Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis and Outcome Measures
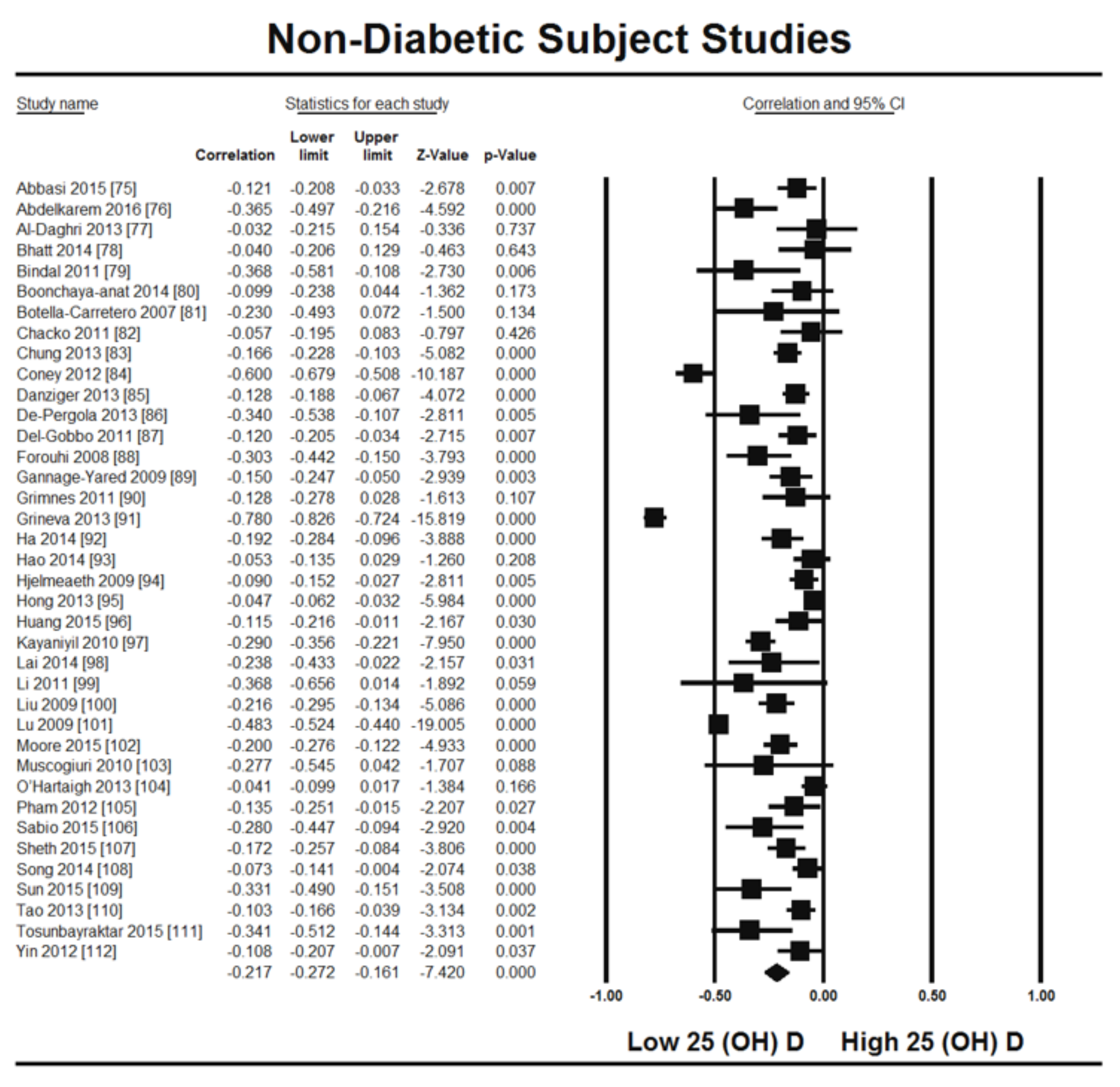
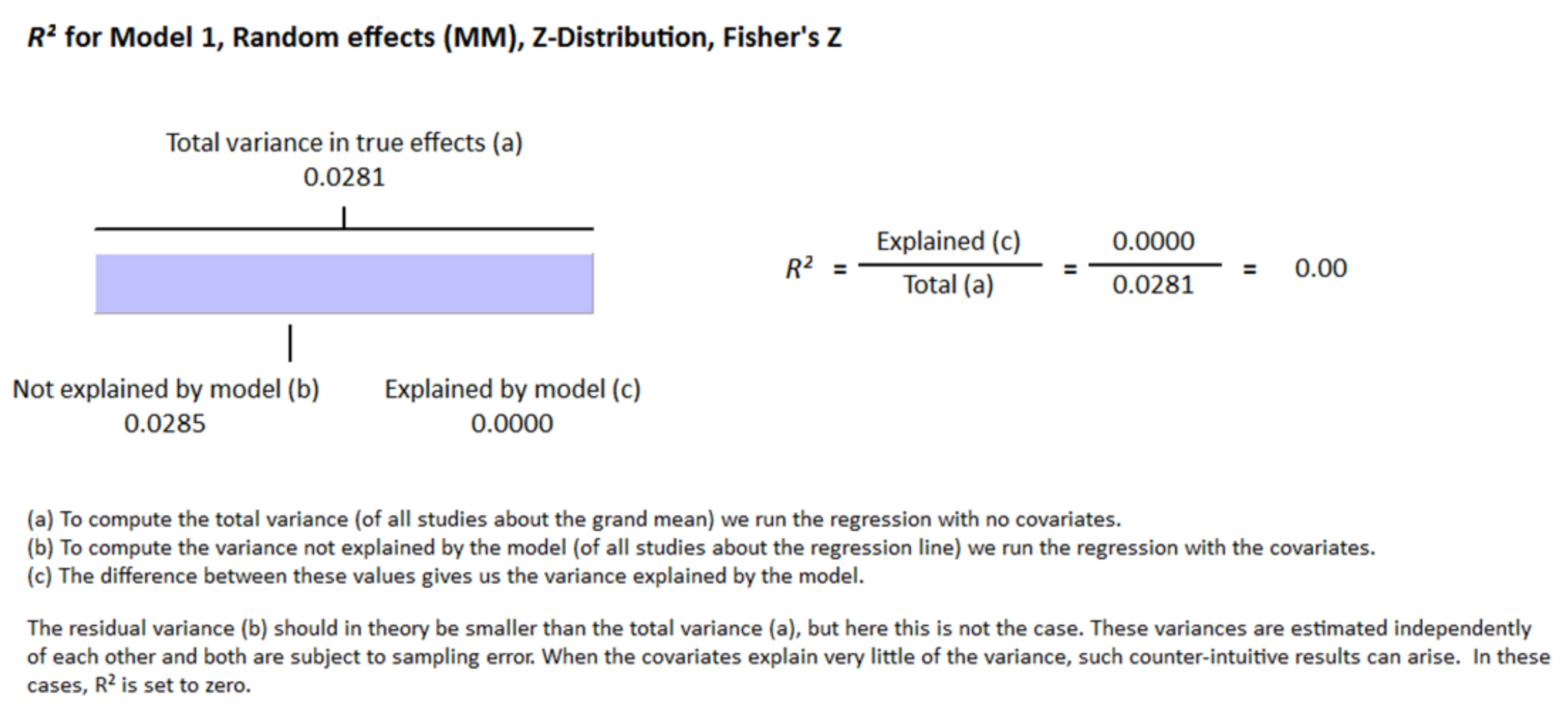
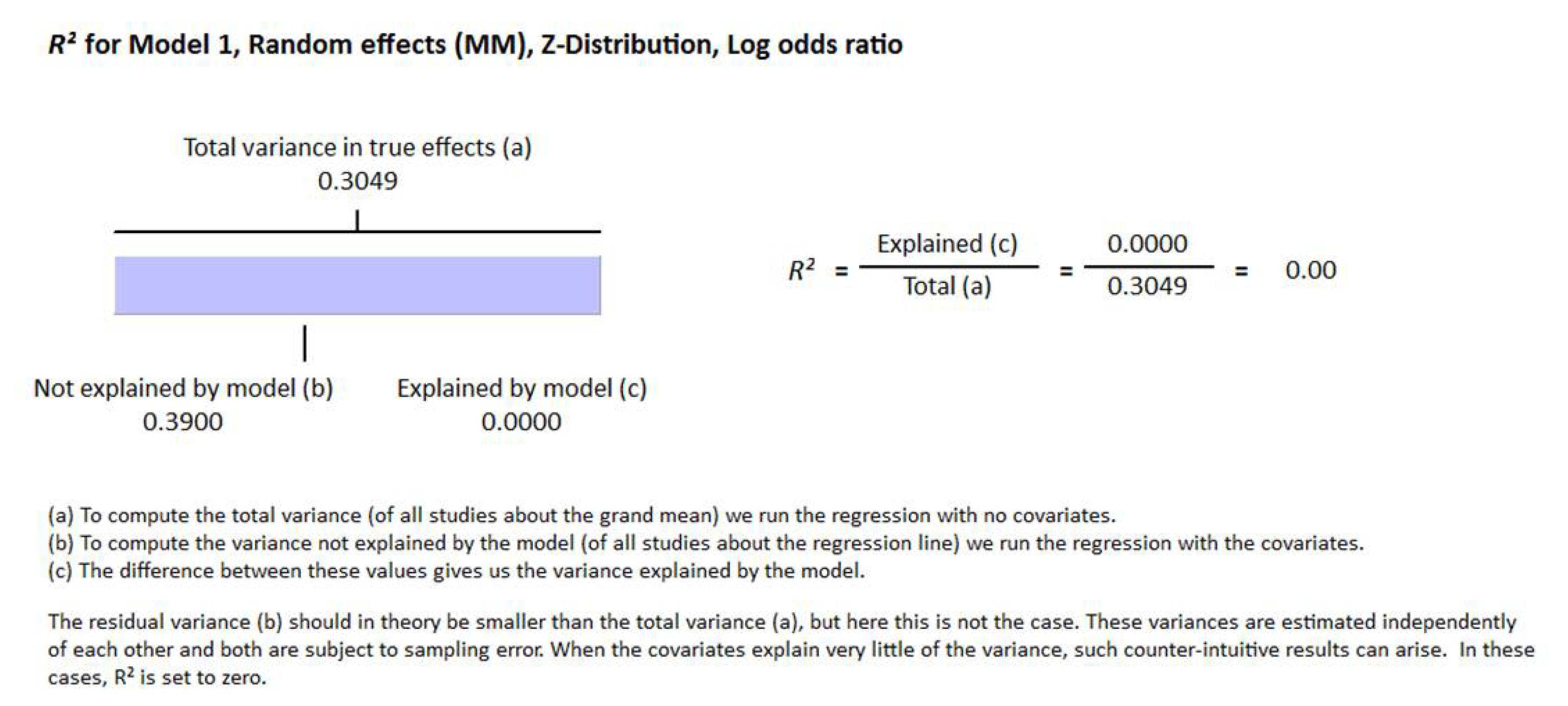
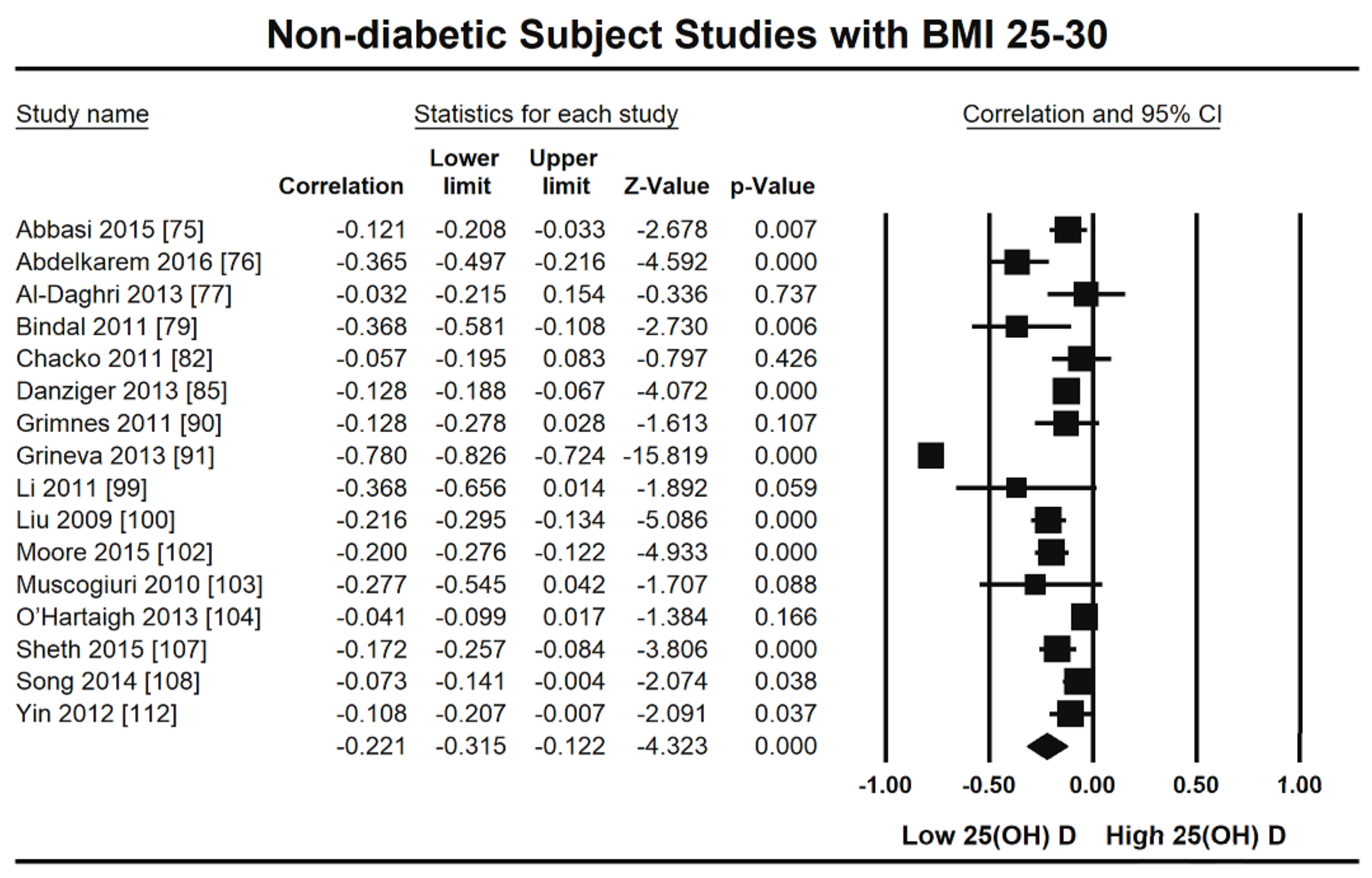
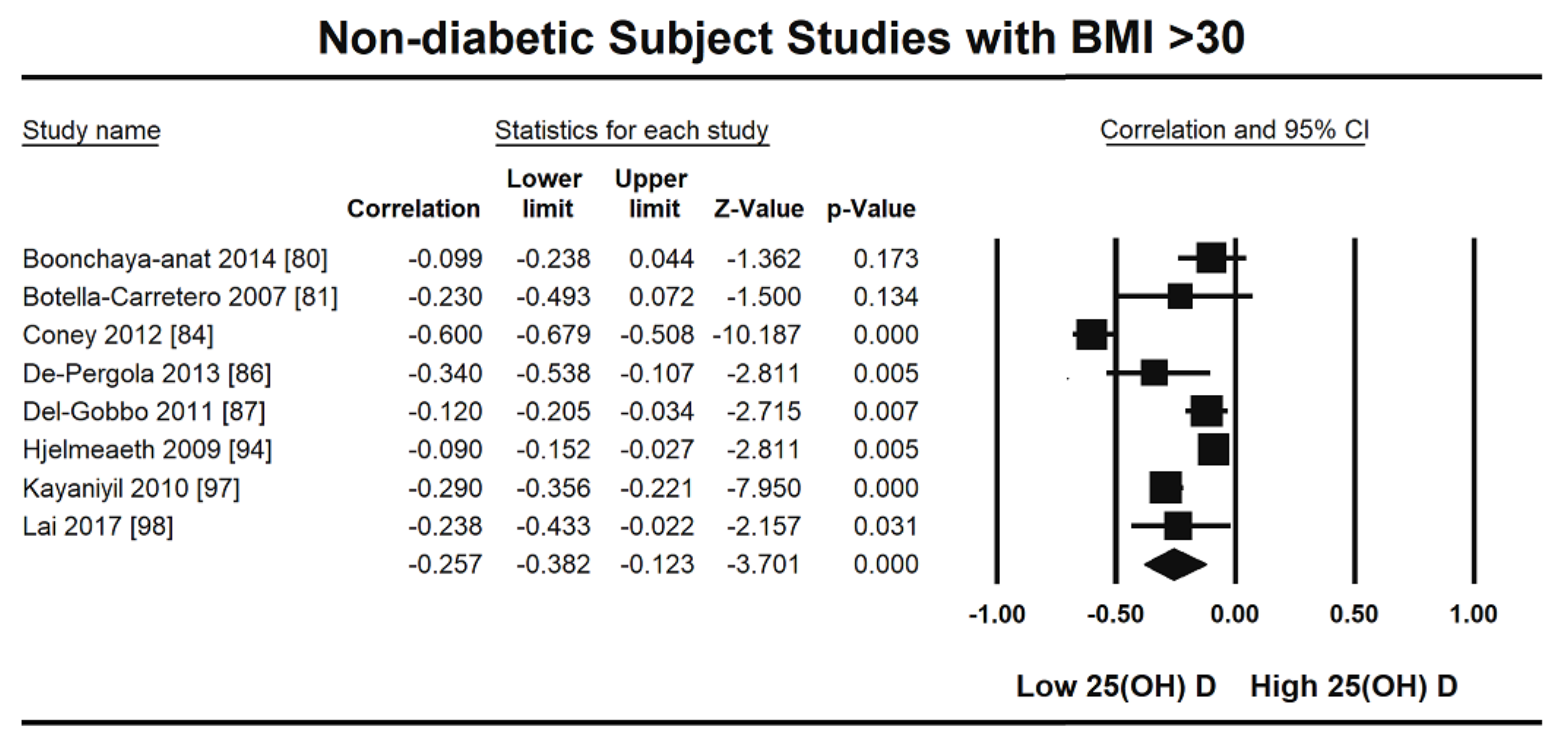
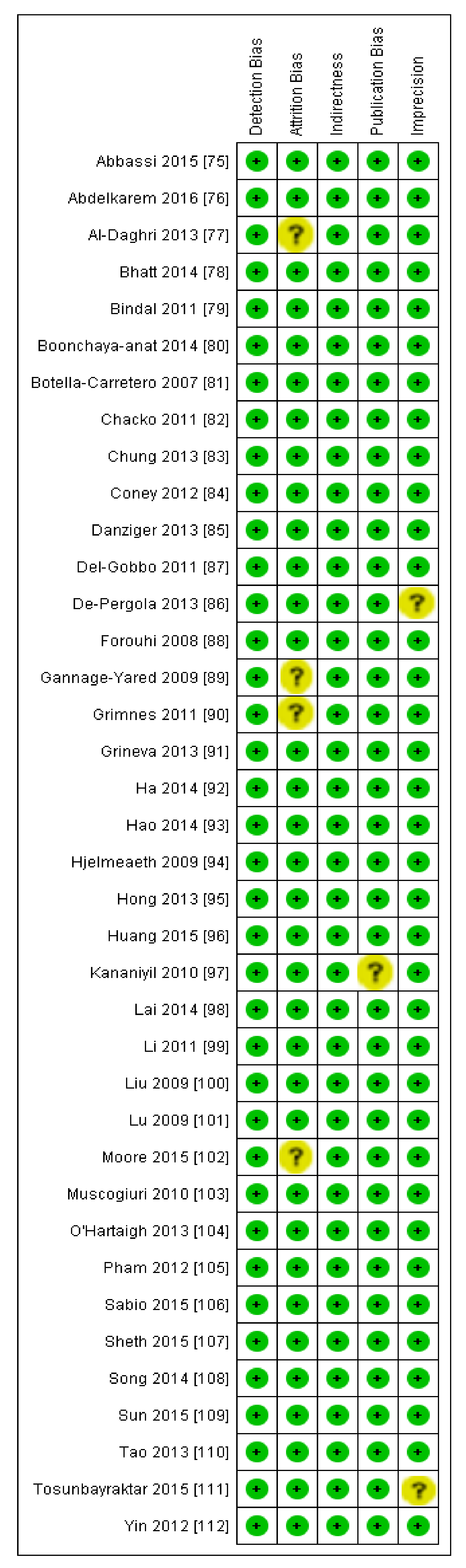
3. Results
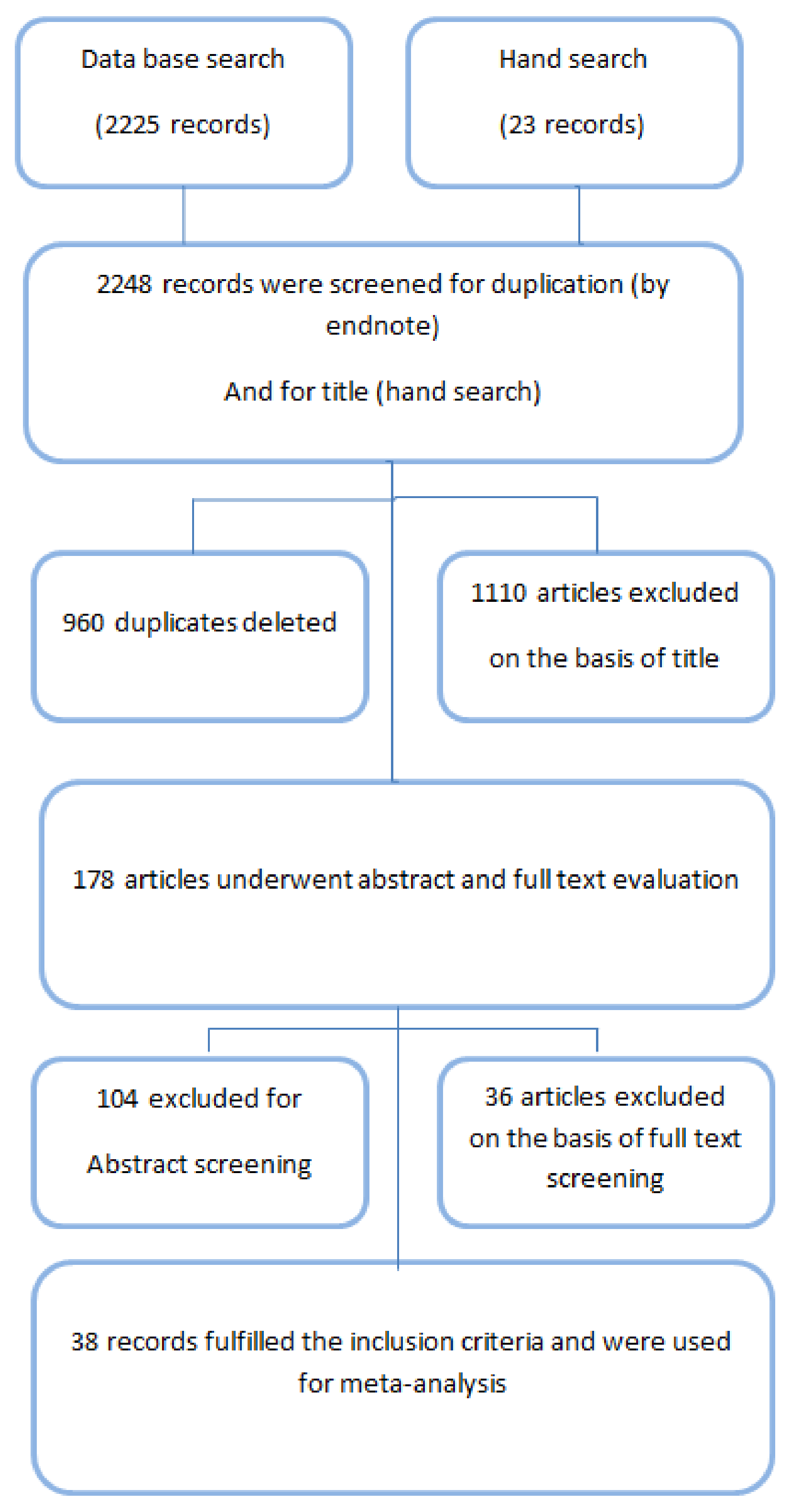
3.1. Excluded Studies
3.2. Included Studies
4. Discussion
Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Durmuller, U.; Stahelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.C.; Liu, M.E.; Ku, C.S.; Liu, T.Y.; Lin, S.L. Relationship between vitamin D deficiency and cardiovascular disease. World J. Cardiol. 2013, 5, 337–346. [Google Scholar] [CrossRef]
- Hahn, S.; Haselhorst, U.; Tan, S.; Quadbeck, B.; Schmidt, M.; Roesler, S.; Kimmig, R.; Mann, K.; Janssen, O.E. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 577–583. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, G.S.; Kim, S.G.; Moon, A.E. The relationship between metabolic syndrome and increase of metabolic syndrome score and serum vitamin D levels in Korean adults: 2012 Korean National Health and Nutrition Examination Survey. J. Clin. Biochem. Nutr. 2015, 57, 82–87. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.C.; Soares de Oliveira, A.R.; Pereira Pinto, D.; Silva Morais, J.B.; da Silva Lima, F.; Colli, C.; Torres-Leal, F.L.; do Nascimento Marreiro, D. Influence of magnesium on insulin resistance in obese women. Biol. Trace Elem. Res. 2014, 160, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Campbel, L.V. Insulin resistance and obesity. Clin. Dermatol. 2004, 22, 289–295. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdelrahman, M.M. Role of insulin and other related hormones in energy metabolism-A review. Cogent Food Agric. 2016, 2, 1267691. [Google Scholar] [CrossRef]
- Ravier, M.A.; Rutter, G.A. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 2006, 54, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Browning, E.T.; Olson, M. Interrelations between fatty acid oxidation and the control of gluconeogenesis in perfused rat liver. Adv. Enzym. Regul. 1968, 6, 67–100. [Google Scholar] [CrossRef]
- Boucher, B.J.; John, W.G.; Noonan, K. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004, 80, 1666. [Google Scholar] [CrossRef]
- Saintonge, H.B.; Gerber, L.M. Implications of a new definition of vitamin D deficiency in a multiracial US adolescent population: The National Health and Nutrition Examination Survey III. Pediatrics 2009, 123, 797–803. [Google Scholar] [CrossRef]
- Brancati, F.L.; Kao, W.H.L.; Folsom, A.R.; Watson, R.L.; Szklo, M. Incident type 2 diabetes mellitus in African American and white adults: The atherosclerosis risk in communities study. J. Am. Med. Assoc. 2000, 283, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.J.; Meier, A.H. Circadian and seasonal variations of plasma insulin and cortisol concentrations in the Syrian hamster, Mesocricetus auratus. Chronobiol. Int. 1987, 4, 141–151. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.N.; Geloneze, B.; Tambascia, M.A.; Lerário, A.C.; Halpern, A.; Cozzolino, S.M.F. Participação do Zinco na Resistência à Insulina. Arq. Bras. Endocrinol. Metabol. 2004, 48, 234–239. [Google Scholar]
- Salehpour, A.; Hosseinpanah, F.; Shidfar, F.; Vafa, M.; Razaghi, M.; Dehghani, S.; Gohari, M. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr. J. 2012, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Is hypovitaminosis D related to incidence of type 2 diabetes and high fasting glucose level in healthy subjects: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef]
- Szlagatys-Sidorkiewicz, A.; Brzeziński, M.; Jankowska, A.; Metelska, P.; Słomińska-Frączek, M.; Socha, P. Long-term effects of vitamin D supplementation in vitamin D deficient obese children participating in an integrated weight-loss programme (a double-blind placebo-controlled study)—Rationale for the study design. BMC Pediatr. 2017, 17, 97. [Google Scholar] [CrossRef]
- Roth, C.L.; Elfers, C.; Kratz, M.; Hoofnagle, A.N. Vitamin D deficiency in obese children and its relationship to insulin resistance and adipokines. J. Obes. 2011, 2011, 495101. [Google Scholar] [CrossRef]
