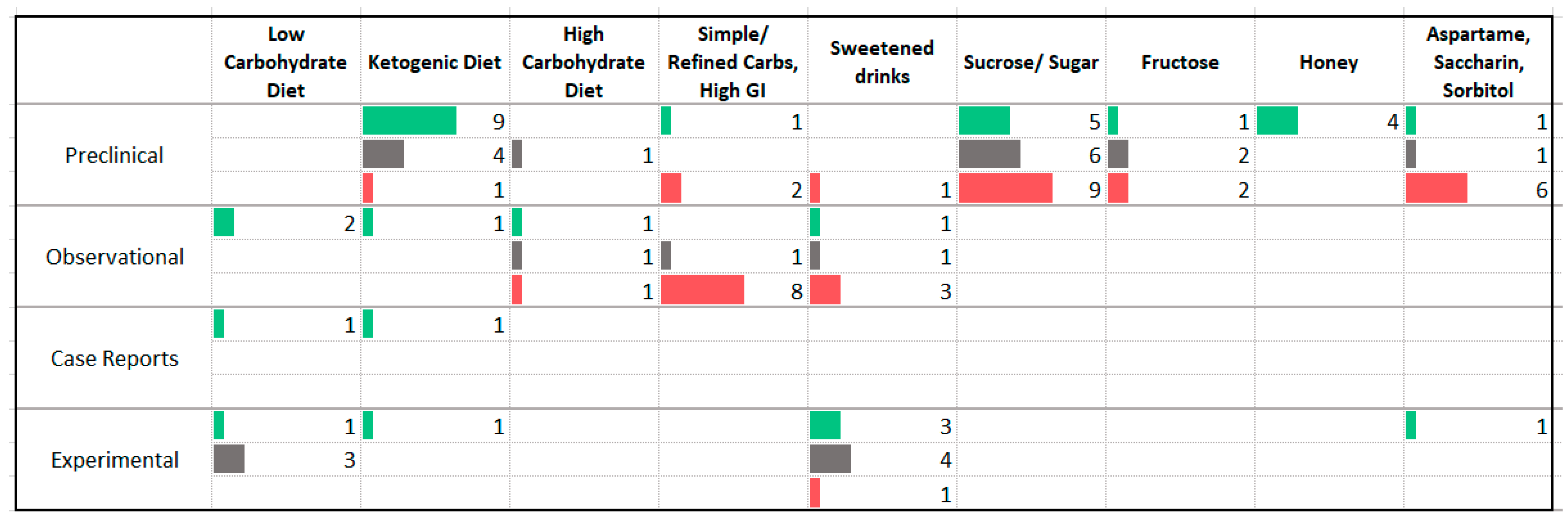
3.4. Carbohydrates
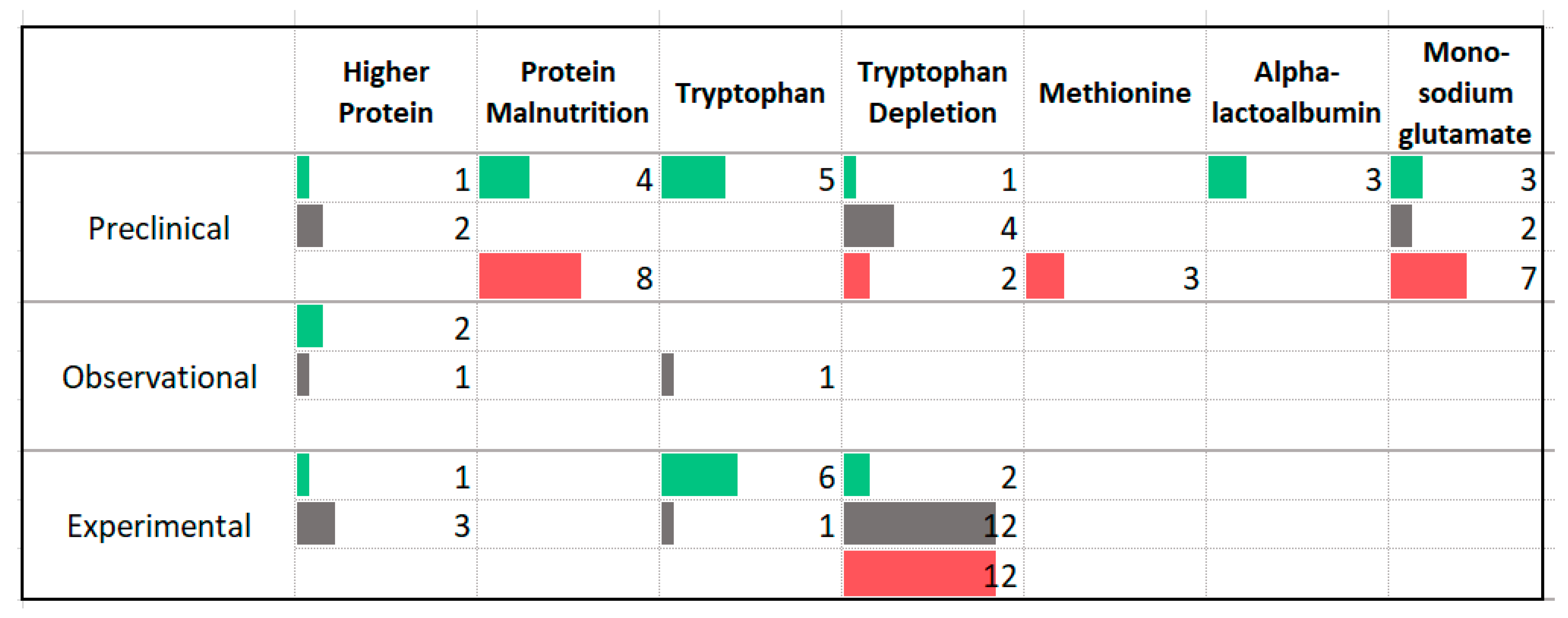
3.5. Protein
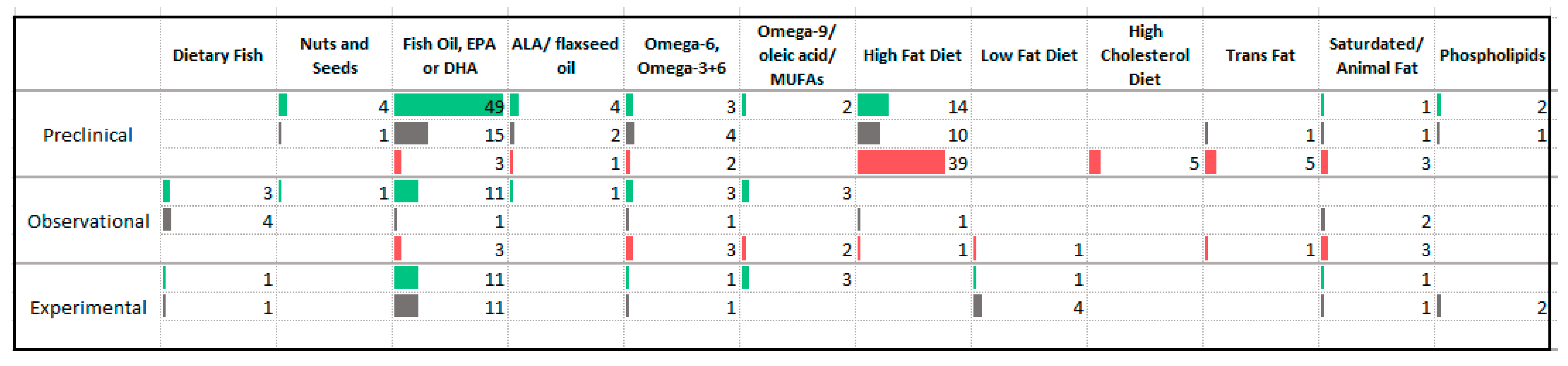
3.6. Fats
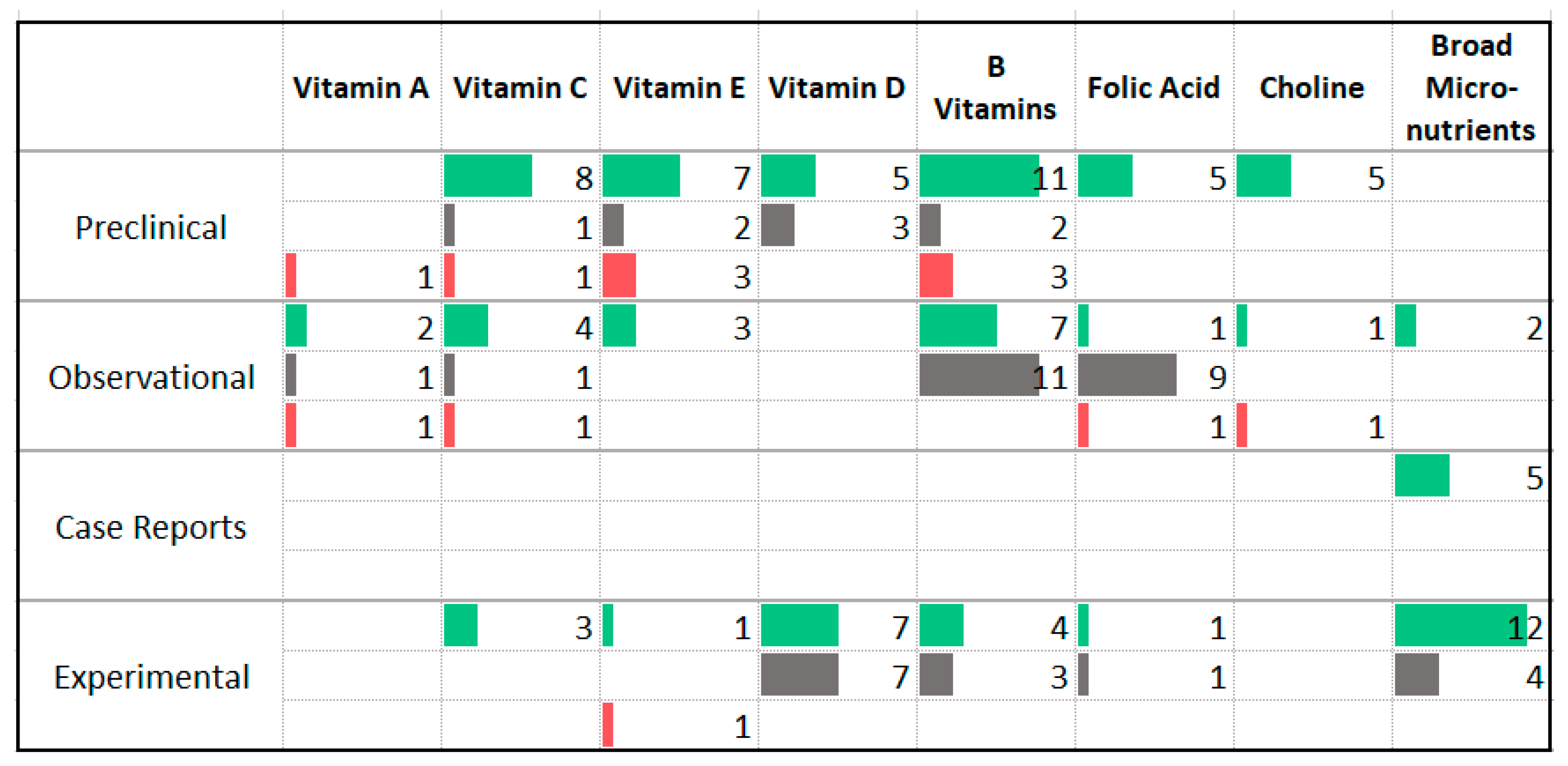
3.7. Vitamins
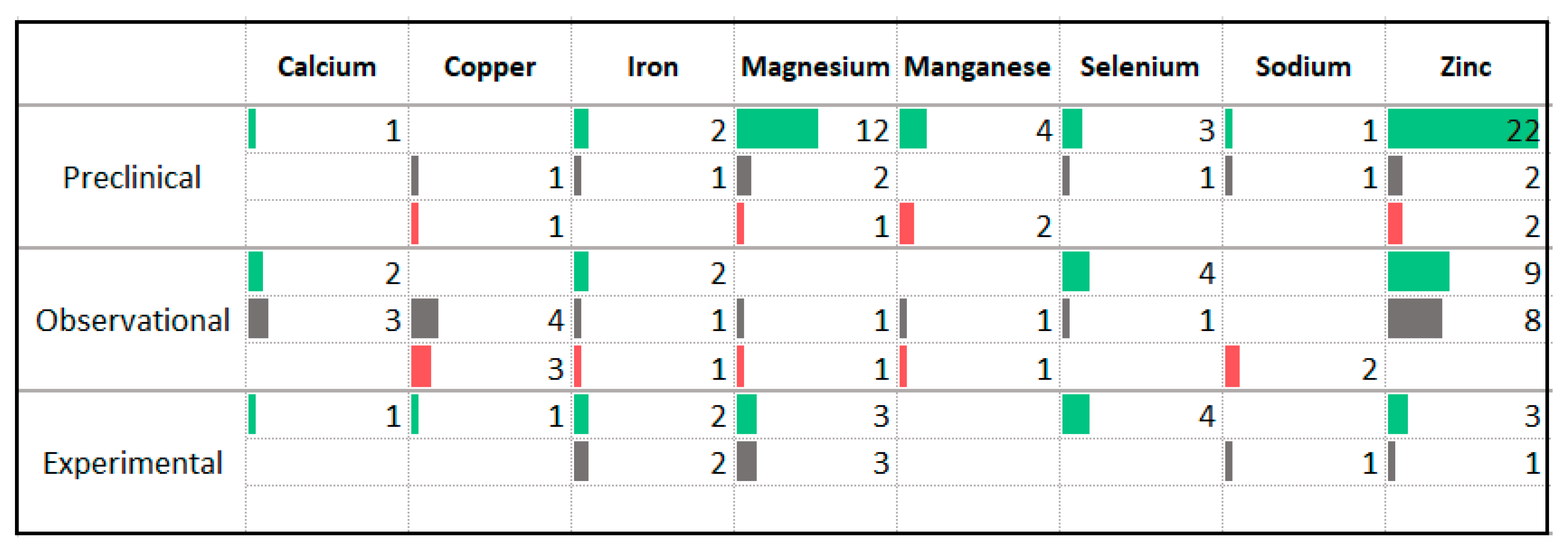
3.8. Minerals
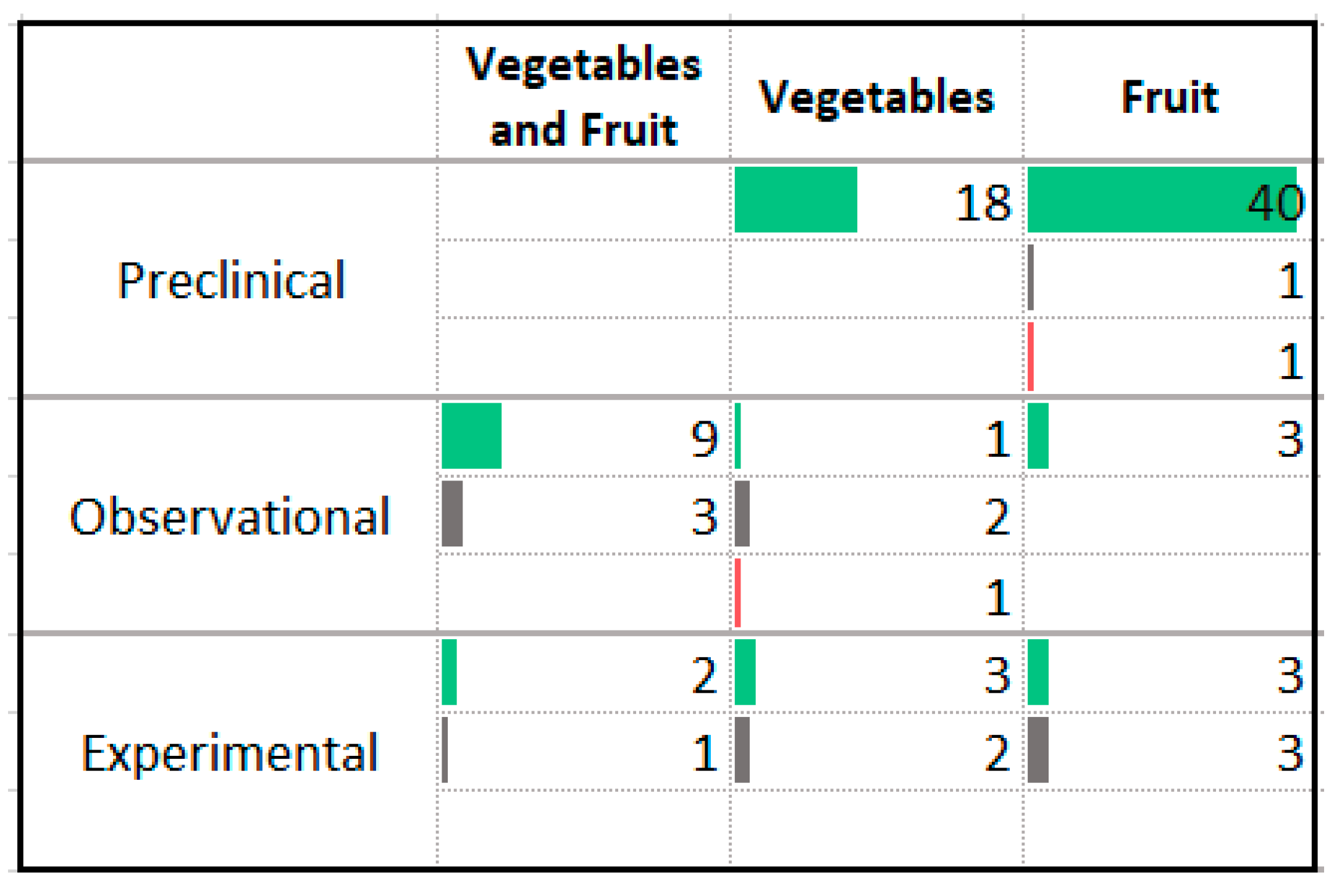
3.9. Vegetables and Fruit
3.10. Phytochemicals
3.11. Food Allergy and Intolerance
3.12. Gut Microbiome
4. Discussion
4.1. Dietary Patterns
4.2. Carbohydrates
4.3. Protein
4.4. Fats
4.5. Vitamins and Minerals
4.6. Vegetables, Fruits, and Phytochemicals
4.7. Food Allergy and Intolerance
4.8. Gut Microbiome
4.9. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gale, C.K.; Millichamp, J. Generalised anxiety disorder. BMJ Clin. Evid. 2011, 2011, 1002. [Google Scholar]
- Kim, Y.-K. Panic Disorder: Current Research and Management Approaches. Psychiatry Investig. 2019, 16, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, H.-U.; Gloster, A.T.; Beesdo-Baum, K.; Fava, G.A.; Craske, M.G. Agoraphobia: A review of the diagnostic classificatory position and criteria. Depress. Anxiety 2010, 27, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Canton, J.; Scott, K.M. Optimal treatment of social phobia: Systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2012, 8, 203–215. [Google Scholar] [CrossRef][Green Version]
- Eaton, W.W.; Bienvenu, O.J.; Miloyan, B. Specific phobias. Lancet Psychiatry 2018, 5, 678–686. [Google Scholar] [CrossRef]
- Louise, P.; Siobhan, O.; Louise, M.; Jean, G.; Pelletier, L.; O’Donnell, S.; McRae, L.; Grenier, J. The burden of generalized anxiety disorder in Canada. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 54–62. [Google Scholar] [CrossRef]
- Saarni, S.I.; Suvisaari, J.; Sintonen, H.; Pirkola, S.; Koskinen, S.; Aromaa, A.; Lönnqvist, J. Impact of psychiatric disorders on health-related quality of life: General population survey. Br. J. Psychiatry 2007, 190, 326–332. [Google Scholar] [CrossRef]
- Revicki, D.A.; Travers, K.; Wyrwich, K.W.; Svedsäter, H.; Locklear, J.; Mattera, M.S.; Sheehan, D.V.; Montgomery, S. Humanistic and economic burden of generalized anxiety disorder in North America and Europe. J. Affect. Disord. 2012, 140, 103–112. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.-U.; Kendler, K.S. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Gliatto, M.F. Generalized anxiety disorder. Am. Fam. Physician 2000, 62, 1591–1600. [Google Scholar]
- Collins, K.A.; Westra, H.A.; Dozois, D.J.; Burns, D.D. Gaps in accessing treatment for anxiety and depression: Challenges for the delivery of care. Clin. Psychol. Rev. 2004, 24, 583–616. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zeng, L.; Zou, K.; Shan, S.; Wang, X.; Xiong, J.; Zhao, L.; Zhang, L.; Cheng, G. Role of dietary factors in the prevention and treatment for depression: An umbrella review of meta-analyses of prospective studies. Transl. Psychiatry 2021, 11, 478. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Aucoin, M.; LaChance, L.; Cooley, K.; Kidd, S. Diet and Psychosis: A Scoping Review. Neuropsychobiology 2020, 79, 20–42. [Google Scholar] [CrossRef]
