The Association between Plasma Concentration of Phytoestrogens and Hypertension within the Korean Multicenter Cancer Cohort
Abstract
1. Introduction
2. Materials and Methods
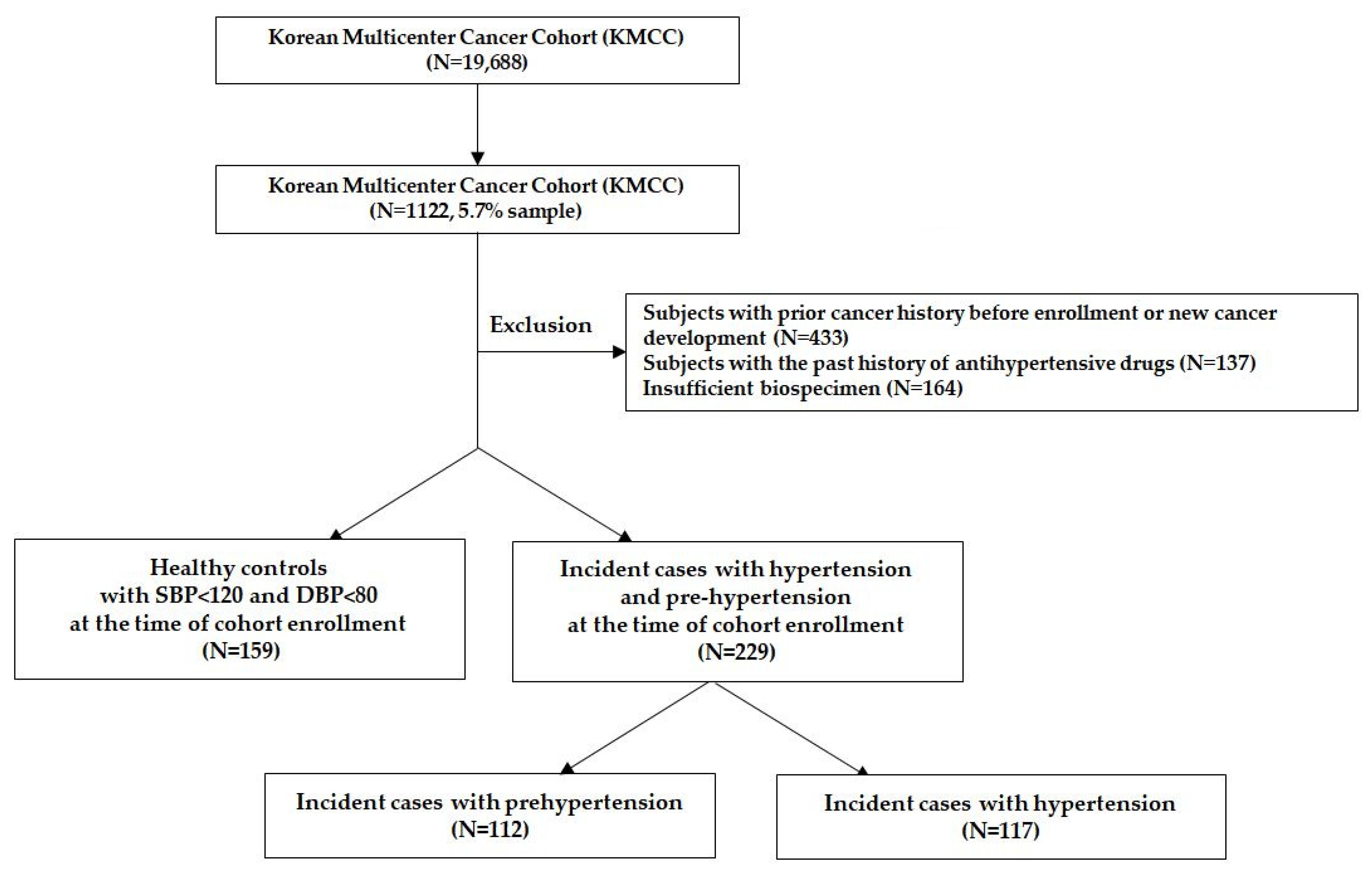
2.1. Study Population
2.2. Assessment of Hypertension
2.3. Measurement of Plasma Concentration of Phytoestrogens
2.4. Statistical Analyses
3. Results
3.1. General Characteristics
3.2. Association between Plasma Phytoestrogen Levels and High BP including Both Prehypertension and Hypertension
3.3. Association with Plasma Phytoestrogen Levels for Hypertension and Prehypertension, Respectively
3.4. Association between Plasma Phytoestrogen Levels and Hypertension According to Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Kang, S.; Kim, H.C. Effects of Income Level on the Association between Hypertension and Depression: 2010–2017 Korea National Health and Nutrition Examination Survey. J. Prev. Med. Public Health 2020, 53, 439. [Google Scholar] [CrossRef]
- Kim, H.C.; Cho, S.M.J.; Lee, H.; Lee, H.-H.; Baek, J.; Heo, J.E. Korea hypertension fact sheet 2020: Analysis of nationwide population-based data. Clin. Hypertens. 2021, 27, 1–4. [Google Scholar] [CrossRef]
- Lee, J.-S.; Park, J.; Kim, J. Dietary factors related to hypertension risk in Korean adults-data from the Korean national health and nutrition examination survey III. Nutr. Res. Pract. 2011, 5, 60–65. [Google Scholar] [CrossRef][Green Version]
- Delia Mirela, Ţ.; Pallag, A.; Iovan, C.; Furău, G.; Furău, C.; Bungău, S. Somatic-vegetative symptoms evolution in postmenopausal women treated with phytoestrogens and hormone replacement therapy. Iran. J. Public Health 2017, 46, 1528. [Google Scholar]
- Bumbu, A.; Pasca, B.; Tit, D.M.; Bungau, S.; Bumbu, G. The effects of soy isoflavones and hormonal replacing therapy on the incidence and evolution of postmenopausal female urinary incontinence. Farmacia 2016, 64, 419–422. [Google Scholar]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Otani, T.; Inoue, M.; Tsugane, S. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol. Prev. Biomark. 2007, 16, 538–545. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, M.; Otani, T.; Sasazuki, S.; Kurahashi, N.; Miura, T.; Yamamoto, S.; Tsugane, S. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: A nested case-control study from the Japan Public Health Center-based prospective study group. J. Clin. Oncol. 2008, 26, 1677–1683. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002, 21, 265–280. [Google Scholar] [CrossRef]
- Verdrengh, M.; Jonsson, I.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef]
- Cornwell, T.; Cohick, W.; Raskin, I. Dietary phytoestrogens and health. Phytochemistry 2004, 65, 995–1016. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Ko, K.-P.; Yeo, Y.; Yoon, J.-H.; Kim, C.-S.; Tokudome, S.; Koriyama, C.; Lim, Y.-K.; Chang, S.-H.; Shin, H.-R.; Kang, D. Plasma phytoestrogens concentration and risk of colorectal cancer in two different Asian populations. Clin. Nutr. 2018, 37, 1675–1682. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Yang, J.J.; Cho, L.Y.; Ko, K.-P.; Shin, A.; Ma, S.H.; Choi, B.Y.; Han, D.S.; Song, K.S.; Kim, Y.S.; Lee, J.-Y. Genetic susceptibility on CagA-interacting molecules and gene-environment interaction with phytoestrogens: A putative risk factor for gastric cancer. PLoS ONE 2012, 7, e31020. [Google Scholar]