- Wamberg, L.; Pedersen, S.B.; Rejnmark, L.; Richelsen, B. Causes of vitamin D deficiency and effect of vitamin D supplementation on metabolic complications in obesity: A review. Curr. Obes. Rep. 2015, 4, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, J.; Pande, J.N.; Bhartia, A. A double-blind, randomized, placebocontrolled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet. Med. 2009, 26, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.A.; Tosh, A.K.; Belenchia, A.M. Vitamin D insufficiency and insulin resistance in obese adolescents. Ther. Adv. Endocrinol. Metab. 2014, 5, 166–189. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Rad, E.; Djalali, M.; Koohdani, F.; Saboor-Yaraghi, A.A.; Eshraghian, M.R.; Javanbakht, M.H.; Saboori, S.; Zarei, M.; Hosseinzadeh-Attar, M.J. The effects of vitamin D supplementation on glucose control and insulin resistance in patients with diabetes type 2: A randomized clinical trial study. Iran. J. Public Health 2014, 43, 1651–1656. [Google Scholar] [PubMed]
- Lazear, J.; Kapustin, J. Vitamin D deficiency and type 2 diabetes: A retrospective review. J. Nurse Pract. 2014, 10, 175–182. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Bush, N.C.; Choquette, S.S.; Hunter, G.R.; Darnell, B.E.; Oster, R.A.; Gower, B.A. Vitamin D intake is associated with insulin sensitivity in African American, but not European American, women. Nutr. Metab. 2010, 7, 28. [Google Scholar] [CrossRef]
- Dutta, D.; Maisnam, I.; Shrivastava, A.; Sinha, A.; Ghosh, S.; Mukhopadhyay, P.; Mukhopadhyay, S.; Chowdhury, S. Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J. Med. Res. 2013, 138, 853–860. [Google Scholar]
- Kabadi, S.; Lee, B.; Liu, L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes. Diabetes Care 2012, 35, 2048–2054. [Google Scholar] [CrossRef]
- Kim, S.; Lim, J.; Kye, S.; Joung, H. Association between vitamin D status and metabolic syndrome risk among Korean population: Based on the Korean National Health and Nutrition Examination Survey IV-2, 2008. Diabetes Res. Clin. Pract. 2012, 9, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kobzaa, V.M.; Feet, J.C.; Zhoua, J.; Conley, T.B.; Peacock, M.; Reger, H.B.I.; Palmad, G.D.; Campbell, W.W. Vitamin D status and resistance exercise training independently affect glucose tolerance in older adults. Nutr. Res. 2013, 3, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Li, X.; Elli, E.F.; Ayloo, S.M.; Castellanos, K.J.; Fantuzzi, G.; Freels, S.; Braunschweig, C.L. Vitamin D, inflammation, and relations to insulin resistance in premenopausal women with morbid obesity. Obesity 2015, 23, 1591–1597. [Google Scholar] [CrossRef]
- Scragg, R.; Sowers, M.R.; Bell, C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [Google Scholar] [CrossRef]
- Weiler, H.A.; Lowea, J.; Krahn, J.; Leslie, W.D. Osteocalcin and vitamin D status are inversely associated with homeostatic model assessment of insulin resistance in Canadian Aboriginal and white women: The First Nations Bone Health Study. J. Nutr. Biochem. 2013, 24, 412–418. [Google Scholar] [CrossRef]
- Al-Sultan, A.I.; Amin, T.T.; Abou-Seif, M.A.; Al Naboli, M.R. Vitamin D, parathyroid hormone levels and insulin sensitivity among obese young adult Saudis. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 135–147. [Google Scholar] [PubMed]
- Bilge, U.; Unalacak, M.; Unluoglu, I.; Ipek, M.; Celer, O.; Akalın, A. Relationship between 1,25-dihydroxy Vitamin D levels and homeostatic model assessment insulin resistance values in obese subjects. Niger. J. Clin. Pract. 2015, 18, 377–380. [Google Scholar] [CrossRef]
- Gedik, O.; Akalin, S. Effect of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 1986, 29, 142–145. [Google Scholar] [CrossRef]
- Justice, J.N.; Pierpoint, L.A.; Mani, D.; Schwartz, R.S.; Enoka, R.M. Motor function is associated with 1,25(OH)2D and indices of insulin–glucose dynamics in non-diabetic older adults. Aging Clin. Exp. Res. 2014, 26, 249–254. [Google Scholar] [CrossRef]
- Kabadi, S.M.; Liu, L.; Auchincloss, A.H.; Zakeri, I.F. Multivariate path analysis of serum 25-hydroxyvitamin D concentration, inflammation, and risk of type 2 diabetes mellitus. Dis. Mark. 2013, 35, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kayaniyil, R.R.; Harris, S.B.; Vieth, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Prospective associations of vitamin D with b-cell function and glycemia, the PROspective metabolism and ISlet cell evaluation (PROMISE) cohort study. Diabetes 2011, 60, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Park, S.; Kim, Y. Age- and gender-specific associations between low serum 25-hydroxyvitamin D level and type 2 diabetes in the Korean general population: Analysis of 2008-2009 Korean National Health and Nutrition Examination. Survey data. Asia Pac. J. Clin. Nutr. 2012, 21, 536–546. [Google Scholar]