- Gates, A.; Johnson, C.; Hartling, L. Technology-assisted title and abstract screening for systematic reviews: A retrospective evaluation of the Abstrackr machine learning tool. Syst. Rev. 2018, 7, 45. [Google Scholar] [CrossRef]
- Rathbone, J.; Hoffmann, T.; Glasziou, P. Faster title and abstract screening? Evaluating Abstrackr, a semi-automated online screening program for systematic reviewers. Syst. Rev. 2015, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Gates, A.; Gates, M.; Sebastianski, M.; Guitard, S.; Elliott, S.A.; Hartling, L. The semi-automation of title and abstract screening: A retrospective exploration of ways to leverage Abstrackr’s relevance predictions in systematic and rapid reviews. BMC Med. Res. Methodol. 2020, 20, 139. [Google Scholar] [CrossRef]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef]
- Hunsche, C.; de Toda, I.M.; De la Fuente, M. Impacts of the late adulthood diet-induced obesity onset on behavior, immune function, redox state and life span of male and female mice. Brain Behav. Immun. 2019, 78, 65–77. [Google Scholar] [CrossRef]
- Souza, C.; Moreira, J.D.; Siqueira, I.; Pereira, A.; Rieger, D.; Souza, D.; Souza, T.; Portela, L.; Perry, M. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007, 81, 198–203. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J. Multidisciplinary intervention in the treatment of mixed anxiety and depression disorder. Physiol. Behav. 2020, 219, 112858. [Google Scholar] [CrossRef]
- Null, G.; Pennesi, L.; Feldman, M. Nutrition and Lifestyle Intervention on Mood and Neurological Disorders. J. Evid.-Based Integr. Med. 2017, 22, 68–74. [Google Scholar] [CrossRef]
- Cooley, K.; Szczurko, O.; Perri, D.; Mills, E.J.; Bernhardt, B.; Zhou, Q.; Seely, D. Naturopathic Care for Anxiety: A Randomized Controlled Trial ISRCTN78958974. PLoS ONE 2009, 4, e6628. [Google Scholar] [CrossRef]
- Forsyth, A.; Deane, F.P.; Williams, P. A lifestyle intervention for primary care patients with depression and anxiety: A randomised controlled trial. Psychiatry Res. 2015, 230, 537–544. [Google Scholar] [CrossRef]
- Laporte, D.J. Treatment response in obese binge eaters: Preliminary results using a very low calorie diet (VLCD) and behavior therapy. Addict. Behav. 1992, 17, 247–257. [Google Scholar] [CrossRef]
- Ein, N.; Armstrong, B.; Vickers, K. The effect of a very low calorie diet on subjective depressive symptoms and anxiety: Meta-analysis and systematic review. Int. J. Obes. 2018, 43, 1444–1455. [Google Scholar] [CrossRef]
- Jebeile, H.; Gow, M.L.; Baur, L.A.; Garnett, S.P.; Paxton, S.J.; Lister, N.B. Association of Pediatric Obesity Treatment, Including a Dietary Component, with Change in Depression and Anxiety: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, e192841. [Google Scholar] [CrossRef] [PubMed]
- Hillman, L.C.; Stace, N.H.; Pomare, E.W. Irritable bowel patients and their long-term response to a high fiber diet. Am. J. Gastroenterol. 1984, 79, 1–7. [Google Scholar]
