Hemp Seeds in Post-Arthroplasty Rehabilitation: A Pilot Clinical Study and an In Vitro Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Study Design
2.1.1. Raw Material for Pasta Manufacturing and Protein Content Analysis
2.1.2. Biochemical Assessments
2.1.3. Bioavailability Assessment
2.2. In Vitro Study
2.2.1. Chemicals, Reagents, and Materials
2.2.2. Hemp Seeds’ Extraction
2.2.3. Determination of Proteins, Carbohydrates, and Total Phenolic Content
A1 = Absorbance of extracts/standards.
2.2.4. Cell Line and Culture Conditions
2.2.5. Cell Viability Assay
2.2.6. Western Blotting
2.2.7. Alkaline Phosphatase (ALP) Activity
2.2.8. Real Time-PCR
2.3. Statistical Analysis
3. Results
3.1. Bioavailability of Proteins after HS Pasta Consumption
3.2. Clinical Characteristics of Participants in the Pilot Study
3.3. Clinical Characteristics Changes at Follow-Up and Outcomes of the Study
3.4. Characterization of Hemp Seeds’ Extract
3.5. Hemp Seeds’ Extract Does Not Act on Osteoblasts’ Proliferation In Vitro
3.6. Hemp Seeds’ Extract Induces Higher β-Catenin and p-ERK1/2 Levels in Saos-2
3.7. Hemp Seeds’ Extract Increases Activity and Protein Levels of Alkaline Phosphatase in Saos-2
3.8. Hemp Seeds’ Extract Decreases RANKL and Increases RUNX2 and Osteocalcin mRNA Expression Levels in Saos-2
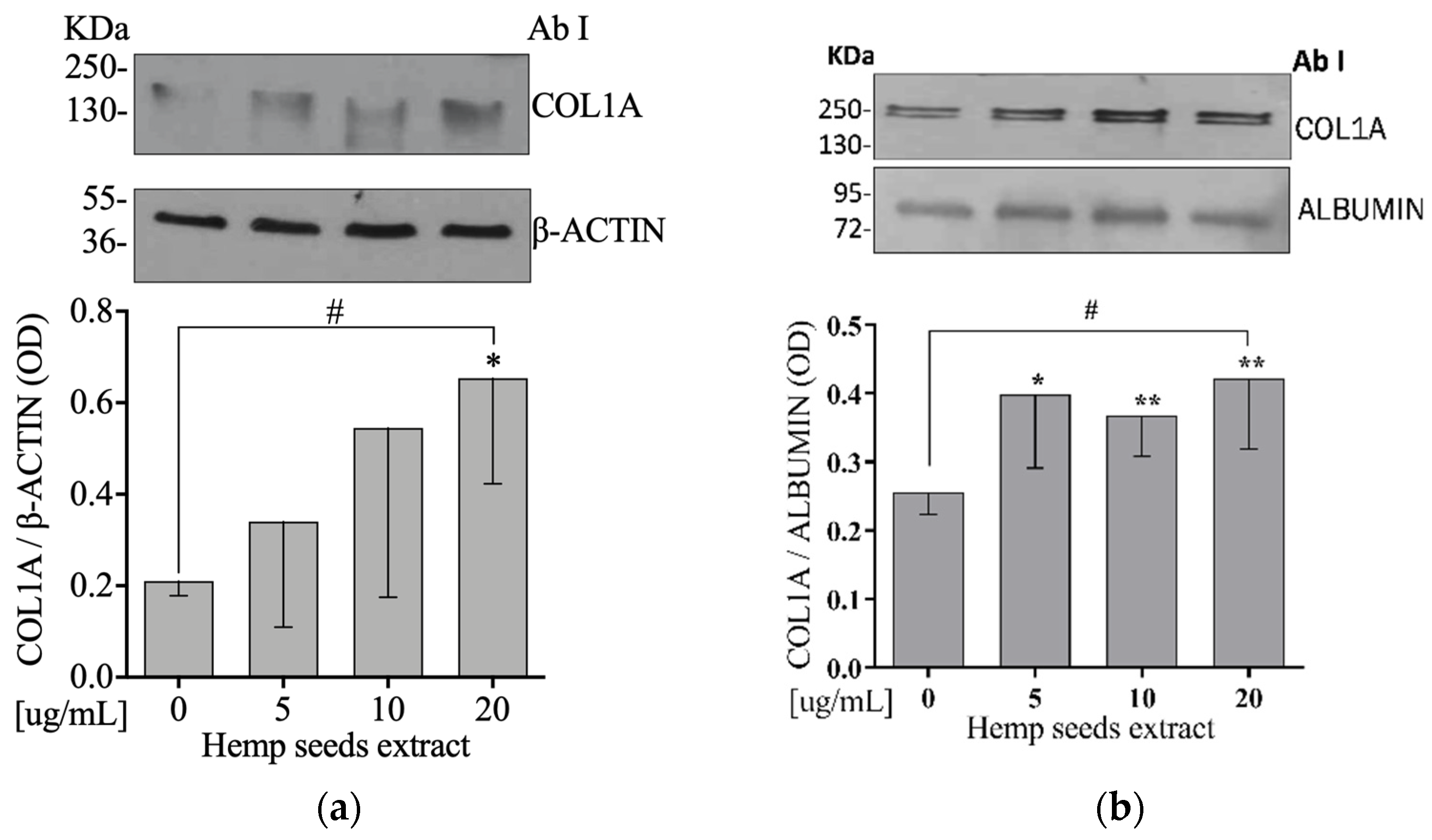
3.9. Hemp Seeds’ Extract Increases Extracellular and Intracellular COL1A Protein Levels in Saos-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabriel, S.E.; Crowson, C.S.; Campion, M.E.; O’Fallon, W.M. Direct medical costs unique to people with arthritis. J. Rheumatol. 1997, 24, 719–725. [Google Scholar] [PubMed]
- Murphy, L.; Helmick, C.G. The Impact of Osteoarthritis in the United States: A Population-Health PerspectiveA population-based review of the fourth most common cause of hospitalization in US adults. Orthopaed. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef]
- Looker, K.; Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Int. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zhu, S.; Bi, R. Comparison of early-stage changes of osteoarthritis in cartilage and subchondral bone between two different rat models. PeerJ 2020, 8, e8934. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.; Andreassen, O.; Christensen, P.; Conaghan, P.G.; Doherty, M.; Geenen, R.; Hammond, A.; Kjeken, I. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Diseases 2013, 72, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Hegmann, K.T.; Biggs, J.J.; Cox, C.M.; Portmann, A.J.; Gildea, J.H.; Gren, L.H.; Lyon, J.L. Relationships between body mass indices and surgical replacements of knee and hip joints. Am. J. Prevent. Med. 2003, 25, 290–295. [Google Scholar] [CrossRef]