- Lu, L.; Wu, Y.; Qi, Q.; Liu, C.; Gan, W.; Zhu, J.; Li, H.; Lin, X. Associations of type 2 diabetes with common variants in PPARD and the modifying effect of vitamin D among middle-aged and elderly Chinese. PLoS ONE 2012, 7, e34895. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Vollenweider, P.; Guessous, I.; Henry, H.; Boulat, O.; Waeber, G.; Jornayvaz, F.R. Serum vitamin D concentrations are not associated with insulin resistance in Swiss adults. J. Nutr. 2015, 145, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Renzaho, A.M.N.; Nowson, C.; Kaur, A.; Halliday, J.A.; Fong, D.; DeSilva, J. Prevalence of vitamin D insufficiency and risk factors for type 2 diabetes and cardiovascular disease among African migrant and refugee adults in Melbourne. Asia Pac. J. Clin. Nutr. 2011, 20, 397–403. [Google Scholar]
- Scott, D.; Joham, A.; Teede, H.; Gibson-Helm, M.; Harrison, C.; Cassar, S.; Hutchison, S.; Ebeling, P.R.; Stepto, N.; de Courten, B. Associations of vitamin D with inter- and intra-muscular adipose tissue and insulin resistance in women with and without polycystic ovary syndrome. Nutrients 2016, 8, 774. [Google Scholar] [CrossRef]
- Sorkin, J.D.; Vasaitis, T.S.; Streeten, E.; Ryan, A.S.; Goldberg, A.P. Evidence for threshold effects of 25-hydroxyvitamin D on glucose tolerance and insulin resistance in black and white obese postmenopausal women. J. Nutr. 2014, 144, 734–742. [Google Scholar] [CrossRef]
- Stadlmayr, A.; Aigner, E.; Huber-Schonauer, U.; Niederseer, D.; Zwerina, J.; Husar-Memmer, E.; Hohla, F.; Schett, G.; Patsch, W.; Datz, C. Relations of vitamin D status, gender and type 2 diabetes in middle-aged Caucasians. Acta Diabetol. 2015, 52, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tzotzas, T.; Papadopoulou, F.G.; Tziomalos, K.; Karras, S.; Gastaris, K.; Perros, P.; Krassas, G.E. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J. Clin. Endocrinol. Metab. 2010, 95, 4251–4257. [Google Scholar] [CrossRef]
- Vigna, L.; Cassinelli, L.; Tirelli, A.S.; Felicetta, I.; Napolitano, F.; Tomaino, L.; Mutti, M.; Barberi, C.E.; Riboldi, L. 25(OH)D levels in relation to gender, overweight, insulin resistance, and inflammation in a cross- sectional cohort of northern Italian workers: Evidence in support of preventive health care programs. J. Am. Coll. Nutr. 2017, 36, 253–260. [Google Scholar] [CrossRef]
- Vujosevic, S.; Borozan, S.; Radojevic, N.; Aligrudic, S.; Bozovic, D. Relationship between 25-hydroxyvitamin D and newly diagnosed type 2 diabetes mellitus in postmenopausal women with osteoporosis. Med. Princ. Pract. 2014, 23, 229–233. [Google Scholar] [CrossRef]
- Al-Shoumer, K.A.; Al-Asoosi, A.A.; Ali, A.H.; Nair, V.S. Does Insulin Resistance in Type 2 Diabetes Alter Vitamin D Status? Prim. Care Diabetes 2013, 7, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Guzzaloni, G.; Rinaldi, M.; Merlotti, E.; Ferrari, C.; Tagliaferri, A.; Pirisi, M.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Altered glucose metabolism rather than naïve type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovasc. Diabetol. 2014, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Dalgard, C.; Skaalum, M.; Weihe, P.P.; Grandjean, P. DMSC vitamin D status in relation to glucose metabolism and type 2 diabetes in septuagenarians. Diabetes Care 2011, 34, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Esteghamatia, A.; Aryanab, B.; Esteghamatia, A.; Nakhjavania, M. Differences in vitamin D concentration between metabolically healthy andunhealthy obese adults: Associations with inflammatory and cardiometabolicmarkers in 4391 subjects. Diabetes Metab. 2014, 40, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Wang, M.; Ning, H.; Lima, A.; Li, Y.; Sun, C. Lipoprotein lipase links vitamin D, insulin resistance, and type 2 diabetes: A cross-sectional epidemiological study. Cardiovasc. Diabetol. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Peng, S. The relationship between serum vitamin D and HOMA-IR in overweight elderly patients. Int. J. Cardiol. 2014, 177, 1100–1102. [Google Scholar]