- Ko, K.-P.; Park, S.K.; Park, B.; Yang, J.J.; Cho, L.Y.; Kang, C.; Kim, C.S.; Gwack, J.; Shin, A.; Kim, Y. Isoflavones from phytoestrogens and gastric cancer risk: A nested case-control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol. Prev. Biomark. 2010, 19, 1292–1300. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Behloul, N.; Wu, G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013, 698, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hemati, N.; Asis, M.; Moradi, S.; Mollica, A.; Stefanucci, A.; Nikfar, S.; Mohammadi, E.; Farzaei, M.H.; Abdollahi, M. Effects of genistein on blood pressure: A systematic review and meta-analysis. Food Res. Int. 2020, 128, 108764. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes/Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, M.; Sharma, P.L. Ameliorative effect of daidzein: A caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J Exp Biol. 2012, 50, 28–34. [Google Scholar] [PubMed]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Is equol the key to the efficacy of soy foods? Am. J. Clin. Nutr. 2009, 89, 1664S–1667S. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Lee, M.-J.; Sohn, C.-Y.; Park, O.-J. Relation between health status and intake of soy isoflavone among adult women in Seoul. J. East Asian Soc. Diet. Life 2010, 20, 218–230. [Google Scholar]
- Choi, M.-K.; Kim, M.-H.; Sung, C.-J.; Lee, W.-Y.; Park, J.-D. A study on relation among habitual isoflavone intake, blood pressure, and serum lipid parameters in Korean men and women over 20 years old. Korean J. Community Nutr. 2005, 10, 493–500. [Google Scholar]
- Keogh, R.H.; White, I.R.; Rodwell, S.A. Using surrogate biomarkers to improve measurement error models in nutritional epidemiology. Stat. Med. 2013, 32, 3838–3861. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Bergante, S.; Satriano, A.; Pluchinotta, F.R.; Marranzano, M. Dietary phytoestrogen intake is inversely associated with hypertension in a cohort of adults living in the Mediterranean area. Molecules 2018, 23, 368. [Google Scholar] [CrossRef]
- Yoo, K.-Y.; Shin, H.-R.; Chang, S.-H.; Lee, K.-S.; Park, S.; Kang, D.; Lee, D. Korean multi-center cancer cohort study including a biological materials bank (KMCC-I). Asian Pac. J. Cancer Prev. 2002, 3, 85–92. [Google Scholar]
- Wang, G.J.; Lapcík, O.; Hampl, R.; Uehara, M.; Al-Maharik, N.; Stumpf, K.; Mikola, H.; Wähälä, K.; Adlercreutz, H. Time-resolved fluoroimmunoassay of plasma daidzein and genistein. Steroids 2000, 65, 339–348. [Google Scholar] [CrossRef]