- Christensen, L.; Krietsch, K.; White, B.; Stagner, B. Impact of a dietary change on emotional distress. J. Abnorm. Psychol. 1985, 94, 565–579. [Google Scholar] [CrossRef]
- Mohammed, H.; Ghosh, S.; Vuvor, F.; Mensah-Armah, S.; Steiner-Asiedu, M. Dietary intake and the dynamics of stress, hypertension and obesity in a peri-urban community in Accra. Ghana Med. J. 2016, 50, 16–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mesgarani, M.; Hosseinbor, M.; Shafiee, S.; Sarkoubi, R. The Relationship of Parental Mental Health and Dietary Pattern with Adolescent Mental Health. Int. J. High Risk Behav. Addict. 2016, 5, e26616. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.; Hudson, S.; MacKenzie, J. Protein-source tryptophan as an efficacious treatment for social anxiety disorder: A pilot studyThis article is one of a selection of papers published in this special issue (part 1 of 2) on the Safety and Efficacy of Natural Health Products. Can. J. Physiol. Pharmacol. 2007, 85, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Capello, A.E.; Markus, C.R. Effect of sub chronic tryptophan supplementation on stress-induced cortisol and appetite in subjects differing in 5-HTTLPR genotype and trait neuroticism. Psychoneuroendocrinology 2014, 45, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Shewamene, Z.; Galbally, M.; Schmied, V.; Dahlen, H. The effect of complementary medicines and therapies on maternal anxiety and depression in pregnancy: A systematic review and meta-analysis. J. Affect. Disord. 2019, 245, 428–439. [Google Scholar] [CrossRef]
- Deane, K.H.O.; Jimoh, O.F.; Biswas, P.; O’Brien, A.; Hanson, S.; Abdelhamid, A.S.; Fox, C.; Hooper, L. Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: Systematic review and meta-analysis of randomised trials. Br. J. Psychiatry 2021, 218, 135–142. [Google Scholar] [CrossRef]
- Su, K.-P.; Tseng, P.-T.; Lin, P.-Y.; Okubo, R.; Chen, T.-Y.; Chen, Y.-W.; Matsuoka, Y.J. Association of Use of Omega-3 Polyunsaturated Fatty Acids with Changes in Severity of Anxiety Symptoms: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e182327. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovitz, S.; Mostofsky, D.I. Mixture of essential fatty acids lowers test anxiety. Nutr. Neurosci. 2005, 8, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Razzak, K.K.; Almanasrah, S.O.; Obeidat, B.A.; Khasawneh, A.G. Vitamin D is a potential antidepressant in psychiatric outpatients. Int. J. Clin. Pharmacol. Ther. 2018, 56, 585–596. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Andridge, R.; Gorman, B.; Blampied, N.; Gordon, H.; Boggis, A. Shaken but unstirred? Effects of micronutrients on stress and trauma after an earthquake: RCT evidence comparing formulas and doses. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Agrawal, M.; Gautam, M.; Sharma, P.; Gautam, A.S.; Gautam, S. Role of antioxidants in generalised anxiety disorder and depression. Indian J. Psychiatry 2012, 54, 244–247. [Google Scholar] [CrossRef]