- Loveman, E.; Frampton, G.; Shepherd, J.; Picot, J.; Cooper, K.; Bryant, J.; Welch, K.; Clegg, A. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: A systematic review. NIHR Health Technol. Assess. Program. Execut. Sum. 2011, 15, 1–182. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef]
- Vincent, H.K.; Heywood, K.; Connelly, J.; Hurley, R.W. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM&R 2012, 4, S59–S67. [Google Scholar]
- Prior, R.L.; Cao, G. Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006, 8, 1–22. [Google Scholar] [CrossRef]
- Schuelert, N.; McDougall, J.J. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum. 2008, 58, 145–153. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; McDougall, J.J. Cannabis and joints: Scientific evidence for the alleviation of osteoarthritis pain by cannabinoids. Curr. Opin. Pharmacol. 2018, 40, 104–109. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J. Am. Acad. Orthop. Surg. Global Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef]
- Wang, X.-S.; Tang, C.-H.; Yang, X.-Q.; Gao, W.-R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Prot. Protoc. Handb. 2009, 32, 5–8. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Aruwa, C.; Amoo, S.; Kudanga, T. Extractable and macromolecular antioxidants of Opuntia ficus-indica cladodes: Phytochemical profiling, antioxidant and antibacterial activities. South Af. J. Botany 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Trans. Med. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Park, H.-M.; Lee, J.-H.; Lee, Y.-J. Positive association of serum alkaline phosphatase level with severe knee osteoarthritis: A nationwide population-based study. Diagnostics 2020, 10, 1016. [Google Scholar] [CrossRef]
- Kamimura, M.; Uchiyama, S.; Takahara, K.; Hashidate, H.; Kawaguchi, A.; Nakagawa, H. Urinary excretion of type I collagen cross-linked N-telopeptide and serum bone-specific alkaline phosphatase analysis to determine the correlation of age and back-pain related changes in elderly women. J. Bone Miner. Metabol. 2005, 23, 495–500. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Favre, J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Current Rheumatol. Reports 2014, 16, 463. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Hagino, H.; Okano, T.; Enokida, M.; Teshima, R. Role of subchondral bone in osteoarthritis development: A comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. Off. J. Am. College Rheumatol. 2007, 56, 3366–3374. [Google Scholar] [CrossRef]
