Relationship between Oral Hypofunction, and Protein Intake: A Cross-Sectional Study in Local Community-Dwelling Adults
Abstract
:1. Introduction
2. Materials and Methods
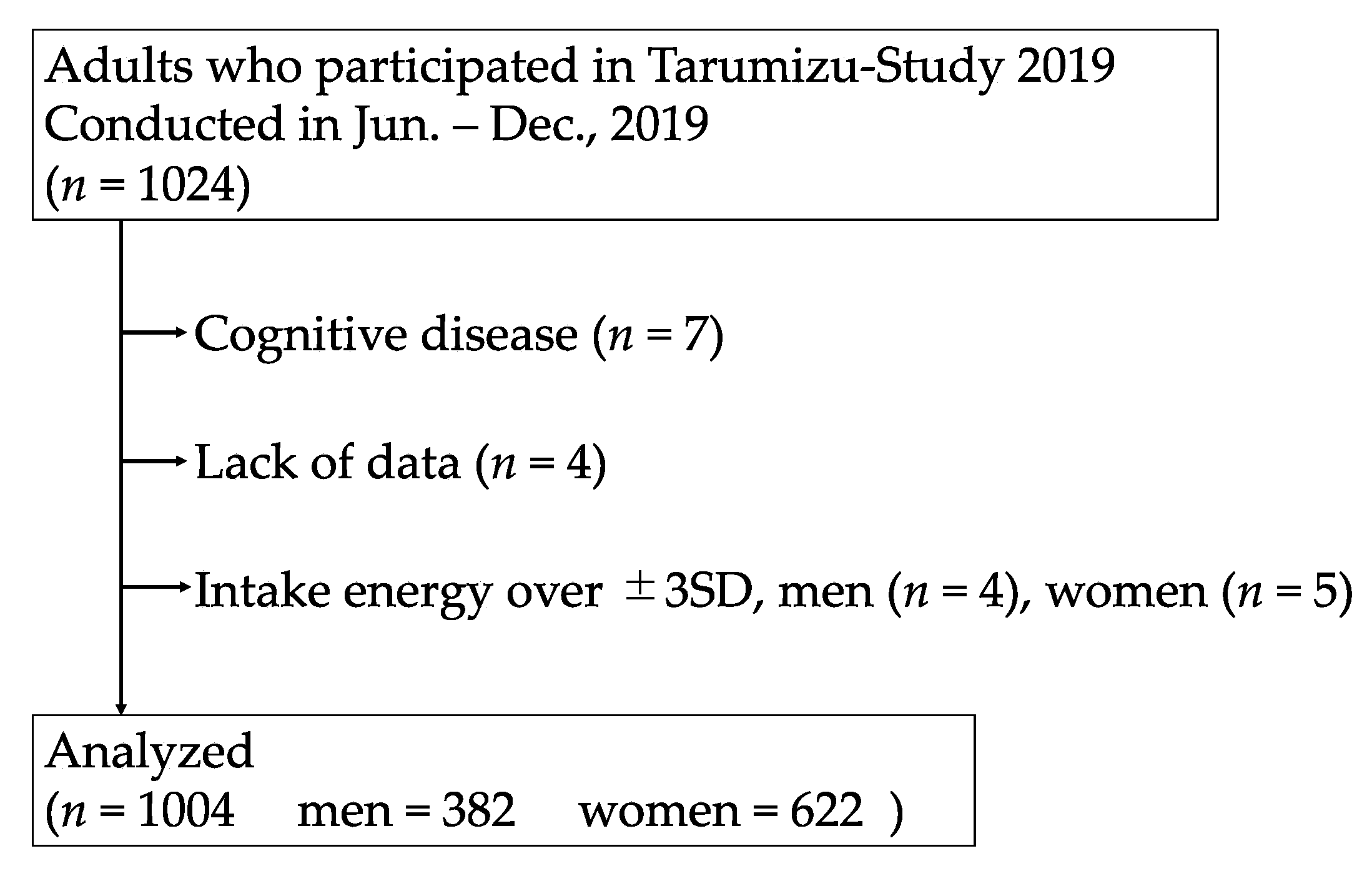
2.1. Participants
2.2. Diagnosis of Oral Hypofunction
2.2.1. Poor Oral Hygiene
2.2.2. Oral Dryness
2.2.3. Reduced Occlusal Force
2.2.4. Decreased Tongue-Lip Motor Function
2.2.5. Tongue Pressure Measurement
2.2.6. Decreased Masticatory Function
2.2.7. Assessing of Swallowing Function
2.3. Nutritional Assessment
2.3.1. Brief-Type Self-Administered Diet History Questionnaire (BDHQ)
2.3.2. Diagnosis of Low Protein Intake
2.4. Other Surveys
2.5. Statistical Analysis
3. Results
3.1. Physical Characteristics and Examination Values by Presence or Absence of Oral Hypofunction
3.2. Nutritional Status and Food Intake Based on the Presence or Absence of Oral Hypofunction
3.3. Physical Characteristics, Nutritional Status, and Social Background Based on Protein Intake
3.4. Oral Hypofunction Evaluation According to Under-DG or Over-DG Protein Intake
3.5. Association with Oral Hypofunction and Protein Intake in Multivariate Binary Logistic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Health, Labour and Welfare. The White Paper on Ministry of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/000735866.pdf (accessed on 2 December 2021).