- Jaatinen, N.; Korpela, R.; Poussa, T.; Turpeinen, A.; Mustonen, S.; Merilahti, J.; Peuhkuri, K. Effects of daily intake of yoghurt enriched with bioactive components on chronic stress responses: A double-blinded randomized controlled trial. Int. J. Food Sci. Nutr. 2013, 65, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Russo, A. Decreased Zinc and Increased Copper in Individuals with Anxiety. Nutr. Metab. Insights 2011, 4, NMI.S6349. [Google Scholar] [CrossRef]
- Choi, E.B.; Lee, J.E.; Hwang, J.-Y. Fruit and vegetable intakes in relation to behavioral outcomes associated with a nutrition education intervention in preschoolers. Nutr. Res. Pract. 2018, 12, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Conner, T.S.; Brookie, K.L.; Carr, A.C.; Mainvil, L.A.; Vissers, M.C.M. Let them eat fruit! The effect of fruit and vegetable consumption on psychological well-being in young adults: A randomized controlled trial. PLoS ONE 2017, 12, e0171206. [Google Scholar] [CrossRef]
- Smith, A.P.; Rogers, R. Positive Effects of a Healthy Snack (Fruit) Versus an Unhealthy Snack (Chocolate/Crisps) on Subjective Reports of Mental and Physical Health: A Preliminary Intervention Study. Front. Nutr. 2014, 1, 10. [Google Scholar] [CrossRef]
- Camfield, D.; Stough, C.; Farrimond, J.; Scholey, A.B. Acute effects of tea constituents L-theanine, caffeine, and epigallocatechin gallate on cognitive function and mood: A systematic review and meta-analysis. Nutr. Rev. 2014, 72, 507–522. [Google Scholar] [CrossRef]
- Hieu, T.H.; Dibas, M.; Dila, K.A.S.; Sherif, N.A.; Hashmi, M.U.; Mahmoud, M.; Trang, N.T.T.; Abdullah, L.; Nghia, T.L.B.; Mai Nhu, Y.; et al. Therapeutic efficacy and safety of chamomile for state anxiety, generalized anxiety disorder, insomnia, and sleep quality: A systematic review and meta-analysis of randomized trials and quasi-randomized trials. Phytotherapy Res. 2019, 33, 1604–1615. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Rocks, T.; Ruusunen, A.; Loughman, A.; Lopresti, A.; Marshall, S.; Berk, M.; Jacka, F.; Dean, O.M. Effect of saffron supplementation on symptoms of depression and anxiety: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 557–571. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Koh, S.S.H.; Chan, H.W.; Ho, C.Y.X. Clinical Use of Curcumin in Depression: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef]
- Keefe, J.R.; Guo, W.; Li, Q.S.; Amsterdam, J.D.; Mao, J.J. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J. Psychiatr. Res. 2018, 96, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, J.D.; Li, Y.; Soeller, I.; Rockwell, K.; Mao, J.J.; Shults, J. A Randomized, Double-Blind, Placebo-Controlled Trial of Oral Matricaria recutita (Chamomile) Extract Therapy for Generalized Anxiety Disorder. J. Clin. Psychopharmacol. 2009, 29, 378–382. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Keefe, J.R.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine 2016, 23, 1735–1742. [Google Scholar] [CrossRef]