- Brouwers, E.; L’homme, R.; Al-Maharik, N.; Lapcík, O.; Hampl, R.; Wähälä, K.; Mikola, H.; Adlercreutz, H. Time-resolved fluoroimmunoassay for equol in plasma and urine. J. Steroid Biochem. Mol. Biol. 2003, 84, 577–587. [Google Scholar] [CrossRef]
- Richardson, S.I.; Steffen, L.M.; Swett, K.; Smith, C.; Burke, L.; Zhou, X.; Shikany, J.M.; Rodriguez, C.J. Dietary total isoflavone intake is associated with lower systolic blood pressure: The Coronary Artery Risk Development in Young Adults (CARDIA) study. J. Clin. Hypertens. 2016, 18, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Jia-Liu, W.; Xin-Yan, W.; Fang-Chao, L.; Ji-Chun, C.; Jie, C.; Jian-Xin, L.; Dong-Sheng, H.; Chong, S.; Fang-Hong, L.; Ying-Xin, Z. Associations of soybean products intake with blood pressure changes and hypertension incidence: The China-PAR project. J. Geriatr. Cardiol. JGC 2020, 17, 384. [Google Scholar]
- Taku, K.; Lin, N.; Cai, D.; Hu, J.; Zhao, X.; Zhang, Y.; Wang, P.; Melby, M.K.; Hooper, L.; Kurzer, M.S. Effects of soy isoflavone extract supplements on blood pressure in adult humans: Systematic review and meta-analysis of randomized placebo-controlled trials. J. Hypertens. 2010, 28, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Woo, J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: A 6-month randomized controlled trial among postmenopausal women. J. Hypertens. 2013, 31, 384–392. [Google Scholar] [CrossRef]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Kraszewska, O.; Nynca, A.; Kaminska, B.; Ciereszko, R. Fitoestrogeny. 1. Wystepowanie, metabolizm i znaczenie biologiczne u samic. Postępy Biol. Komórki 2007, 1, 189–205. [Google Scholar]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [CrossRef]
- Setchell, K.; Borriello, S.; Hulme, P.; Kirk, D.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar] [CrossRef]
- Acharjee, S.; Zhou, J.-R.; Elajami, T.K.; Welty, F.K. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism 2015, 64, 236–243. [Google Scholar] [CrossRef]
- Liu, Z.; Ho, S.; Chen, Y.; Tomlinson, B.; Ho, S.; To, K.; Woo, J. Effect of whole soy and purified daidzein on ambulatory blood pressure and endothelial function—A 6-month double-blind, randomized controlled trial among Chinese postmenopausal women with prehypertension. Eur. J. Clin. Nutr. 2015, 69, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Xie, Y.J.; Huang, Z.-G.; Ling, W.-H. Research protocol: Effect of natural S-equol on blood pressure and vascular function-a six-month randomized controlled trial among equol non-producers of postmenopausal women with prehypertension or untreated stage 1 hypertension. BMC Complement. Altern. Med. 2016, 16, 89. [Google Scholar] [CrossRef]