- Iijima, K.; Arai, H.; Akishita, M.; Endo, T.; Ogasawara, K.; Kashihara, N.; Hayashi, Y.K.; Yumura, W.; Yokode, M.; Ouchi, Y. Toward the development of a vibrant, super-aged society: The future of medicine and society in Japan. Geriatr. Gerontol. Int. 2021, 21, 601–613. [Google Scholar] [CrossRef]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif. Tissue Int. 2019, 104, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanri, H.; Yamada, Y.; Yoshida, T.; Okabe, Y.; Nozawa, Y.; Itoi, A.; Yoshimura, E.; Watanabe, Y.; Yamaguchi, M.; Yokoyama, K.; et al. Sex Difference in the Association Between Protein Intake and Frailty: Assessed Using the Kihon Checklist Indexes Among Older Adults. J. Am. Med. Dir. Assoc. 2018, 19, 801–805. [Google Scholar] [CrossRef]
- Feart, C. Nutrition and frailty: Current knowledge. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109703. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S.; Three-Generation Study of Women on Diets and Health Study Group. High protein intake is associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutr. J. 2013, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Suga, H.; Sasaki, S. Diet with a combination of high protein and high total antioxidant capacity is strongly associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutr. J. 2017, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Júnior, H.J.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health, Labour and Welfare. Dietary Reference Intakes for Japanese (2020). 2020. Available online: https://www.mhlw.go.jp/content/10904750/000586553.pdf (accessed on 2 December 2021).
- Ministry of Health, Labour and Welfare. Eat Well Preveny Frailty. 2020. Available online: https://www.mhlw.go.jp/content/000620855.pdf (accessed on 2 December 2021).