- Park, H.Y.; Lim, Y.; Kim, J.H.; Bae, S.; Oh, S.; Hong, Y. Association of serum 25-hydroxyvitamin D levels with markers for metabolic syndrome in the elderly: A repeated measure analysis. J. Korean Med. Sci. 2012, 27, 653–660. [Google Scholar] [CrossRef]
- Kavadar, G.; Demircioglu, D.T.; Ozgonenel, L.; Emre, T.Y. The relationship between vitamin D status, physical activity and insulin resistance in overweight and obese subjects. Bosn. J. Basic Med. Sci. 2015, 15, 62–66. [Google Scholar] [CrossRef]
- Kim, M.K.; Kang, M.I.; Oh, K.W.; Kwon, H.S.; Lee, J.H.; Lee, W.C.; Yoon, K.; Ho, Y. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin. Endocrinol. 2010, 73, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.O.; Bjerregaard, P.; Rønn, P.F.; Friis, H.; Andersen, S.; Melbye, M. Associations between Vitamin D status and type 2 diabetes measures among Inuit in Greenland may be affected by other factors. PLoS ONE 2016, 11, e0152763. [Google Scholar] [CrossRef]
- Pinelli, N.R.; Jaber, L.A.; Brown, M.B.; Herman, W.H. 3serum 25-hydroxy vitamin D and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care 2010, 33, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Wright, O.R.L.; Hickman, I.J.; Petchey, W.G.; Sullivan, C.M.; Ong, C.; Rose, F.J.; Ng, C.; Prins, J.B.; Whitehead, J.P.; Moore-Sullivan, T.M. The effect of 25-hydroxyvitamin D on insulin sensitivity in obesity: Is it mediated via adiponectin? Can. J. Physiol. Pharmacol. 2013, 91, 496–501. [Google Scholar] [CrossRef]
- Chonchol, M.; Cigolini, M.; Targher, G. Association between 25-hydroxyvitamin D deficiency and cardiovascular disease in type 2 diabetic patients with mild kidney dysfunction. Nephrol. Dial. Transplant. 2008, 23, 269–274. [Google Scholar] [CrossRef][Green Version]
- Esteghamati, A.; Aryan, Z.; Esteghamati, A.R.; Nakhjavani, M. Vitamin D deficiency is associated with insulin resistance in nondiabetics and reduced insulin production in type 2 diabetics. Horm. Metab. Res. 2015, 47, 273–279. [Google Scholar] [CrossRef]
- Nama, G.E.; Kima, D.H.; Choa, K.H.; Parkb, Y.G.; Hanb, K.D.; Choi, Y.S.; Kim, S.M.; Ko, B.J.; Kim, Y.H.; Lee, K.S. Estimate of a predictive cut-off value for serum 25-hydroxyvitamin D reflecting abdominal obesity in Korean adolescents. Nutr. Res. 2012, 32, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Al-Okail, M.S.; Alkharfy, K.M.; Al-Yousef, M.A.; Nadhrah, H.M.; Sabico, S.B.; Chrousos, G.P. Severe hypovitaminosis D is widespread and more common in non-diabetics than diabetics in Saudi adults. Saudi Med. J. 2010, 31, 775–780. [Google Scholar] [PubMed]
- Eraslan, S.; Kizilgul, M.; Uzunlulu, M.; Colak, Y.; Ozturk, O.; Tuncer, I. Frequency of metabolic syndrome and 25-hydroxyvitamin D3 levels in patients with non-alcoholic fatty liver disease. Minerva Med. 2013, 104, 447–453. [Google Scholar]
- Hutchinson, M.S.; Figenschau, Y.; Almås, B.; Njølstad, I.; Jorde, R. Serum 25-hydroxyvitamin D levels in subjects with reduced glucose tolerance and type 2 diabetes—The Tromsø OGTT-study. Int. J. Vitam. Nutr. Res. 2011, 81, 317–327. [Google Scholar] [CrossRef]
- Imura, H.; Seino, Y.; Ishida, H. Osteopenia and circulating levels of vitamin D metabolites in diabetes mellitus. J. Nutr. Sci. Vitaminol. 1985, 31, 27–32. [Google Scholar] [CrossRef]
- Inomata, S.; Kadowaki, S.; Yamatani, T.; Fukase, M.; Fujita, T. Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986, 1, 187–192. [Google Scholar]
- Mhatre, M.; Hall, M. Student forum: Does calcium and vitamin D intake affect incidence of type 2 diabetes mellitus and insulin resistance syndrome? Consult. Pharm. 2010, 25, 379–381. [Google Scholar] [CrossRef]
- Mirzaei, K.; Hossein-Nezhad, A.; Keshavarz, S.A.; Eshaghi, S.M.; Koohdani, F.; Saboor-Yaraghi, A.A.; Hosseini, S.; Tootee, A.; Djalali, M. Insulin resistance via modification of PGC1α function identifying a possible preventive role of vitamin D analogues in chronic inflammatory state of obesity. A double blind clinical trial study. Minerva Med. 2014, 105, 63–78. [Google Scholar]
- Abbasi, F.; Blasey, C.; Feldman, D.; Caulfield, C.P.; Hantash, F.M.; Reaven, G.M. Low circulating 25-hydroxyvitamin D concentrations are associated with defects in insulin action and insulin secretion in persons with prediabetes. J. Nutr. 2015, 145, 714–719. [Google Scholar] [CrossRef]