- Xu, J.-W.; Ikeda, K.; Yamori, Y. Genistein inhibits expressions of NADPH oxidase p22phox and angiotensin II type 1 receptor in aortic endothelial cells from stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2004, 27, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Pessa, L.R.; Tanus-Santos, J.E. Isoflavone genistein inhibits the angiotensin-converting enzyme and alters the vascular responses to angiotensin I and bradykinin. Eur. J. Pharmacol. 2009, 607, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Pesce, N.; Eyster, K.M.; Williams, J.L.; Wixon, R.; Wang, C.; Martin, D.S. Effect of genistein on cardiovascular responses to angiotensin II in conscious unrestrained rats. J. Cardiovasc. Pharmacol. 2000, 36, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Pai, K.V. Angiotensin Converting Enzyme Inhibition Activity of Daidzein. J. Drug Deliv. Ther. 2014, 4, 92–98. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Yang, C.; Li, S.-N. Effects of genistein on angiotensin-converting enzyme in rats. Life Sci. 2006, 79, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, G.; Burguete, M.a.C.; Pérez-Asensio, F.J.; Salom, J.B.; Gil, J.V.; Alborch, E. Pharmacological profile of phytoestrogens in cerebral vessels: In vitro study with rabbit basilar artery. Eur. J. Pharmacol. 2003, 482, 227–234. [Google Scholar] [CrossRef]
- Wang, H.-p.; Mei, R.-h.; Li, X.-y.; Zhao, M.-h.; Lu, Y.; Xia, Q.; Bruce, I. Endothelium-independent vasorelaxant effect of the phyto-oestrogen biochanin A on rat thoracic aorta. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005; pp. 2244–2247. [Google Scholar]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors α and β. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Joy, S.; Siow, R.C.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cell. J. Biol. Chem. 2006, 281, 27335–27345. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, M.Y.; Park, H.M. The effect of eqoul, a metabolite of isoflavone, on endothelial cell-independent vasodilatation of human uterine artery in vitro. J. Bone Metab. 2015, 22, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Chen, J.; Sun, K.; Wang, X.; Wang, X.; Hui, R. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Sonta, T.; Inoguchi, T.; Tsubouchi, H.; Sekiguchi, N.; Kobayashi, K.; Matsumoto, S.; Utsumi, H.; Nawata, H. Evidence for contribution of vascular NAD (P) H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic. Biol. Med. 2004, 37, 115–123. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Barton, M.; Carmona, R.; Ortmann, J.; Krieger, J.E.; Traupe, T. Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. Int. J. Biochem. Cell Biol. 2003, 35, 826–837. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.; Emeis, J.; Coppack, S. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Tounian, P.; Aggoun, Y.; Dubern, B.; Varille, V.; Guy-Grand, B.; Sidi, D.; Girardet, J.-P.; Bonnet, D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: A prospective study. The Lancet 2001, 358, 1400–1404. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Davies, M.J.; Schriver, S.D.; Lauterio, T.J.; Prewitt, R.L. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 2001, 37, 554–560. [Google Scholar] [CrossRef]