- Minakuchi, S.; Tsuga, K.; Ikebe, K.; Ueda, T.; Tamura, F.; Nagao, K.; Furuya, J.; Matsuo, K.; Yamamoto, K.; Kanazawa, M.; et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology 2018, 35, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, F.F.; Bernabé, E.; Sabbah, W. Association between oral health and frailty: A systematic review of longitudinal studies. Gerodontology 2019, 36, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Horibe, Y.; Ueda, T.; Watanabe, Y.; Motokawa, K.; Edahiro, A.; Hirano, H.; Shirobe, M.; Ogami, K.; Kawai, H.; Obuchi, S.; et al. A 2-year longitudinal study of the relationship between masticatory function and progression to frailty or pre-frailty among community-dwelling Japanese aged 65 and older. J. Oral Rehabil. 2018, 45, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satake, A.; Kobayashi, W.; Tamura, Y.; Oyama, T.; Fukuta, H.; Inui, A.; Sawada, K.; Ihara, K.; Noguchi, T.; Murashita, K.; et al. Effects of oral environment on frailty: Particular relevance of tongue pressure. Clin. Interv. Aging 2019, 14, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Machida, N.; Tohara, H.; Hara, K.; Kumakura, A.; Wakasugi, Y.; Nakane, A.; Minakuchi, S. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr. Gerontol. Int. 2017, 17, 295–301. [Google Scholar] [CrossRef]
- Hoeksema, A.R.; Spoorenberg, S.; Peters, L.L.; Meijer, H.; Raghoebar, G.M.; Vissink, A.; Wynia, K.; Visser, A. Elderly with remaining teeth report less frailty and better quality of life than edentulous elderly: A cross-sectional study. Oral Dis. 2017, 23, 526–536. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Nonoyama, T.; Tsushita, K.; Arai, H.; Matsushita, K.; Uchibori, N. Oral hypofunction and its association with frailty in community-dwelling older people. Geriatr. Gerontol. Int. 2020, 20, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Obuchi, S.; Kawai, H.; Fujiwara, Y.; et al. Relationship between Oral Hypofunction and Sarcopenia in Community-Dwelling Older Adults: The Otassha Study. Int. J. Environ. Res. Public Health 2021, 18, 6666. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hamada, T.; Tanaka, A.; Nishi, K.; Kume, K.; Goto, Y.; Beppu, M.; Hijioka, H.; Higashi, Y.; Tabata, H.; et al. Association of Oral Hypofunction with Frailty, Sarcopenia, and Mild Cognitive Impairment: A Cross-Sectional Study of Community-Dwelling Japanese Older Adults. J. Clin. Med. 2021, 10, 1626. [Google Scholar] [CrossRef]
- Motokawa, K.; Mikami, Y.; Shirobe, M.; Edahiro, A.; Ohara, Y.; Iwasaki, M.; Watanabe, Y.; Kawai, H.; Kera, T.; Obuchi, S.; et al. Relationship between Chewing Ability and Nutritional Status in Japanese Older Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1216. [Google Scholar] [CrossRef]