- Abdelkarem, H.M.; El-Sherif, M.A.; Gomaa, S.B. Vitamin D status and insulin resistance among young obese Saudi females. Saudi Med. J. 2016, 37, 561–566. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Al-Othman, A.; Draz, H.M.; Yakout, S.M.; Al-Saleh, Y.; Al-Yousef, M.; Sabico, S.; et al. Hypovitaminosis D associations with adverse metabolic parameters are accentuated in patients with Type 2 diabetes mellitus: A body mass index-independent role of adiponectin? J. Endocrinol. Investig. 2013, 36, 1–6. [Google Scholar]
- Bhatt, S.P.; Misra, A.; Sharma, M.; Guleria, R.; Pandey, R.M.; Luthra, K.; Vikram, N.K. Vitamin D insufficiency is associated with abdominal obesity in urban asian indians without diabetes in north india. Diabetes Technol. Ther. 2014, 16, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Bindal, M.E.; Taskapan, H. Hypovitaminosis D and insulin resistance in peritoneal dialysis patients. Int. Urol. Nephrol. 2011, 43, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Boonchaya-anant, P.; Holick, F.M.; Apovian, C.M. Serum 25-hydroxyvitamin D levels and metabolic health status in extremely obese individuals. Obesity 2014, 22, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
- Botella-Carretero, J.I.; Alvarez-Blasco, F.; Villafruela, J.J.; Balsa, J.A.; Vazquez, C.; Escobar-Morreale, H.F. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin. Nutr. 2007, 26, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Song, Y.; Manson, J.E.; Horn, L.V.; Eaton, C.; Martin, L.W.; McTiernan, A.; Curb, J.D.; Wylie-Rosett, J.; Phillips, L.S.; et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am. J. Clin. Nutr. 2011, 94, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Hong, S. Vitamin D status and its association with cardiometabolic risk factors in Korean adults based on a 2008-2010 Korean national health and nutrition examination survey nutrition research and practice. Nutr. Res. Pract. 2013, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Coney, P.; Demers, L.M.; Dodson, W.C.; Kunselman, A.R.; Ladson, G.; Legro, R.S. Determination of vitamin D in relation to body mass index and race in a defined population of black and white women. Int. J. Gynaecol. Obstet. 2012, 119, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Danziger, J.; Biggs, M.L.; Niemi, M.; Ix, J.H.; Kizer, J.R.; Djoussé, L.; de Boer, I.H.; Siscovick, D.S.; Kestenbaum, B.; Mukamal, K.J. Circulating 25-hydroxyvitamin D is associated with insulin resistance cross-sectionally but not longitudinally in older adults: The Cardiovascular Health Study. Metab. Clin. Exp. 2013, 62, 1788–1794. [Google Scholar] [CrossRef][Green Version]
- De Pergola, G.; Nitti, A.; Bartolomeo, N.; Gesuita, A.; Giagulli, V.A.; Triggiani, V.; Guastamacchia, E.; Silvestris, F. Possible role of hyperinsulinemia and insulin resistance in lower vitamin D levels in overweight and obese patients. Biomed. Res. Int. 2013, 92, 1348. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Song, Y.; Dannenbaum, D.A.; Dewailly, E.; Egeland, G.M. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta cell function in Canadian Cree. J. Nutr. 2011, 141, 290–295. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef]
- Gannage-Yared, M.L.; Chedid, R.; Khalife, S.; Azzi, E.; Zoghbi, F.; Halaby, G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur. J. Endocrinol. 2009, 160, 965–971. [Google Scholar] [CrossRef]
- Grimnes, G.; Figenschau, Y.; Almas, B.; Jorde, R. Vitamin D, insulin secretion, sensitivity, and lipids results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011, 60, 2748–2757. [Google Scholar] [CrossRef]
- Grineva, E.N.; Karonova, T.; Micheeva, E.; Belyaeva, O.; Nikitina, I.L. Vitamin D deficiency is a risk factor for obesity and diabetes type 2 in women at late reproductive age. AGING 2013, 5, 557–581. [Google Scholar]
- Ha, C.; Han, T.; Lee, S.; Cho, J.; Kang, H. Association between serum vitamin D status and metabolic syndrome in Korean young men. Epidemiology 2014, 46, 513–519. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, X.; Shen, Y.; Ni, J.; Luo, Y.; Xiao, Y.; Bao, Y.; Jia, W. Associations of serum 25-hydroxyvitamin D3 levels with Visceral adipose tissue in Chinese men with normal glucose tolerance. PLoS ONE 2014, 9, e86773. [Google Scholar] [CrossRef] [PubMed]