- Asmar, R.; Beebe-Dimmer, J.; Korgavkar, K.; Keele, G.; Cooney, K. Hypertension, obesity and prostate cancer biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 2013, 16, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, S.; Tenaglia, R.L. Metabolic syndrome and aggressive prostate cancer at initial diagnosis. Horm. Metab. Res. 2017, 49, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.; Mukherjee, S.; Ray, G.; Ray, A. Dietary factors and cancer chemoprevention: An overview of obesity-related malignancies. J. Postgrad. Med. 2009, 55, 45. [Google Scholar]
- Kreijkamp-Kaspers, S.; Kok, L.; Bots, M.L.; Grobbee, D.E.; van der Schouw, Y.T. Dietary phytoestrogens and vascular function in postmenopausal women: A cross-sectional study. J. Hypertens. 2004, 22, 1381–1388. [Google Scholar] [CrossRef]
- Cornish, S.M.; Chilibeck, P.D.; Paus-Jennsen, L.; Biem, H.J.; Khozani, T.; Senanayake, V.; Vatanparast, H.; Little, J.P.; Whiting, S.J.; Pahwa, P. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl. Physiol. Nutr. Metab. 2009, 34, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. Cardiometabolic risk factors are associated with high urinary enterolactone concentration, independent of urinary enterodiol concentration and dietary fiber intake in adults. J. Nutr. 2014, 144, 1445–1453. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A. Dietary lignans: Definition, description and research trends in databases development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.N.; Nicolle, C.; Mila, I.; Lapierre, C.; Nagano, K.; Fukushima, K.; Heinonen, S.-M.; Adlercreutz, H.; Rémésy, C.; Scalbert, A. Dietary lignins are precursors of mammalian lignans in rats. J. Nutr. 2004, 134, 120–127. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A.; Wen, W.; Xiang, Y.-B.; Barnes, S.; Liu, D.; Cai, Q.; Zheng, W.; Shu, X.O. Assessment of dietary isoflavone intake among middle-aged Chinese men. J. Nutr. 2007, 137, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Valentin-Blasini, L.; Blount, B.C.; Caudill, S.P.; Needham, L.L. Urinary and serum concentrations of seven phytoestrogens in a human reference population subset. J. Expo. Sci. Environ. Epidemiol. 2003, 13, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.B.; Taylor, J.I.; Low, Y.-L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.-T.; Wareham, N.J. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol. Prev. Biomark. 2004, 13, 698–708. [Google Scholar]
- Wiseman, H.; Casey, K.; Bowey, E.A.; Duffy, R.; Davies, M.; Rowland, I.R.; Lloyd, A.S.; Murray, A.; Thompson, R.; Clarke, D.B. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am. J. Clin. Nutr. 2004, 80, 692–699. [Google Scholar] [CrossRef]
- Heald, C.; Ritchie, M.; Bolton-Smith, C.; Morton, M.; Alexander, F. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br. J. Nutr. 2007, 98, 388–396. [Google Scholar] [CrossRef]
| Healthy Controls (n = 159) | Hypertension 1 including Prehypertension (n = 229) | p-Value | |
|---|---|---|---|
| Age(years), mean (SD) | 62.5 (7.5) | 63.9 (8.9) | 0.09 |
| Sex, n (%) | 0.40 | ||
| Men | 104 (65.4) | 159 (69.4) | |
| Women | 55 (34.6) | 70 (30.6) | |