- Okamoto, N.; Amano, N.; Nakamura, T.; Yanagi, M. Relationship between tooth loss, low masticatory ability, and nutritional indices in the elderly: A cross-sectional study. BMC Oral Health 2019, 19, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, Y.; Kakuta, E.; Okada, A.; Otsuka, R.; Shimada, M.; Tomizawa, Y.; Taguchi, C.; Arikawa, K.; Daikoku, H.; Sato, T.; et al. Effects of self-assessed chewing ability, tooth loss and serum albumin on mortality in 80-year-old individuals: A 20-year follow-up study. BMC Oral Health 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.H. The effect of mastication on food intake, satiety and body weight. Physiol. Behav. 2018, 193, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Hirano, H.; Edahiro, A.; Motokawa, K.; Shirobe, M.; Watanabe, Y.; Motohashi, Y.; Ohara, Y.; Iwasaki, M.; Maruoka, Y.; et al. Association between Oral Frailty and Dietary Variety among Community-Dwelling Older Persons: A Cross-Sectional Study. J. Nutr. Health Aging 2021, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Inagaki, H.; Edahiro, A.; Ohara, Y.; Hirano, H.; Shinkai, S.; Awata, S. Association between Oral Frailty and Nutritional Status among Community-Dwelling Older Adults: The Takashimadaira Study. J. Nutr. Health Aging 2020, 24, 1003–1010. [Google Scholar] [CrossRef]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Ohara, Y.; Edahiro, A.; Kawai, H.; Fujiwara, Y.; Kim, H.; Ihara, K.; et al. Oral hypofunction and malnutrition among community-dwelling older adults: Evidence from the Otassha study. Gerodontology 2021. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ueda, T.; Sakurai, K. New method for evaluation of tongue-coating status. J. Oral Rehabil 2007, 34, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Kaimoto, K.; Yamashita, M.; Suzuki, T.; Makizako, H.; Koriyama, C.; Kubozono, T.; Takenaka, T.; Ohishi, M.; Kanouchi, H.; The Tarumizu Study Diet Group. Association of Protein and Magnesium Intake with Prevalence of Prefrailty and Frailty in Community-Dwelling Older Japanese Women. J. Nutr. Sci. Vitaminol. 2021, 67, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Calvani, R.; Picca, A.; Gonçalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Kikutani, T.; Yoshikawa, M.; Tsuga, K.; Kimura, M.; Akagawa, Y. Correlation between dental and nutritional status in community-dwelling elderly Japanese. Geriatr. Gerontol. Int. 2011, 11, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Watanabe, R.; Nishimuta, M.; Hanada, N.; Miyazaki, H. The relationship between dietary intake and the number of teeth in elderly Japanese subjects. Gerodontology 2005, 22, 211–218. [Google Scholar] [CrossRef]
