Text Messages to Curb Sugar-Sweetened Beverage Consumption among Pregnant Women and Mothers: A Mobile Health Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants and Setting
2.3. Interventions
- Graphic health warning labels (Ix 1)
- 2.
- Beverage sugar content imagery (Ix 2)
- 3.
- Attention control (AC)
2.4. Outcomes
2.5. Sample Size
2.6. Randomization
2.7. Statistical Analysis
2.7.1. Primary Analysis
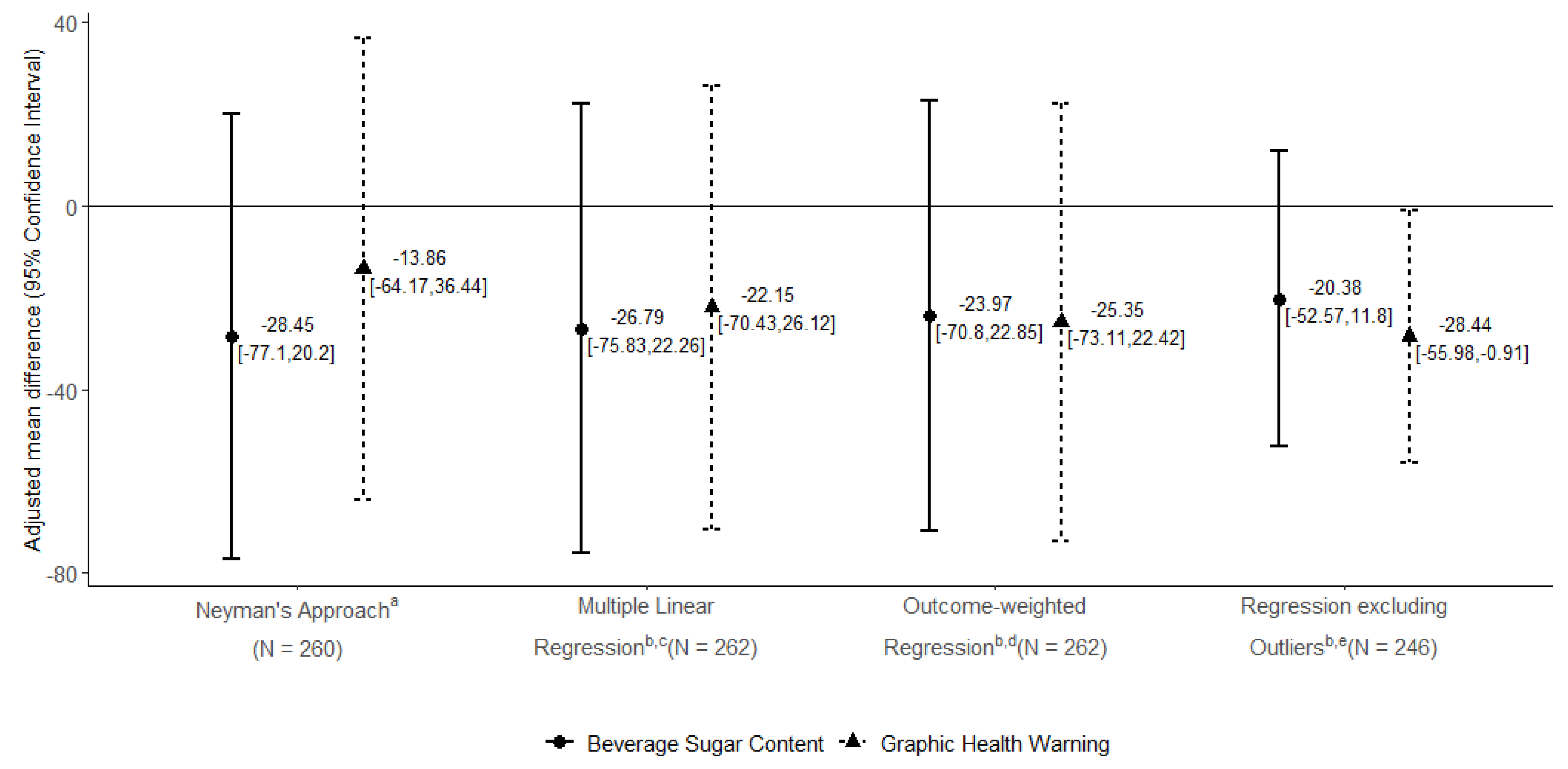
2.7.2. Sensitivity Analyses
2.7.3. Secondary Analyses
3. Results
3.1. Study Participants
3.2. Changes in Maternal SSB Consumption and Other Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.2.3. Process Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988-1994 Through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Gillman, M.W.; Rifas-Shiman, S.L.; Sherry, B.; Kleinman, K.; Taveras, E.M. Decreasing prevalence of obesity among young children in Massachusetts from 2004 to 2008. Pediatrics 2012, 129, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederick, C.B.; Snellman, K.; Putnam, R.D. Increasing socioeconomic disparities in adolescent obesity. Proc. Natl. Acad. Sci. USA 2014, 111, 1338–1342. [Google Scholar] [CrossRef] [Green Version]
- May, A.L.; Pan, L.; Sherry, B.; Blanck, H.M.; Galuska, D.; Dalenius, K.; Polhamus, B.; Kettel-Khan, L.; Grummer-Strawn, L.M. Vital Signs: Obesity Among Low-Income, Preschool-Aged Children—United States, 2008–2011. MMWR. Morb. Mortal. Wkly. Rep. 2013, 62, 629–634. [Google Scholar]
- Ogden, C.L.; Fryar, C.D.; Martin, C.B.; Freedman, D.S.; Carroll, M.D.; Gu, Q.; Hales, C.M. Trends in Obesity Prevalence by Race and Hispanic Origin—1999–2000 to 2017–2018. JAMA 2020, 324, 1208–1210. [Google Scholar] [CrossRef]
- Taveras, E.M.; Gillman, M.W.; Kleinman, K.; Rich-Edwards, J.W.; Rifas-Shiman, S.L. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics 2010, 125, 686–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennison, B.A.; Erb, T.A.; Jenkins, P.L. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics 2002, 109, 1028–1035. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bleich, S.N.; Gortmaker, S.L. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008, 121, e1604–e1614. [Google Scholar] [CrossRef]
- Piernas, C.; Popkin, B.M. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in US children and adolescents: Patterns and trends by age group and sociodemographic characteristics, 1977–2006. Am. J. Clin. Nutr. 2011, 94, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Whitt-Glover, M.C.; Taylor, W.C.; Floyd, M.F.; Yore, M.M.; Yancey, A.K.; Matthews, C.E. Disparities in physical activity and sedentary behaviors among US children and adolescents: Prevalence, correlates, and intervention implications. J. Public Health Policy 2009, 30 (Suppl. S1), S309–S334. [Google Scholar] [CrossRef]