| Enrollment year, mean (SD) | 1996.3 (1.8) | 1996.0 (2.4) | 0.15 |
| Educated years, n (%) | 0.63 | ||
| ≤6 (≤Elementary school) | 45 (28.3) | 67 (29.3) | |
| 7–12 (Middle–High school) | 111 (69.8) | 155 (67.7) | |
| ≥13 (≥University) | 2 (1.3) | 6 (2.6) | |
| Diabetes history, n (%) | 0.54 | ||
| Without diabetes | 150 (95.0) | 208 (94.1) | |
| Diabetes | 7 (4.4) | 13 (5.9) | |
| Smoking history, n (%) | 0.26 | ||
| Non-smokers | 66 (41.5) | 82 (35.8) | |
| Cigarette smokers | 93 (58.5) | 147 (64.2) | |
| Alcohol drinking history, n (%) | <0.01 | ||
| Non-drinkers | 79 (49.7) | 80 (34.9) | |
| Alcohol drinkers | 80 (50.3) | 149 (65.1) | |
| Obesity, n (%) | <0.01 | ||
| BMI < 25 kg/m2 | 138 (86.8) | 173 (75.6) | |
| BMI ≥ 25 kg/m2 | 21 (13.2) | 56 (24.4) |
| Phytoestrogens (nmol/L) | Healthy Controls (n = 159) | Hypertension 1 including Prehypertension (n = 229) | |
|---|---|---|---|
| n (%) | n (%) | OR (95% CI) 2 | |
| Isoflavones | |||
| Genistein | |||
| 1T (<101) | 53 (33.3) | 64 (28.0) | 1.00 |
| 2T (101–296.4) | 36 (22.6) | 86 (37.5) | 1.49 (0.83–2.66) |
| 3T (≥296.5) | 70 (44.0) | 79 (34.5) | 0.80 (0.47–1.37) |
| p-trend | 0.36 | ||
| Daidzein | |||
| 1T (<57.5) | 45 (28.3) | 73 (31.9) | 1.00 |
| 2T (57.5–208.9) | 56 (35.2) | 83 (36.2) | 0.74 (0.43–1.30) |
| 3T (≥209) | 58 (36.5) | 73 (31.9) | 0.68 (0.39–1.19) |
| p-trend | 0.11 | ||
| Equol | |||
| 1T (<20.5) | 36 (22.6) | 81 (35.4) | 1.00 |
| 2T (20.5–58.9) | 53 (33.3) | 76 (33.2) | 0.54 (0.32–0.92) |
| 3T (≥59) | 70 (44.0) | 72 (31.4) | 0.34 (0.20–0.57) |
| p-trend | <0.01 | ||
| Lignans | |||
| Enterolactone | |||
| 1T (<31.6) | 37 (23.3) | 88 (38.4) | 1.00 |
| 2T (31.6–76.35) | 49 (30.8) | 78 (34.1) | 0.66 (0.37–1.17) |
| 3T (≥76.4) | 73 (45.9) | 63 (27.5) | 0.32 (0.18–0.57) |
| p-trend | <0.01 |
| Phytoestrogens (nmol/L) | Healthy Controls (n = 159) | Prehypertension Cases 1 (n = 112) | Hypertension Cases 2 (n = 117) | |||
|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) 3 | n (%) | OR (95% CI) 3 | p-Ordinal | |
| Isoflavones | ||||||
| Genistein | ||||||
| 1T (<101) | 53 (33.3) | 31 (27.7) | 1.00 | 33 (28.2) | 1.00 | 0.67 |
| 2T (101–296.4) | 36 (22.6) | 51 (45.5) | 1.91 (0.98–3.73) | 35 (29.9) | 1.08 (0.54–2.16) | |
| 3T (≥296.5) | 70 (44.0) | 30 (26.8) | 0.68 (0.35–1.33) | 49 (41.9) | 0.89 (0.48–1.65) | |
| p-trend | 0.70 | 0.23 | ||||
| Daidzein | ||||||
| 1T (<57.5) | 45 (28.3) | 36 (32.1) | 1.00 | 37 (31.6) | 1.00 | 0.69 |
| 2T (57.5–208.9) | 56 (35.2) | 42 (37.5) | 0.75 (0.39–1.45) | 49 (41.9) | 0.87 (0.46–1.65) | |
| 3T (≥209) | 58 (36.5) | 34 (30.4) | 0.60 (0.31–1.18) | 31 (26.5) | 0.57 (0.29–1.13) | |
| p-trend | 0.76 | 0.12 | ||||
| Equol | ||||||
| 1T (<20.5) | 36 (22.6) | 40 (35.7) | 1.00 | 41 (35.0) | 1.00 | <0.01 |
| 2T (20.5–58.9) | 53 (33.3) | 36 (32.1) | 0.65 (0.33–1.27) | 40 (34.2) | 0.62 (0.32–1.19) | |
| 3T (≥59) | 70 (44.0) | 36 (32.1) | 0.50 (0.26–0.96) | 36 (30.8) | 0.42 (0.22–0.81) | |
| p-trend | 0.01 | 0.04 | ||||
| Lignans | ||||||
| Enterolactone | ||||||