- Takeuchi, H.; Terada, M.; Kobayashi, K.; Uraguchi, M.; Nomura, Y.; Hanada, N. Influences of Masticatory Function Recovery Combined with Health Guidance on Body Composition and Metabolic Parameters. Open Dent. J. 2019, 13, 124–136. [Google Scholar] [CrossRef]
- Nomura, Y.; Takeuchi, H.; Shigemoto, S.; Okada, A.; Shigeta, Y.; Ogawa, T.; Hanada, N. Secondary Endpoint of the Prosthodontics. Int. J. Clin. Case Stud. 2017, 3, 117. [Google Scholar] [CrossRef]
- Nomura, Y.; Ishii, Y.; Suzuki, S.; Morita, K.; Suzuki, A.; Suzuki, S.; Tanabe, J.; Ishiwata, Y.; Yamakawa, K.; Chiba, Y.; et al. Nutritional Status and Oral Frailty: A Community Based Study. Nutrients 2020, 12, 2886. [Google Scholar] [CrossRef] [PubMed]
| Evaluation | Criteria | |
|---|---|---|
| 1 | Poor oral hygiene | TCI was more than 50%. |
| 2 | Oral dryness | The oral moisture checker value was less than 27. |
| 3 | Occlusal force | The number of remaining teeth was less than 20. |
| 4 | ODK (/pa/, /ta/, /ka/) | The rate of repetitions performed was less than 6 per second. |
| 5 | Tongue pressure | Tongue pressure was less than 30 kPa. |
| 6 | Masticatory function | The participants answered difficulty chewing the hard foods. |
| 7 | Swallowing function | The total score of EAT-10 was more than 3. |
| Men (n = 382) | Women (n = 622) | |||||
|---|---|---|---|---|---|---|
| No Oral Hypofunction (n = 202) | Oral Hypofunction (n = 180) | p-Value | No Oral Hypofunction (n = 333) | Oral Hypofunction (n = 289) | p-Value | |
| Age (y) | 63.3 ± 11.3 | 72.3 ± 9.2 | <0.0001 †† | 64.2 ± 10.0 | 74.0 ± 9.3 | <0.0001 |
| BW (kg) | 65.6 ± 10.0 | 63.4 ± 8.8 | 0.0218 | 54.7 ± 9.6 | 51.7 ± 8.6 | <0.0001 |
| BMI (kg/m2) | 23.7 ± 3.1 | 23.5 ± 2.8 | 0.5786 | 23.1 ± 3.7 | 23.0 ± 3.5 | 0.7556 |
| SMI (kg/m2) | 7.6 ± 0.7 | 7.3 ± 0.7 | <0.0001 | 6.1 ± 0.7 | 5.8 ± 0.6 | <0.0001 |
| Oral hygiene (%) | 44.8 ± 29.4 | 63.3 ± 29.3 | <0.0001 | 33.6 ± 26.9 | 52.0 ± 31.6 | <0.0001 |
| Oral dryness | 27.6 ± 2.4 | 26.6 ± 2.7 | <0.0001 | 27.2 ± 2.3 | 26.3 ± 2.5 | <0.0001 |
| Occlusal force (number) | 24.5 ± 6.2 | 14.3 ± 9.7 | <0.0001 | 24.0 ± 6.2 | 13.9 ± 9.8 | <0.0001 |
| ODK (pa) (/s) | 6.3 ± 1.1 | 5.7 ± 1.1 | <0.0001 | 6.5 ± 0.7 | 5.7 ± 0.8 | <0.0001 |
| ODK (ta) (/s) | 6.4 ± 0.9 | 5.7 ± 1.0 | <0.0001 | 6.6 ± 0.8 | 5.7 ± 0.8 | <0.0001 |
| ODK (ka) (/s) | 5.8 ± 1.1 | 5.0 ± 1.1 | <0.0001 | 6.2 ± 0.7 | 5.3 ± 0.8 | <0.0001 |
| Tongue pressure (kPa) | 40 ± 8.9 | 32.2 ± 10.8 | <0.0001 | 37.6 ± 7.4 | 30.4 ± 10.0 | <0.0001 |
| Masticatory function (n) | 3 | 49 | <0.0001 † | 24 | 93 | <0.0001 |
| Swallowing function | 0.6 ± 1.2 | 1.6 ± 2.8 | <0.0001 | 0.8 ± 2.0 | 1.7 ± 3.0 | <0.0001 |