- Nevarez, M.D.; Rifas-Shiman, S.L.; Kleinman, K.P.; Gillman, M.W.; Taveras, E.M. Associations of early life risk factors with infant sleep duration. Acad. Pediatr. 2010, 10, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Bleich, S.N.; Ard, J.D. COVID-19, Obesity, and Structural Racism: Understanding the Past and Identifying Solutions for the Future. Cell Metab. 2021, 33, 234–241. [Google Scholar] [CrossRef]
- Grier, S.A.; Kumanyika, S. Targeted marketing and public health. Annu. Rev. Public Health 2010, 31, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Glantz, S.A.; Palmer, C.N.; Schmidt, L.A. Transferring Racial/Ethnic Marketing Strategies From Tobacco to Food Corporations: Philip Morris and Kraft General Foods. Am. J. Public Health 2020, 110, 329–336. [Google Scholar] [CrossRef]
- Pan, L.; Li, R.; Park, S.; Galuska, D.A.; Sherry, B.; Freedman, D.S. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014, 134 (Suppl. S1), S29–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillman, M.W.; Rifas-Shiman, S.L.; Fernandez-Barres, S.; Kleinman, K.; Taveras, E.M.; Oken, E. Beverage Intake During Pregnancy and Childhood Adiposity. Pediatrics 2017, 140, e20170031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleich, S.N.; Vercammen, K.A. The negative impact of sugar-sweetened beverages on children’s health: An update of the literature. BMC Obes. 2018, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.F.W.; Rifas-Shiman, S.L.; Young, J.; Oken, E. Associations of Prenatal and Child Sugar Intake With Child Cognition. Am. J. Prev. Med. 2018, 54, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Lundeen, E.A.; Park, S.; Baidal, J.A.W.; Sharma, A.J.; Blanck, H.M. Sugar-Sweetened Beverage Intake Among Pregnant and Non-pregnant Women of Reproductive Age. Matern. Child Health J. 2020, 24, 709–717. [Google Scholar] [CrossRef]
- Duffy, E.W.; Kay, M.C.; Jacquier, E.; Catellier, D.; Hampton, J.; Anater, A.S.; Story, M. Trends in Food Consumption Patterns of US Infants and Toddlers from Feeding Infants and Toddlers Studies (FITS) in 2002, 2008, 2016. Nutrients 2019, 11, 2807. [Google Scholar] [CrossRef] [Green Version]
- Thompson, I.J.B.; Ritchie, L.D.; Bradshaw, P.T.; Mujahid, M.S.; Au, L.E. Earlier Introduction to Sugar-Sweetened Beverages Associated with Lower Diet Quality among WIC Children at Age 3 Years. J. Nutr. Educ. Behav. 2021, 53, 912–920. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Morel, K.; Nichols, K.; Elbel, E.; Charles, N.; Goldsmith, J.; Chen, L.; Taveras, E. Sugar-Sweetened Beverage Attitudes and Consumption During the First 1000 Days of Life. Am. J. Public Health 2018, 108, 1659–1665. [Google Scholar] [CrossRef]
- Sonneville, K.R.; Rifas-Shiman, S.L.; Kleinman, K.P.; Gortmaker, S.L.; Gillman, M.W.; Taveras, E.M. Associations of obesogenic behaviors in mothers and obese children participating in a randomized trial. Obesity 2012, 20, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Morel, K.; Nichols, K.; Nong, Y.; Charles, N.; Price, S.; Taveras, E.; Goldman, R.; Baidal, J.A.W. Parental and Provider Perceptions of Sugar-Sweetened Beverage Interventions in the First 1000 Days: A Qualitative Study. Acad. Pediatr. 2019, 19, 748–755. [Google Scholar] [CrossRef]