- Jafarnia, N.; Ghorbani, Z.; Nokhostin, M.; Manayi, A.; Nourimajd, S.; Jahromi, S.R. Effect of Saffron (Crocus satious L.) as an Add-On Therapy to Sertraline in Mild to Moderate Generalized Anxiety Disorder: A Double Blind Randomized Controlled Trial. Arch. Neurosci. 2017, 4, e14332. [Google Scholar] [CrossRef]
- Milajerdi, A.; Jazayeri, S.; Shirzadi, E.; Hashemzadeh, N.; Azizgol, A.; Djazayery, A.; Esmaillzadeh, A.; Akhondzadeh, S. The effects of alcoholic extract of saffron (Crocus satious L.) on mild to moderate comorbid depression-anxiety, sleep quality, and life satisfaction in type 2 diabetes mellitus: A double-blind, randomized and placebo-controlled clinical trial. Complement. Ther. Med. 2018, 41, 196–202. [Google Scholar] [CrossRef]
- Sudheeran, S.P.; Jacob, D.; Mulakal, J.N.; Nair, G.G.; Maliakel, A.; Maliakel, B.; Ramadasan, K.; Krishnakumar, I.M. Safety, Tolerance, and Enhanced Efficacy of a Bioavailable Formulation of Curcumin with Fenugreek Dietary Fiber on Occupational Stress: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2016, 36, 236–243. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Cribb, L.; Oliver, G.; Murphy, J.; Macdonald, P.; Nazareth, S.; Karamacoska, D.; Galea, S.; Short, A.; et al. L-theanine in the adjunctive treatment of generalized anxiety disorder: A double-blind, randomised, placebo-controlled trial. J. Psychiatr. Res. 2019, 110, 31–37. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Eskandarzadeh, S.; Effatpanah, M.; Khosravi-Darani, K.; Askari, R.; Hosseini, A.F.; Reisian, M.; Jazayeri, S. Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: A double blind, randomized, placebo-controlled trial. Nutr. Neurosci. 2021, 24, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, Y.; Wang, M.; Liu, J.; Ju, Y.; Zhang, Y.; Liu, T.; Li, L.; Li, Q. Efficacy of probiotics on anxiety-A meta-analysis of randomized controlled trials. Depress. Anxiety 2018, 35, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef]
- Del Olmo, N.; Blanco-Gandia, M.C.; Mateos-Garcia, A.; Del Rio, D.; Minarro, J.; Ruiz-Gayo, M.; Rodríguez-Arias, M. Differential Impact of Ad Libitum or Intermittent High-Fat Diets on Bingeing Ethanol-Mediated Behaviors. Nutrients 2019, 11, 2253. [Google Scholar] [CrossRef]
- Dornellas, A.P.S.; Boldarine, V.T.; Pedroso, A.P.; Carvalho, L.O.T.; de Andrade, I.S.; Vulcani-Freitas, T.M.; dos Santos, C.C.C.; da Penha Oller do Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B. High-Fat Feeding Improves Anxiety-Type Behavior Induced by Ovariectomy in Rats. Front. Neurosci. 2018, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Segat, H.; Barcelos, R.; Metz, V.; Rosa, H.; Roversi, K.; Antoniazzi, C.; Vey, L.; Kronbauer, M.; Veit, J.; Piccolo, J.; et al. Influence of physical activity on addiction parameters of rats exposed to amphetamine which were previously supplemented with hydrogenated vegetable fat. Brain Res. Bull. 2017, 135, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Segev, Y.; Livne, A.; Mints, M.; Rosenblum, K. Concurrence of High Fat Diet and APOE Gene Induces Allele Specific Metabolic and Mental Stress Changes in a Mouse Model of Alzheimer’s Disease. Front. Behav. Neurosci. 2016, 10, 170. [Google Scholar] [CrossRef]