| Number of indicators of poor functioning in the oral hypofunction evaluation (n) | 1.5 ± 0.7 | 3.8 ± 0.9 | <0.0001 | 1.3 ± 0.7 | 3.8 ± 0.9 | <0.0001 |
| Educational history (y) | 13.5 ± 2.6 | 12.0 ± 2.7 | <0.0001 | 12.4 ± 2.0 | 11.0 ± 1.9 | <0.0001 |
| Living alone (n) | 25 | 37 | 0.0305 † | 64 | 98 | <0.0001 † |
| Men (n = 382) | Women (n = 622) | |||||
|---|---|---|---|---|---|---|
| No Oral Hypofunction (n = 202) | Oral Hypofunction (n = 180) | p-Value | No Oral Hypofunction (n = 333) | Oral Hypofunction (n = 289) | p-Value | |
| Energy (kcal) | 2099.7 ± 554.7 | 2105.8 ± 584.6 | 0.9165 | 1672.8 ± 454.6 | 1770.2 ± 524.3 | 0.0143 †† |
| Protein (% energy) | 15.4 ± 2.9 | 15.2 ± 2.9 | 0.4966 | 17.0 ± 3.0 | 17.0 ± 3.2 | 0.9704 |
| Fat (% energy) | 26.7 ± 5.5 | 25.5 ± 5.3 | 0.0267 | 29.7 ± 5.3 | 28.8 ± 5.1 | 0.0358 |
| Carbohydrate (% energy) | 49.4 ± 8.7 | 50.7 ± 8.6 | 0.1453 | 51.2 ± 7.5 | 52.4 ± 7.4 | 0.0333 |
| Total protein (g/day) | 81.4 ± 16.5 | 81.2 ± 15.6 | 0.8920 | 73.7 ± 13.0 | 74.1 ± 14.6 | 0.6872 |
| Cereals (g/day) | 440.2 ± 133.8 | 426.7 ± 117.2 | 0.2970 | 320.2 ± 96.0 | 336.0 ± 98.9 | 0.0451 |
| Potatoes (g/day) | 50.2 ± 44.0 | 58.7 ± 56.0 | 0.1150 | 59.0 ± 41.5 | 61.2 ± 50.2 | 0.5417 |
| Sugar (g/day) | 4.6 ± 3.7 | 5.5 ± 4.4 | 0.0303 | 4.1 ± 2.9 | 4.2 ± 2.8 | 0.8778 |
| Beans (g/day) | 90.4 ± 47.6 | 91.3 ± 57.0 | 0.8671 †† | 89.4 ± 48.2 | 80.6 ± 45.1 | 0.0197 |
| Vegetables (green and yellow) (g/day) | 120.0 ± 78.1 | 129.6 ± 78.0 | 0.2272 | 133.8 ± 70.0 | 136.2 ± 68.1 | 0.6677 |
| Vegetables (other) (g/day) | 191.3 ± 107.0 | 188.0 ± 94.1 | 0.7472 | 197.6 ± 88.9 | 179.2 ± 83.8 | 0.0083 |
| Fruits (g/day) | 111.3 ± 102.2 | 118.9 ± 90.1 | 0.4492 | 134.6 ± 90.2 | 150.5 ± 103.9 | 0.0415 |
| Seafoods (g/day) | 91.0 ± 50.3 | 95.9 ± 57.5 | 0.3732 | 83.1 ± 41.3 | 87.8 ± 53.2 | 0.2085 †† |
| Meats (g/day) | 85.8 ± 38.6 | 76.9 ± 38.8 | 0.0265 | 75.7 ± 33.0 | 72.5 ± 41.8 | 0.2852 |
| Eggs (g/day) | 54.9 ± 27.5 | 54.5 ± 30.3 | 0.8941 | 46.8 ± 23.6 | 47.0 ± 23.0 | 0.9371 |
| Dairy products (g/day) | 149.3 ± 110.4 | 154.0 ± 118.1 | 0.6857 | 151.9 ± 87.2 | 158.2 ± 97.4 | 0.3967 |
| Oil and fat (g/day) | 13.4 ± 5.3 | 12.4 ± 5.4 | 0.0810 | 11.6 ± 4.9 | 10.8 ± 5.0 | 0.0466 |
| Sweets (g/day) | 36.6 ± 27.8 | 43.6 ± 37.7 | 0.0403 †† | 45.6 ± 30.2 | 41.5 ± 35.8 | 0.1193 |
| Beverages (g/day) | 877.4 ± 375.9 | 834.4 ± 359.5 | 0.2559 | 673.8 ± 298.2 | 664.1 ± 261.0 | 0.6685 |
| Seasoning and spices (g/day) | 285.2 ± 126.5 | 315.6 ± 122.1 | 0.0178 | 222.7 ± 5.6 | 236.3 ± 6.0 | 0.0945 |
| Albumin (g/dL) | 4.373 ± 0.3 | 4.243 ± 0.3 | <0.0001 | 4.348 ± 0.3 | 4.259 ± 0.3 | <0.0001 |
| Men (n = 382) | Women (n = 622) | |||||
|---|---|---|---|---|---|---|