- Zickuhr, K.; Smith, A. Digital differences. In Pew Research Center’s Internet &American Life Project; Pew Research Center: Washington, DC, USA, 2012. [Google Scholar]
- Ajzen, I. The Theory of Planned Behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Glass, T.A.; McAtee, M.J. Behavioral science at the crossroads in public health: Extending horizons, envisioning the future. Soc. Sci. Med. 2006, 62, 1650–1671. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Blake-Lamb, T.L.; Locks, L.M.; Perkins, M.E.; Baidal, J.A.W.; Cheng, E.R.; Taveras, E.M. Interventions for Childhood Obesity in the First 1000 Days A Systematic Review. Am. J. Prev. Med. 2016, 50, 780–789. [Google Scholar] [CrossRef] [Green Version]
- California Senate Bill-347 Sugar-Sweetened Beverages: Safety Warnings. Available online: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB347 (accessed on 1 June 2019).
- American Academy of Pediatrics. Healthychildren.org-Safety & Prevention. 2020. Available online: https://www.healthychildren.org/english/safety-prevention/Pages/default.aspx (accessed on 10 July 2020).
- Story, M.; Fox, F.; Corbett, A. Recommendations for Healthier Beverages; Healthy Eating Research, Robert Wood Johnson Foundation, Duke Global Health Institute, Duke University: Durham, NC, USA, 2013. [Google Scholar]
- Hedrick, V.E.; Savla, J.; Comber, D.L.; Flack, K.D.; Estabrooks, P.A.; Nsiah-Kumi, P.A.; Ortmeier, S.; Davy, B.M. Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): Sugar-sweetened beverages and total beverage energy intake. J. Acad. Nutr. Diet. 2012, 112, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lora, K.R.; Davy, B.; Hedrick, V.; Ferris, A.M.; Anderson, M.P.; Wakefield, D. Assessing Initial Validity and Reliability of a Beverage Intake Questionnaire in Hispanic Preschool-Aged Children. J. Acad. Nutr. Diet. 2016, 116, 1951–1960. [Google Scholar] [CrossRef]
- Hedrick, V.E.; Comber, D.L.; Estabrooks, P.A.; Savla, J.; Davy, B.M. The beverage intake questionnaire: Determining initial validity and reliability. J. Am. Diet. Assoc. 2010, 110, 1227–1232. [Google Scholar] [CrossRef] [Green Version]
- Marshall, T.A.; Gilmore, J.M.E.; Broffitt, B.; Stumbo, P.J.; Levy, S.M. Relative validity of the Iowa Fluoride Study targeted nutrient semi-quantitative questionnaire and the block kids’ food questionnaire for estimating beverage, calcium, and vitamin D intakes by children. J. Am. Diet. Assoc. 2008, 108, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Fein, S.B.; Labiner-Wolfe, J.; Shealy, K.R.; Li, R.; Chen, J.; Grummer-Strawn, L.M. Infant Feeding Practices Study II: Study methods. Pediatrics 2008, 122 (Suppl. S2), S28–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, M.B.; Kaar, J.L.; Welsh, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Munos, J.C.; Krebs, N.F.; Xanthakos, S.A.; et al. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1017–e1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Escamilla, R.; Segura-Perez, S.; Lott, M.; on behalf of the RWJF HER Expert Panel. Best Practices for Promoting Healthy Nutrition, Feeding Patterns, and Weight Status for Infants and Toddlers from Birth to 24 Months. In Feeding Guidelines for Infants an Young Toddlers: A Responsive Parenting Approach; Healthy Eating Research, Robert Wood Johnson Foundation, Duke Global Health Institute, Duke University: Durham, NC, USA, 2017. [Google Scholar]
- Chernick, L.S.; Stockwell, M.S.; Gonzalez, A.; Mitchell, J.; Ehrhardt, A.; Bakken, S.; Westhoff, C.L.; Santelli, J.; Dayan, P.S. A User-Informed, Theory-Based Pregnancy Prevention Intervention for Adolescents in the Emergency Department: A Prospective Cohort Study. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2021, 68, 705–712. [Google Scholar] [CrossRef]
- Roberto, C.A.; Wong, D.; Musicus, A.; Hammond, D. The Influence of Sugar-Sweetened Beverage Health Warning Labels on Parents’ Choices. Pediatrics 2016, 137, e20153185. [Google Scholar] [CrossRef] [Green Version]
- Grummon, A.H.; Hall, M.G.; Taillie, L.S.; Brewer, N.T. How should sugar-sweetened beverage health warnings be designed? A randomized experiment. Prev. Med. 2019, 121, 158–166. [Google Scholar] [CrossRef]
- Grummon, A.H.; Hall, M.G. Sugary drink warnings: A meta-analysis of experimental studies. PLoS Med. 2020, 17, e1003120. [Google Scholar] [CrossRef]
- Donnelly, G.E.; Zatz, L.Y.; Svirsky, D.; John, L.K. The Effect of Graphic Warnings on Sugary-Drink Purchasing. Psychol. Sci. 2018, 29, 1321–1333. [Google Scholar] [CrossRef]
- Hall, M.G.; Lazard, A.J.; Grummon, A.H.; Higgins, I.C.A.; Bercholz, M.; Richter, A.P.C.; Taillie, L.S. Designing warnings for sugary drinks: A randomized experiment with Latino parents and non-Latino parents. Prev. Med. 2021, 148, 106562. [Google Scholar] [CrossRef]
- An, R.; Liu, J.; Liu, R.; Barker, A.R.; Figueroa, R.B.; McBride, T.D. Impact of Sugar-Sweetened Beverage Warning Labels on Consumer Behaviors: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2021, 60, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Wolfson, J.A.; Hsu, R.; Soster, K.; Mangan, S.; Falbe, J. Warning Labels Reduce Sugar-Sweetened Beverage Intake among College Students. J. Nutr. 2021, 151, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Baltimore City Council. Sugar-Sweetened Beverages-Warning Labels. 2016. Available online: https://baltimore.legistar.com/LegislationDetail.aspx?ID=2547410&GUID=BF49C0ED-0647-4625-B7AE-C2592FCAFD7C&Options=ID%7CText%7C&Search=ssb (accessed on 21 July 2021).
- Falbe, J.; Montuclard, A.; Engelman, A.; Adler, S.; Roesler, A. Developing sugar-sweetened beverage warning labels for young adults. Public Health Nutr. 2021, 24, 4765–4775. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. The New Nutrition Facts Label. Content Current as of 6/29/2020. Available online: https://www.fda.gov/food/nutrition-education-resources-materials/new-nutrition-facts-label (accessed on 1 July 2021).