- Otsuka, A.; Shiuchi, T.; Chikahisa, S.; Shimizu, N.; Séi, H. Sufficient intake of high-fat food attenuates stress-induced social avoidance behavior. Life Sci. 2019, 219, 219–230. [Google Scholar] [CrossRef]
- Haleem, D.J.; Mahmood, K. Brain serotonin in high-fat diet-induced weight gain, anxiety and spatial memory in rats. Nutr. Neurosci. 2021, 24, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of Dietary Fat and Risk of Coronary Heart Disease: A Critical Review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.; Broste, S.K.; Frantz, R.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.; Chelmi, M.-E.; Liampas, A.; Yfanti, C.-M.; Panagouli, E.; Vlachopapadopoulou, E.; Michalacos, S.; Bacopoulou, F.; Psaltopoulou, T.; Tsitsika, A. Vegetarian Diets and Eating Disorders in Adolescents and Young Adults: A Systematic Review. Children 2020, 8, 12. [Google Scholar] [CrossRef]
- Swinbourne, J.M.; Touyz, S. The co-morbidity of eating disorders and anxiety disorders: A review. Eur. Eat. Disord. Rev. 2007, 15, 253–274. [Google Scholar] [CrossRef]
- Woo, K.S.; Kwok, T.C.; Celermajer, D.S. Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health. Nutrients 2014, 6, 3259–3273. [Google Scholar] [CrossRef]
- Lakin, V.; Haggarty, P.; Abramovich, D.; Ashton, J.; Moffat, C.; McNeill, G.; Danielian, P.; Grubb, D. Dietary intake and tissue concentration of fatty acids in omnivore, vegetarian and diabetic pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 1998, 59, 209–220. [Google Scholar] [CrossRef]
- Anderson, R.J.; Grigsby, A.B.; Freedland, K.E.; De Groot, M.; McGill, J.B.; Clouse, R.E.; Lustman, P.J. Anxiety and poor glycemic control: A meta-analytic review of the literature. Int. J. Psychiatry Med. 2002, 32, 235–247. [Google Scholar] [CrossRef]
- Moja, E.A.; Cipolla, P.; Castoldi, D.; Tofanetti, O. Dose-response decrease in plasma tryptophan and in brain tryptophan and serotonin after tryptophan-free amino acid mixtures in rats. Life Sci. 1989, 44, 971–976. [Google Scholar] [CrossRef]
- Stein, D.J.; Stahl, S. Serotonin and anxiety: Current models. Int. Clin. Psychopharmacol. 2000, 15, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L.; Bier, D.M.; Kadowaki, M.; Morris, J.S.M.; Elango, R.; Smriga, M. Proposals for Upper Limits of Safe Intake for Arginine and Tryptophan in Young Adults and an Upper Limit of Safe Intake for Leucine in the Elderly. J. Nutr. 2016, 146, 2652S–2654S. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, S.; Dewart, D.; Pollack, R.L.; Jackson, E. The effects of dietary tryptophan on chronic maxillofacial pain and experimental pain tolerance. J. Psychiatr. Res. 1982, 17, 181–186. [Google Scholar] [CrossRef]
- Alusik, S.; Kalatova, D.; Paluch, Z. Serotonin syndrome. Neuroendocrinol. Lett. 2014, 35, 265–273. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: Fdc.nal.usda.gov (accessed on 22 September 2021).