- Hjelmesæth, J.; Hofsø, D.; Aasheim, E.T.; Jenssen, T.; Moan, J.; Hager, H.; Røislien, J.; Bollerslev, J. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: A cross-sectional study. Cardiovasc. Diabetol. 2009, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.C.; Lee, J.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and Nutrition Examination Survey. Metab. Clin. Exp. 2013, 62, 1305–1312. [Google Scholar] [CrossRef]
- Huang, C.; Chang, H.; Lu, C.; Tseng, F.; Lee, L.; Huang, K. Vitamin D status and risk of metabolic syndrome among non-diabetic young adults. Clin. Nutr. 2015, 34, 484–489. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and β-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Coppola, B.; Dimko, M.; Galani, A.; Innico, G.; Frassetti, N.; Mariotti, A. Vitamin D deficiency, insulin resistance, and ventricular hypertrophy in the early stages of chronic kidney disease. Ren. Fail. 2014, 36, 58–64. [Google Scholar] [CrossRef]
- Li, H.W.R.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K.M. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metab. Clin. Exp. 2011, 60, 1475–1481. [Google Scholar] [CrossRef]
- Liu, E.; Meigs, J.B.; Pittas, A.G.; McKeown, N.M.; Economos, C.D.; Booth, S.L.; Jacques, P.F. Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J. Nutr. 2009, 139, 329–334. [Google Scholar] [CrossRef]
- Lu, L.; Yu, Z.; Pan, A.; Hu, F.B.; Franco, O.H.; Li, H.; Li, X.; Yang, X.; Chen, Y.; Lin, X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 2009, 32, 1278–1283. [Google Scholar] [CrossRef]
- Moore, A.; Hochner, H.; Sitlani, C.M.; Williams, M.A.; Hoofnagle, A.N.; de Boer, I.H.; Kestenbaum, B.; Siscovick, D.S.; Friedlander, Y.; Enquobahrie, D.A. Plasma vitamin D is associated with fasting insulin and homeostatic model assessment of insulin resistance in young adult males, but not females, of the Jerusalem Perinatal Study. Public Health Nutr. 2015, 18, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Sorice, G.P.; Prioletta, A.; Policola, C.; Casa, S.D.; Pontecorvi, A.; Giaccari, A. 25-hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity 2010, 18, 1906–1910. [Google Scholar] [CrossRef]
- O’Hartaigh, B.; Thomas, G.N.; Silbernagel, G.N.; Bosch, J.A.; Pilz, S.; Loerbroks, A.; Kleber, M.E.; Grammer, T.B.; Bohm, B.O.; Marz, W. Association of 25-hydroxyvitamin D with type 2 diabetes among patients undergoing coronary angiography: Cross-sectional findings from the LUdwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin. Endocrinol. 2013, 79, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Akter, S.; Kurotani, K.; Nanri, A.; Sato, M.; Hayabuchi, H.; Yasuda, K.; Mizoue, T. Serum 25-hydroxyvitamin D and markers of insulin resistance in a Japanese working population. Eur. J. Clin. Nutr. 2012, 66, 1323–1328. [Google Scholar] [CrossRef]
- Sabio, J.M.; Vargas-Hitos, J.A.; Martinez-Bordonado, J.; Navarrete-Navarrete, N.; Chamorro-Diaz, A.D.; Olvera-Porcel, C.; Zamora, M.; Jimenez-Alonso, J. Association between low 25-hydroxyvitamin D, insulin resistance and arterial stiffness in nondiabetic women with systemic lupus erythematosus. Lupus 2015, 24, 155–163. [Google Scholar] [CrossRef]
- Sheth, J.J.; Shah, A.; Sheth, F.J.; Trivedi, S.; Lele, M.; Shah, N.; Thakor, P.; Vaidya, R. Does vitamin D play a significant role in type 2diabetes? BMC Endocr. Disord. 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, B.M.; Rhee, Y.; Kim, C.O.; Youm, Y.; Kim, K.M.; Lee, E.Y.; Lee, J.M.; Yoon, Y.M.; Kim, H.C. Urban-rural differences explain the association between serum 25-hydroxyvitamin D level and insulin resistance in Korea. Nutrients 2014, 6, 5806–5818. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.; Tanisawa, K.; Ito, T.; Oshima, S.; Higuchi, M. The relationship between serum 25-hydroxyvitamin D concentration, cardiorespiratory fitness, and insulin resistance in Japanese men. Nutrients 2015, 7, 91–102. [Google Scholar] [CrossRef]
- Tao, M.; Zhang, Z.; Yao-hua, K.E.; Jin-wei, H.E.; Wen-zhen, F.U.; Zhang, C.; Zhang, Z. Association of serum 25-hydroxyvitamin D with insulin resistance and β-cell function in a healthy Chinese female population. Acta Pharmacol. Sin. 2013, 34, 1070–1074. [Google Scholar] [CrossRef]