| Over DG (n = 240) | Under DG (n = 142) | p-Value | Over DG (n = 495) | Under DG (n = 127) | p-Value | |
| Age (y) | 67.3 ± 11.8 | 68.0 ± 10.5 | 0.5744 | 68.6 ± 10.8 | 69.4 ± 11.1 | 0.4326 |
| BW (kg) | 64.4 ± 9.4 | 65.0 ± 9.6 | 0.5515 | 53.2 ± 9.2 | 53.6 ± 9.6 | 0.7072 |
| BMI (kg/m2) | 23.6 ± 2.9 | 23.6 ± 3.1 | 0.8419 | 23.0 ± 3.5 | 23.4 ± 4.1 | 0.2963 |
| SMI (kg/m2) | 7.5 ± 0.7 | 7.5 ± 0.7 | 0.6731 | 6.0 ± 0.7 | 6.0 ± 0.7 | 0.7887 |
| Energy (kcal) | 2151.4 ± 548.2 | 2019.9 ± 593.5 | 0.0286 | 1719.1 ± 484.7 | 1713.9 ± 513.4 | 0.9148 |
| Protein (% energy) | 15.4 ± 2.9 | 15.2 ± 3.0 | <0.0001 | 18.0 ± 2.6 | 13.1 ± 1.5 | <0.0001 |
| Fat (% energy) | 28.3 ± 4.7 | 22.4 ± 4.7 | <0.0001 | 30.3 ± 4.7 | 25.3 ± 5.4 | <0.0001 |
| Carbohydrate (% energy) | 48.5 ± 7.3 | 52.7 ± 10.1 | <0.0001 | 50.1 ± 6.3 | 58.5 ± 7.7 | <0.0001 |
| Total protein (g/day) | 89.5 ± 13.3 | 67.5 ± 9.5 | <0.0001 | 78.1 ± 11.6 | 57.5 ± 7.4 | <0.0001 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.5114 | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.6465 |
| Educational history (y) | 13.1 ± 2.8 | 12.4 ± 2.7 | 0.0100 | 11.8 ± 2.1 | 11.4 ± 2.2 | 0.0471 |
| Living alone (n) | 33 | 29 | 0.0909 † | 129 | 33 | 0.9860 † |
| Men (n = 382) | Women (n = 622) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Over DG (n = 240) | Under DG (n = 142) | p-Value | Over DG (n = 495) | Under DG (n= 127) | p-Value | Over DG (n = 735) | Under DG (n = 269) | p-Value | |
| Oral hypofunction (n) | 97 | 83 | 0.0006 † | 219 | 70 | 0.0284 † | 316 | 153 | <0.0001 † |
| Number of indicators of poor functioning in the oral hypofunction evaluation (n) | 2.3 ± 1.4 | 2.9 ± 1.4 | 0.0007 | 2.4 ± 1.5 | 2.8 ± 1.4 | 0.0054 | 2.4 ± 1.4 | 2.8 ± 1.4 | <0.0001 |
| Oral hygiene (%) | 51.7 ± 30.1 | 56.6 ± 31.6 | 0.1347 | 42.4 ± 30.4 | 41.3 ± 31.4 | 0.7295 | 45.4 ± 30.6 | 49.4 ± 32.4 | 0.0756 |
| Oral dryness | 27.1 ± 2.5 | 27.0 ± 2.7 | 0.7519 | 26.8 ± 2.4 | 26.7 ± 2.4 | 0.8187 | 26.9 ± 2.5 | 26.9 ± 2.5 | 0.9818 |
| Occlusal force (number) | 20.4 ± 9.5 | 18.5 ± 9.6 | 0.0550 | 19.8 ± 9.3 | 17.2 ± 10.1 | 0.0054 | 19.8 ± 9.3 | 17.2 ± 10.1 | 0.0015 |
| ODK (/pa/) (syllables/sec) | 6.0 ± 1.3 | 6.0 ± 0.9 | 0.9190 | 6.2 ± 0.9 | 6.0 ± 0.9 | 0.0014 | 6.1 ± 1.0 | 6.0 ± 0.9 | 0.0316 |
| ODK (/ta/) (syllables/sec) | 6.1 ± 1.1 | 6.0 ± 1.0 | 0.1798 | 6.2 ± 0.9 | 6.1 ± 0.9 | 0.1444 | 6.2 ± 1.0 | 6.0 ± 0.9 | 0.0193 |
| ODK (/ka/) (syllables/sec) | 5.5 ± 1.2 | 5.4 ± 1.1 | 0.3183 | 5.8 ± 0.9 | 5.7 ± 0.8 | 0.1101 | 5.7 ± 1.0 | 5.5 ± 1.0 | 0.0005 |
| Tongue pressure (kPa) | 36.9 ± 10.6 | 36.0 ± 10.7 | 0.4586 | 34.2 ± 9.5 | 34.4 ± 9.1 | 0.8632 | 35.1 ± 10.0 | 35.2 ± 10.0 | 0.8098 |