- Firth, J.; Veronese, N.; Cotter, J.; Shivappa, N.; Hebert, J.R.; Ee, C.; Smith, L.; Stubbs, B.; Jackson, S.E.; Sarris, J. What Is the Role of Dietary Inflammation in Severe Mental Illness? A Review of Observational and Experimental Findings. Front. Psychiatry 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Chugh, G.; Asghar, M. Inflammation in Anxiety. Adv. Protein Chem. Struct. Biol. 2012, 88, 1–25. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Zong, Y.; Yang, X. DHA Protects Hepatocytes from Oxidative Injury through GPR120/ERK-Mediated Mitophagy. Int. J. Mol. Sci. 2021, 22, 5675. [Google Scholar] [CrossRef]
- DeLion, S.; Chalon, S.; Hérault, J.; Guilloteau, D.; Besnard, J.C.; Durand, G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J. Nutr. 1994, 124, 2466–2476. [Google Scholar] [CrossRef]
- Marino, A.; Cuzzocrea, S. n-3 Fatty Acids: Role in Neurogenesis and Neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef]
- Gałecki, P.; Mossakowska-Wójcik, J.; Talarowska, M. The anti-inflammatory mechanism of antidepressants—SSRIs, SNRIs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I.; Santarelli, S.; Capoccia, S.; Poggini, S.; D’Andrea, I.; Cirulli, F.; Alleva, E. Antidepressant Treatment Outcome Depends on the Quality of the Living Environment: A Pre-Clinical Investigation in Mice. PLoS ONE 2013, 8, e62226. [Google Scholar] [CrossRef]
- Solomons, N.W. Dietary Sources of Zinc and Factors Affecting its Bioavailability. Food Nutr. Bull. 2001, 22, 138–154. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Kaplan, B.J. Broad-spectrum micronutrient formulas for the treatment of psychiatric symptoms: A systematic review. Expert Rev. Neurother. 2013, 13, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Smith, C.; Faraone, S.; Shafa, R.; Stone, W.; Glatt, S.; Tsuang, M.T. Methylomics in psychiatry: Modulation of gene-environment interactions may be through DNA methylation. Am. J. Med. Genet. 2004, 127B, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Trovato, C.M.; Raucci, U.; Valitutti, F.; Montuori, M.; Villa, M.P.; Cucchiara, S.; Parisi, P. Neuropsychiatric manifestations in celiac disease. Epilepsy Behav. 2019, 99, 106393. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, J.; Khan, B.; Saleem, F.; Abbas, G.; Ahmed, A. Gut microbiome interferes with host tryptophan metabolism pathway and regulate basal anxiety–like behavior. Int. J. Infect. Dis. 2020, 101, 5. [Google Scholar] [CrossRef]
- Arbour-Nicitopoulos, K.P.; Faulkner, G.; Irving, H.M. Multiple Health-Risk Behaviour and Psychological Distress in Adolescence. J. Can. Acad. Child Adolesc. Psychiatry 2012, 21, 171–178. [Google Scholar] [PubMed]
- Grønning, K.; Espnes, G.A.; Nguyen, C.; Rodrigues, A.M.F.; Gregorio, M.J.; Sousa, R.; Canhão, H.; André, B. Psychological distress in elderly people is associated with diet, wellbeing, health status, social support and physical functioning—A HUNT3 study. BMC Geriatr. 2018, 18, 205. [Google Scholar] [CrossRef]