- Tosunbayraktar, G.; Bas, M.; Kut, A.; Buyukkaragoz, A.H. Low serum 25(OH)D levels are assocıated to hıgher BMI and metabolic syndrome parameters in adults subjects in Turkey. Afr. Health Sci. 2015, 15, 1161–1169. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Q.; Zhang, X.; Lu, Y.; Sun, C.; Cui, Y.; Wang, S. Serum 25(OH)D is inversely associated with metabolic syndrome risk profile among urban middle-aged Chinese population. Nutr. J. 2012, 11, 68. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med. J. 2005, 98, 1024–1027. [Google Scholar] [CrossRef]
- dos Santos, L.R.; Lima, A.G.A.; Braz, A.F.; de Sousa Melo, S.R.; Morais, J.B.S.; Severo, J.S.; de Oliveira, A.R.S.; Cruz, K.J.C.; Marreiro, D.d.N. Role of vitamin D in insulin resistance in obese individuals. Nutrire 2017, 42, 17. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P.B. Body mass index, vitamin D, and type 2 diabetes: A systematic review and meta-analysis. Nutrients 2018, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Strain, G.; Sinha, N.; Ortiz, D.; Pomp, A.; Dakin, G.; McMahon, D.J.; Bockman, R.; Silverberg, S.J. Vitamin D insufficiency prior to bariatric surgery: Risk factors and a pilot treatment study. Clin. Endocrinol. 2009, 71, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Adipose tissue and the insulin resistance syndrome. Proc. Nutr. Soc. 2001, 60, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Walker, G.; Vietti, R.; Cattaldo, S.; Mele, C.; Priano, L.; Marzullo, P. Acute vitamin D3 supplementation in severe obesity: Evaluation of multimeric adiponectin. Nutrients 2017, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Khosravi, Z.; Kafeshani, M.; Tavasoli, P.; Zadeh, A. Effect of Vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: A clinical trial study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [CrossRef]
- Petersen, K.F.; Oral, E.A.; Dufour, S.; Befroy, D.; Ariyan, C.; Yu, C.; Cline, G.W.; DePaoli, A.M.; Taylor, S.I.; Gorden, P.; et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Investig. 2002, 109, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.A.; Gavin, I.J.R.; Aguilar, R.B.; Herman, M.E. A Unified pathophysiological construct of diabetes and its complications. Trends Endocrinol. Metab. 2017, 28, 645–655. [Google Scholar] [CrossRef]
- Kasuga, M. Insulin resistance and pancreatic beta cell failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nistico, S.P. Role of vitamins in skin health: A systematic review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.A.; Eun, C.R.; Cho, H.; Lee, S.K.; Yoo, H.J.; Kim, S.G. Low vitamin D status is associated with non-alcoholic fatty liver disease independent of visceral obesity in Korean adults. PLoS ONE 2013, 8, e75197. [Google Scholar]
- Tomson, J.; Emberson, J.; Hill, M.; Gordon, A.; Armitage, J.; Shipley, M. Vitamin D and risk of death from vascular and non-vascular causes in the whitehall study and meta-analyses of 12,000 Deaths. Eur. Heart J. 2013, 34, 1365–1374. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.S.; Tangpricha, V.; Quyyumi, A.A. Vitamin D and cardiovascular disease: Is the evidence solid? Eur. Heart J. 2013, 34, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Chu, A.; Go, V.L.W.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Oosterwerff, M.M.; Eekhoff, E.M.W.; Schoor, N.M.V.; Boeke, A.J.P.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D–deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial1–4. Am. J. Clin. Nutr. 2014, 100, 152–160. [Google Scholar] [CrossRef]
- Kositsawat, J.; Freeman, V.; Gebber, B.; Geraci, S. Association of A1c levels with vitamin D status in U.S. Adults. Diabetes Care 2010, 33, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Power, C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: The role of obesity. Diabetes Care 2006, 29, 2244–2246. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Maximos, M.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Subbarayan, S.; Correa, M.; Lo, M.; Suman, A.; Cusi, K. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J. Hepatol. 2015, 62, 405–411. [Google Scholar] [CrossRef]
- Chung, S.J.; Lee, Y.A.; Hong, H.; Kang, M.J.; Kwon, H.J.; Shin, C.H.; Yang, S.W. Inverse relationship between vitamin D status and insulin resistance and the risk of impaired fasting glucose in Korean children and adolescents: The Korean National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Public Health Nutr. 2014, 17, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Insulin resistance is inversely associated with the status of vitamin D in both diabetic and non-diabetic populations. Nutrients 2021, 13, 1742. [Google Scholar] [CrossRef] [PubMed]