| 1T (<31.6) | 37 (23.3) | 44 (39.3) | 1.00 | 44 (37.6) | 1.00 | <0.01 |
| 2T (31.6–76.35) | 49 (30.8) | 37 (33.0) | 0.66 (0.34–1.30) | 41 (35.0) | 0.66 (0.35–1.29) | |
| 3T (≥76.4) | 73 (45.9) | 31 (27.7) | 0.38 (0.19–0.75) | 32 (27.4) | 0.28 (0.14–0.54) | |
| p-trend | <0.01 | <0.01 |
| Non-Obese (BMI < 25 kg/m2) | Obese (BMI ≥ 25 kg/m2) | |||||
|---|---|---|---|---|---|---|
| Phytoestrogens (nmol/L) | Healthy Controls (n = 138) | Hypertension 1 Including Prehypertension (n = 173) | Healthy Controls (n = 21) | Hypertension 1 Including Prehypertension (n = 56) | ||
| n (%) | n (%) | OR 2 (95% CI) | n (%) | n (%) | OR 2 (95% CI) | |
| Isoflavones | ||||||
| Genistein | ||||||
| 1T (<101) | 49 (35.5) | 48 (27.7) | 1.00 | 4 (19.1) | 16 (28.6) | 1.00 |
| 2T (101–296.4) | 27 (19.6) | 64 (37.0) | 1.69 (0.87–3.27) | 9 (42.9) | 22 (39.3) | 0.49 (0.11–2.18) |
| 3T (≥296.5) | 62 (44.9) | 61 (35.3) | 0.86 (0.47–1.55) | 8 (38.1) | 18 (32.1) | 0.51 (0.12–2.22) |
| p-trend | 0.55 | 0.41 | ||||
| Daidzein | ||||||
| 1T (<57.5) | 39 (28.3) | 56 (32.4) | 1.00 | 6 (28.6) | 17 (30.4) | 1.00 |
| 2T (57.5–208.9) | 49 (35.5) | 50 (28.9) | 0.49 (0.25–0.94) | 7 (33.3) | 23 (41.1) | 1.24 (0.31–4.95) |
| 3T (≥209) | 50 (36.2) | 67 (38.7) | 0.71 (0.38–1.34) | 8 (38.1) | 16 (28.6) | 0.72 (0.19–2.70) |
| p-trend | 0.33 | 0.57 | ||||
| Equol | ||||||
| 1T (<20.5) | 34 (24.6) | 60 (34.7) | 1.00 | 2 (9.5) | 21 (37.5) | 1.00 |
| 2T (20.5–58.9) | 44 (31.9) | 50 (28.9) | 0.63 (0.33–1.21) | 9 (42.9) | 26 (46.4) | 0.30 (0.06–1.67) |
| 3T (≥59) | 60 (43.5) | 63 (36.4) | 0.63 (0.34–1.12) 3 | 10 (47.6) | 9 (16.1) | 0.06 (0.01–0.35) 3 |
| p-trend | 0.14 | <0.01 | ||||
| Lignans | ||||||
| Enterolactone | ||||||
| 1T (<31.6) | 34 (24.6) | 59 (34.1) | 1.00 | 3 (14.3) | 29 (51.8) | 1.00 |
| 2T (31.6–76.35) | 42 (30.4) | 60 (34.7) | 0.80 (0.42–1.52) | 7 (33.3) | 18 (32.1) | 0.27 (0.06–1.29) |
| 3T (≥76.4) | 62 (44.9) | 54 (31.2) | 0.46 (0.24–0.88) 3 | 11 (52.4) | 9 (16.1) | 0.07 (0.13–0.34) 3 |
| p-trend | 0.01 | <0.01 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kang, J.-Y.; Ko, K.-P.; Park, S.-K. The Association between Plasma Concentration of Phytoestrogens and Hypertension within the Korean Multicenter Cancer Cohort. Nutrients 2021, 13, 4366. https://doi.org/10.3390/nu13124366
Lee J, Kang J-Y, Ko K-P, Park S-K. The Association between Plasma Concentration of Phytoestrogens and Hypertension within the Korean Multicenter Cancer Cohort. Nutrients. 2021; 13(12):4366. https://doi.org/10.3390/nu13124366
Chicago/Turabian StyleLee, Juyeon, Ju-Young Kang, Kwang-Pil Ko, and Sue-Kyung Park. 2021. "The Association between Plasma Concentration of Phytoestrogens and Hypertension within the Korean Multicenter Cancer Cohort" Nutrients 13, no. 12: 4366. https://doi.org/10.3390/nu13124366
APA StyleLee, J., Kang, J.-Y., Ko, K.-P., & Park, S.-K. (2021). The Association between Plasma Concentration of Phytoestrogens and Hypertension within the Korean Multicenter Cancer Cohort. Nutrients, 13(12), 4366. https://doi.org/10.3390/nu13124366