| Masticatory function (n) | 29 | 23 | 0.2572 † | 88 | 29 | 0.1933 † | 117 | 52 | 0.2006 † |
| Swallowing function | 1.1 ± 2.1 | 1.0 ± 2.2 | 0.9727 | 1.3 ± 2.3 | 1.5 ± 3.3 | 0.1283 | 1.1 ± 2.2 | 1.3 ± 2.8 | 0.3458 |
| Men | Women | Total | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | Crude OR | 95% CI | Adjusted OR a | 95% CI | |
| Oral hypofunction | 2.07 | 1.36–3.16 | 1.55 | 1.05–2.29 | 1.70 | 1.21–2.35 |
| Number of indicators of poor functioning in the oral hypofunction evaluation | 1.30 | 1.11–1.51 | 1.20 | 1.05–1.37 | 1.24 | 1.10–1.40 |
| Oral hygiene | 1.00 | 1.00–1.01 | 1.00 | 0.99–1.00 | 1.00 | 1.00–1.01 |
| Oral dryness | 0.99 | 0.91–1.07 | 0.99 | 0.91–1.07 | 0.99 | 0.93–1.05 |
| Occlusal force (number) | 0.98 | 0.96–1.01 | 0.97 | 0.95–1.00 | 0.98 | 0.96–1.00 |
| ODK (/pa/) | 1.18 | 0.87–1.61 | 0.65 | 0.47–0.90 | 0.95 | 0.75–1.19 |
| ODK (/ta/) | 0.80 | 0.53–1.20 | 1.25 | 0.81–1.92 | 0.94 | 0.69–1.27 |
| ODK (/ka/) | 1.02 | 0.75–1.39 | 1.00 | 0.67–1.50 | 1.00 | 0.78–1.30 |
| Tongue pressure | 1.00 | 0.97–1.02 | 1.01 | 0.99–1.04 | 1.00 | 0.99–1.02 |
| Masticatory function | 1.32 | 0.70–2.52 | 1.06 | 0.63–1.77 | 1.10 | 0.72–1.65 |
| Swallowing function | 0.98 | 0.98–1.09 | 1.04 | 0.97–1.14 | 1.03 | 0.96–1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, K.; Kanouchi, H.; Tanaka, A.; Nakamura, M.; Hamada, T.; Mishima, Y.; Goto, Y.; Kume, K.; Beppu, M.; Hijioka, H.; et al. Relationship between Oral Hypofunction, and Protein Intake: A Cross-Sectional Study in Local Community-Dwelling Adults. Nutrients 2021, 13, 4377. https://doi.org/10.3390/nu13124377
Nishi K, Kanouchi H, Tanaka A, Nakamura M, Hamada T, Mishima Y, Goto Y, Kume K, Beppu M, Hijioka H, et al. Relationship between Oral Hypofunction, and Protein Intake: A Cross-Sectional Study in Local Community-Dwelling Adults. Nutrients. 2021; 13(12):4377. https://doi.org/10.3390/nu13124377
Chicago/Turabian StyleNishi, Keitaro, Hiroaki Kanouchi, Akihiko Tanaka, Maya Nakamura, Tomofumi Hamada, Yumiko Mishima, Yuichi Goto, Kenichi Kume, Mahiro Beppu, Hiroshi Hijioka, and et al. 2021. "Relationship between Oral Hypofunction, and Protein Intake: A Cross-Sectional Study in Local Community-Dwelling Adults" Nutrients 13, no. 12: 4377. https://doi.org/10.3390/nu13124377
APA StyleNishi, K., Kanouchi, H., Tanaka, A., Nakamura, M., Hamada, T., Mishima, Y., Goto, Y., Kume, K., Beppu, M., Hijioka, H., Tabata, H., Mori, K., Uchino, Y., Yamashiro, K., Matsumura, Y., Higashi, Y., Makizako, H., Kubozono, T., Takenaka, T., ... Sugiura, T. (2021). Relationship between Oral Hypofunction, and Protein Intake: A Cross-Sectional Study in Local Community-Dwelling Adults. Nutrients, 13(12), 4377. https://doi.org/10.3390/nu13124377