- Barnard, W.F.; Saxena, V.K.; Wenny, B.N.; DeLuisi, J.J. Daily surface UV exposure and its relationship to surface pollutant measurements. J. Air Waste Manag. Assoc. 2003, 53, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Elminir, H.K. Sensitivity of ultraviolet solar radiation to anthropogenic air pollutants and weather conditions. Atmos. Res. 2007, 84, 250–264. [Google Scholar] [CrossRef]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Seino, Y.; Ishida, M.; Yamaoka, K.; Yabuuchi, H.; Ishida, H.; Seino, S.; Seino, Y.; Imura, H. Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol. 1984, 105, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, S.; Norman, A.W. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J. Clin. Investig. 1984, 73, 759–766. [Google Scholar] [CrossRef]
- Clark, S.A.; Stumpf, W.E.; Sar, M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes 1981, 30, 382–386. [Google Scholar] [CrossRef]
- Bourlon, P.M.; Faure-Dussert, A.; Billaudel, B. The de novo synthesis of numerous proteins is decreased during vitamin D3 deficiency and is gradually restored by 1, 25-dihydroxyvitamin D3 repletion in the islets of langerhans of rats. J. Endocrinol. 1999, 162, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Davila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230. [Google Scholar] [CrossRef]
- Maestro, B.; Molero, S.; Bajo, S.; Davila, N.; Calle, C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem. Funct. 2002, 20, 227–232. [Google Scholar] [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 2003, 17, 509–511. [Google Scholar] [CrossRef]
- Mathieu, C.; Van Etten, E.; Gysemans, C. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J. Bone Miner. Res. 2001, 16, 2057–2065. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Rapid Normalization/stimulation by 1,25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology 1987, 120, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.D.; Frahm, M.A.; DeLuca, H.F. 1,25-dihydroxyvitamin D3 up-regulates the renal vitamin D receptor through indirect gene activation and receptor stabilization. Arch. Biochem. Biophys. 2005, 433, 466–473. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Daghri, N.M.; Yakout, S.M. Calcitriol Attenuates weight-related systemic inflammation and ultrastructural changes of the liver in a rodent model. Basic Clin. Pharmacol. Toxicol. 2012, 112, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Raffel, L.J.; Goodarzi, M.O.; Rotter, J.I. Diabetes mellitus. In Principles and Practice of Medical Genetics; Rimoin, D.L., Connor, J.M., Pyeritz, R.E., Korf, B., Eds.; Churchill Livingstone: London, UK, 1980; pp. 1980–2022. [Google Scholar]
- Wang, Z.H.; Shi, X.; Su, H.; Harshfield, S.; Gutin, G.A.; Snieder, B.; Dong, H. A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J. Pediatr. 2013, 162, 1004–1009. [Google Scholar]
- Yu, F.; Cui, L.; Li, X.; Wang, C.; Ba, Y.; Wang, L.; Li, J.; Li, C.; Dai, L.; Li, W. The genetic polymorphisms in vitamin D receptor and the risk of type 2 diabetes mellitus: An updated meta-analysis. Asia Pac. J. Clin. Nutr. 2016, 25, 614–624. [Google Scholar] [PubMed]
- Jeddi, S.; Syedmoradi, L.; Bagheripour, F.; Ghasemi, A. The effects of vitamin D on insulin release from isolated islets of rats. Int. J. Endocrinol. Metab. 2015, 13, e20620. [Google Scholar] [CrossRef] [PubMed]
- Cade, C.; Norman, A.W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Campion, J.; Davila, N.; Calle, C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptorexpression and insulin responsiveness for glucose transport in U-937 human promonocyticcells. Endocr. J. 2000, 47, 383–391. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. https://doi.org/10.3390/nu13124358
Rafiq S, Jeppesen PB. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients. 2021; 13(12):4358. https://doi.org/10.3390/nu13124358
Chicago/Turabian StyleRafiq, Shamaila, and Per Bendix Jeppesen. 2021. "Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance" Nutrients 13, no. 12: 4358. https://doi.org/10.3390/nu13124358
APA StyleRafiq, S., & Jeppesen, P. B. (2021). Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients, 13(12), 4358. https://doi.org/10.3390/nu13124358