- Steimer, T. Animal models of anxiety disorders in rats and mice: Some conceptual issues. Dialog. Clin. Neurosci. 2011, 13, 495–506. [Google Scholar]
- van der Pols, J.C. Nutrition and mental health: Bidirectional associations and multidimensional measures. Public Health Nutr. 2018, 21, 829–830. [Google Scholar]
- Macht, M.; Roth, S.; Ellgring, H. Chocolate eating in healthy men during experimentally induced sadness and joy. Appetite 2002, 39, 147–158. [Google Scholar] [CrossRef]
- Willner, P.; Benton, D.; Brown, E.; Cheeta, S.; Roderique-Davies, G.; Morgan, J.; Morgan, M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology 1998, 136, 272–283. [Google Scholar] [CrossRef]
- Shobo, M.; Yamada, H.; Mihara, T.; Kondo, Y.; Irie, M.; Harada, K.; Ni, K.; Matsuoka, N.; Kayama, Y. Two models for weight gain and hyperphagia as side effects of atypical antipsychotics in male rats: Validation with olanzapine and ziprasidone. Behav. Brain Res. 2011, 216, 561–568. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; la Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Bell, M.E.; Liang, J.; Soriano, L.; Nagy, T.R.; Dallman, M.F. Corticosterone Facilitates Saccharin Intake in Adrenalectomized Rats: Does Corticosterone Increase Stimulus Salience? J. Neuroendocr. 2000, 12, 453–460. [Google Scholar] [CrossRef]
- Health Canada, Eating Well with Canada’s Food Guide. 2007. Available online: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php (accessed on 9 December 2021).
- Chatterton, M.L.; Mihalopoulos, C.; O’Neil, A.; Itsiopoulos, C.; Opie, R.; Castle, D.; Dash, S.; Brazionis, L.; Berk, M.; Jacka, F. Economic evaluation of a dietary intervention for adults with major depression (the “SMILES” trial). BMC Public Health 2018, 18, 599. [Google Scholar] [CrossRef] [PubMed]
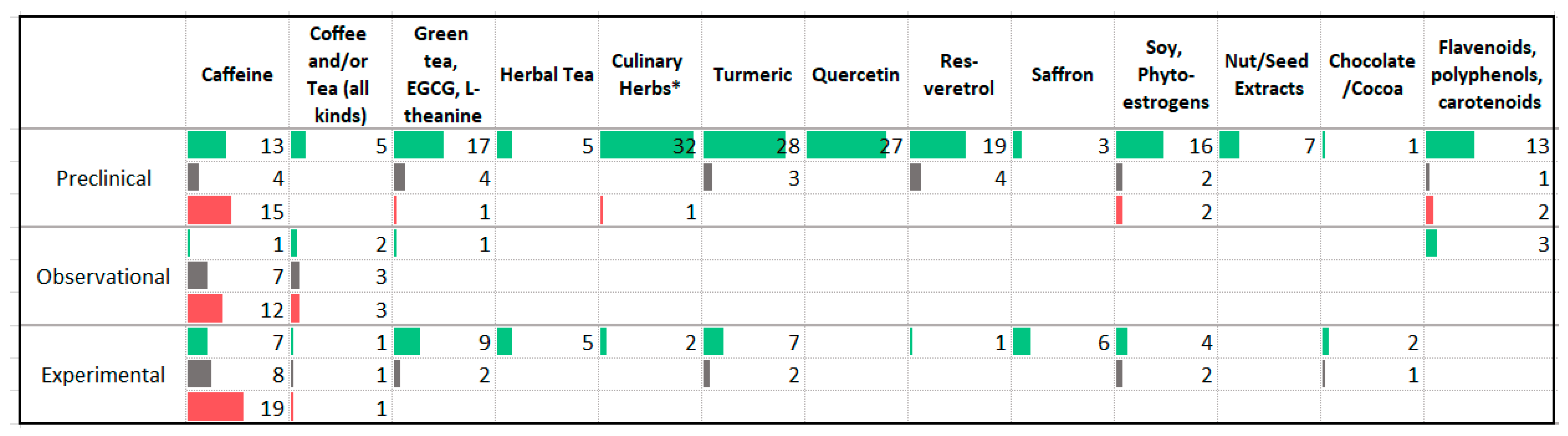
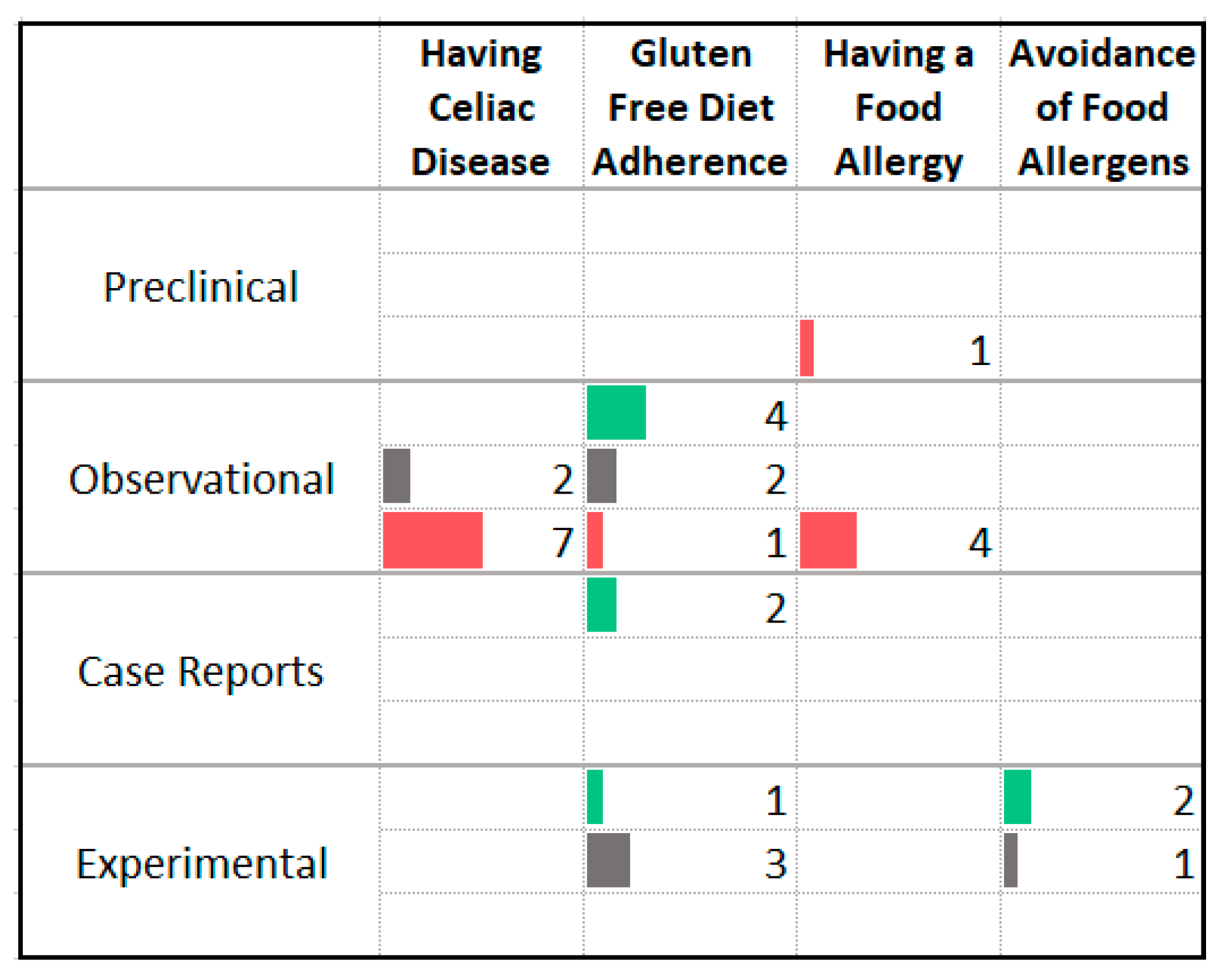
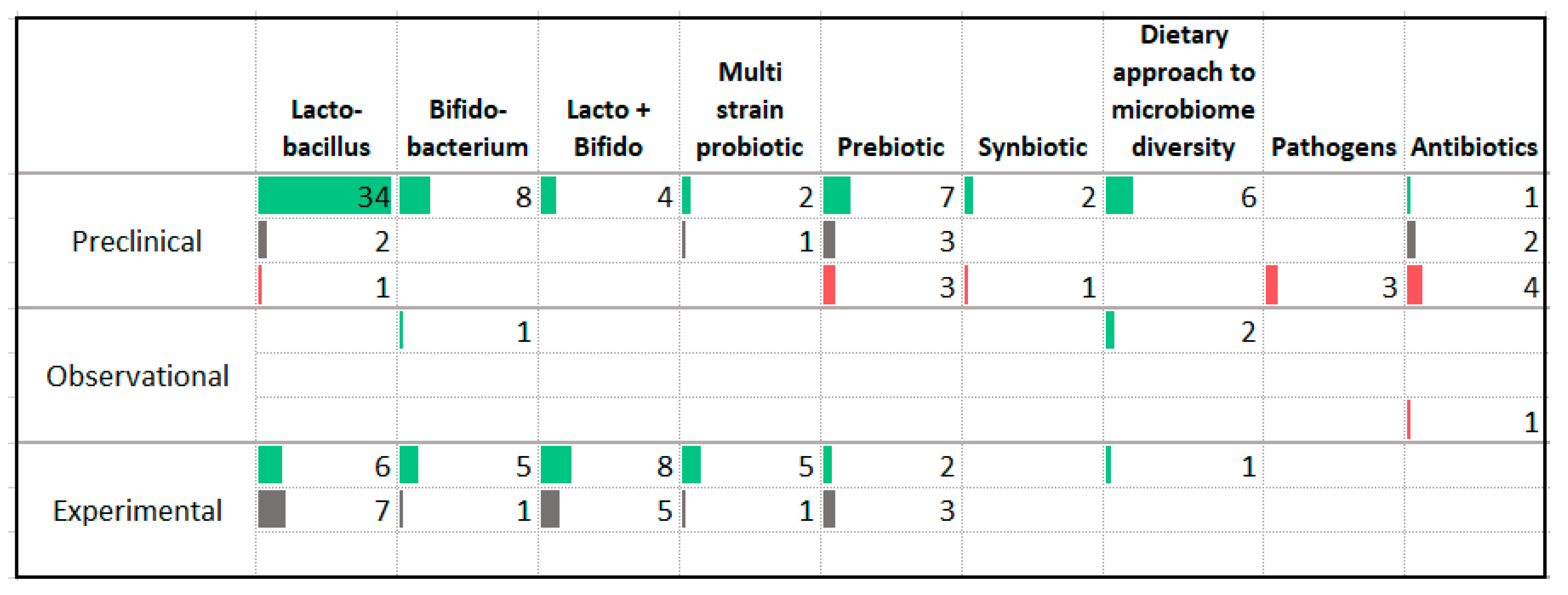
| Association with Less Anxiety | Association with More Anxiety |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aucoin, M.; LaChance, L.; Naidoo, U.; Remy, D.; Shekdar, T.; Sayar, N.; Cardozo, V.; Rawana, T.; Chan, I.; Cooley, K. Diet and Anxiety: A Scoping Review. Nutrients 2021, 13, 4418. https://doi.org/10.3390/nu13124418
Aucoin M, LaChance L, Naidoo U, Remy D, Shekdar T, Sayar N, Cardozo V, Rawana T, Chan I, Cooley K. Diet and Anxiety: A Scoping Review. Nutrients. 2021; 13(12):4418. https://doi.org/10.3390/nu13124418
Chicago/Turabian StyleAucoin, Monique, Laura LaChance, Umadevi Naidoo, Daniella Remy, Tanisha Shekdar, Negin Sayar, Valentina Cardozo, Tara Rawana, Irina Chan, and Kieran Cooley. 2021. "Diet and Anxiety: A Scoping Review" Nutrients 13, no. 12: 4418. https://doi.org/10.3390/nu13124418
APA StyleAucoin, M., LaChance, L., Naidoo, U., Remy, D., Shekdar, T., Sayar, N., Cardozo, V., Rawana, T., Chan, I., & Cooley, K. (2021). Diet and Anxiety: A Scoping Review. Nutrients, 13(12), 4418. https://doi.org/10.3390/nu13124418