- Taillie, L.S.; Reyes, M.; Colchero, M.A.; Popkin, B.; Corvalán, C. An evaluation of Chile’s Law of Food Labeling and Advertising on sugar-sweetened beverage purchases from 2015 to 2017: A before-and-after study. PLoS Med. 2020, 17, e1003015. [Google Scholar] [CrossRef]
- Zoellner, J.M.; You, W.; Estabrooks, P.A.; Chen, Y.; Davy, B.M.; Porter, K.J.; Hedrick, V.E.; Bailey, A.; Kružliaková, N. Supporting maintenance of sugar-sweetened beverage reduction using automated versus live telephone support: Findings from a randomized control trial. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ix 1: Graphic Health Warnings | Ix 2: Sugar Content Messages | AC: Attention Control | |
|---|---|---|---|
| Personalized Text Message | Hey [NAME], did you know that drinking sugary drinks can lead to health problems for you and your baby? | [NAME], What drink has as much sugar as four donuts? Click to see! | Hey [NAME], do you know that babies should always sleep on their backs? Click to learn more! |
| Linked Health Message |  |  |  |
| Overall | Ix 1: Graphic Health Warning | Ix 2: Beverage Sugar Content | AC: Attention Control | |
|---|---|---|---|---|
| N | 290 | 98 | 98 | 94 |
| Maternal/Household Baseline Characteristics | ||||
| Maternal age, mean (SD), years | 30.53 (6.30) | 31.06 (6.30) | 30.17 (6.11) | 30.35 (6.53) |
| Pregnant participant, n (%) | 28 (9.7) | 11 (11.2) | 10 (10.2) | 7 (7.4) |
| Spanish language preference, n (%) | 192 (66.2) | 65 (66.3) | 64 (65.3) | 63 (67.0) |
| Annual household income, n (%) | ||||
| <USD 20,000/y | 123 (42.4) | 40 (40.8) | 48 (49.0) | 35 (37.2) |
| >USD 20,000/y | 90 (31.0) | 35 (35.7) | 21 (21.4) | 34 (36.2) |
| Do not Know | 77 (26.6) | 23 (23.5) | 29 (29.6) | 25 (26.6) |
| Maternal Race/ethnicity, n (%) | ||||
| Hispanic/Latina | 265 (91.4) | 89 (90.8) | 88 (89.8) | 88 (93.6) |
| White, non-Hispanic | 2 (0.7) | 0 (0.0) | 1 (1.0) | 1 (1.1) |
| Black, non-Hispanic | 21 (7.2) | 8 (8.2) | 8 (8.2) | 5 (5.3) |
| Current WIC enrollment, n (%) | 272 (93.8) | 90 (91.8) | 95 (96.9) | 87 (92.6) |
| N, Subset with an Infant | 262 | 87 | 88 | 87 |
| Infant Characteristic | ||||
| Female, n (%) | 139 (53.1) | 44 (50.6) | 45 (51.1) | 50 (57.5) |
| Age at baseline, mean (SD), years | 0.67 (0.51) | 0.70 (0.52) | 0.64 (0.56) | 0.66 (0.46) |
| Main Outcome: Maternal Habitual Daily SSB Consumption (kcal) | ||||
|---|---|---|---|---|
| Baseline | 1 Month | 1-Month Change | Within-Group p-Value a | |
| Intervention Condition | Mean (SD) | Mean (SD) | Mean Change (SD) | |
| Ix 1: Graphic Health Warning | 163.92 (194.27) | 98.43 (127.90) | −65.50 (184.79) | <0.0001 |
| Ix 2: Beverage Sugar Content | 158.93 (185.47) | 79.24 (107.11) | −79.69 (171.31) | <0.0001 |
| AC: Attention Control | 135.83 (192.26) | 90.03 (157.39) | −45.81 (135.12) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo Baidal, J.A.; Nichols, K.; Charles, N.; Chernick, L.; Duong, N.; Finkel, M.A.; Falbe, J.; Valeri, L. Text Messages to Curb Sugar-Sweetened Beverage Consumption among Pregnant Women and Mothers: A Mobile Health Randomized Controlled Trial. Nutrients 2021, 13, 4367. https://doi.org/10.3390/nu13124367
Woo Baidal JA, Nichols K, Charles N, Chernick L, Duong N, Finkel MA, Falbe J, Valeri L. Text Messages to Curb Sugar-Sweetened Beverage Consumption among Pregnant Women and Mothers: A Mobile Health Randomized Controlled Trial. Nutrients. 2021; 13(12):4367. https://doi.org/10.3390/nu13124367
Chicago/Turabian StyleWoo Baidal, Jennifer A., Kelsey Nichols, Nalini Charles, Lauren Chernick, Ngoc Duong, Morgan A. Finkel, Jennifer Falbe, and Linda Valeri. 2021. "Text Messages to Curb Sugar-Sweetened Beverage Consumption among Pregnant Women and Mothers: A Mobile Health Randomized Controlled Trial" Nutrients 13, no. 12: 4367. https://doi.org/10.3390/nu13124367
APA StyleWoo Baidal, J. A., Nichols, K., Charles, N., Chernick, L., Duong, N., Finkel, M. A., Falbe, J., & Valeri, L. (2021). Text Messages to Curb Sugar-Sweetened Beverage Consumption among Pregnant Women and Mothers: A Mobile Health Randomized Controlled Trial. Nutrients, 13(12), 4367. https://doi.org/10.3390/nu13124367