- Donell, S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Kim, H.; Jang, S.; Lee, J.; Baeck, S.; In, S.; Kim, E.; Kim, Y.-u.; Han, E. Concentrations of THC, CBD, and CBN in commercial hemp seeds and hempseed oil sold in Korea. Forensic Sci. Int. 2020, 306, 110064. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Zimmer, A. Cannabinoid receptors and the regulation of bone mass. Br. J. Pharmacol. 2008, 153, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef]
- Hashempur, M.H.; Sadrneshin, S.; Mosavat, S.H.; Ashraf, A. Green tea (Camellia sinensis) for patients with knee osteoarthritis: A randomized open-label active-controlled clinical trial. Clin. Nutr. 2018, 37, 85–90. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Connelly, A.E.; Tucker, A.J.; Tulk, H.; Catapang, M.; Chapman, L.; Sheikh, N.; Yurchenko, S.; Fletcher, R.; Kott, L.S.; Duncan, A.M. High-rosmarinic acid spearmint tea in the management of knee osteoarthritis symptoms. J. Med. Food 2014, 17, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Shell, W.E.; Pavlik, S.; Roth, B.; Silver, M.; Breitstein, M.L.; May, L.; Silver, D. Reduction in pain and inflammation associated with chronic low back pain with the use of the medical food theramine. Am. J. Therap. 2016, 23, e1353. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, A.; Miyakoshi, N.; Shimada, Y.; Kodama, H. The relationship between osteoporosis and osteoarthritis of the knee: A report of 2 cases with suspected osteonecrosis. Case Reports Orthop. 2014, 2014, 514058. [Google Scholar] [CrossRef]
- Osterberg, A.; Thiem, D.; Herlyn, P.; Mittlmeier, T.; Frerich, B.; Müller-Hilke, B. Subchondral bone sclerosis and cancellous bone loss following OA induction depend on the underlying bone phenotype. Joint Bone Spine 2017, 84, 71–77. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Protein concentration and amino acid profile in hemp seed and flaxseed meal. In Proceedings of the Eucarpia International Symposium on Protein Crops, Pontevedra, Spain, 4–7 May 2015; pp. 193–195. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Maeda, K.; Takahashi, N. Roles of Wnt signaling in bone formation and resorption. Japan. Dental Sci. Rev. 2008, 44, 76–82. [Google Scholar] [CrossRef]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005, 8, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; El-Serafi, A.T.; Omar, H.A. Caffeic acid phenethyl ester protects against glucocorticoid-induced osteoporosis in vivo: Impact on oxidative stress and RANKL/OPG signals. Toxicol. Appl. Pharmacol. 2017, 324, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Weitzmann, M.N. The bone anabolic carotenoid p-hydroxycinnamic acid promotes osteoblast mineralization and suppresses osteoclast differentiation by antagonizing NF-κB activation. Int. J. Mol. Med. 2012, 30, 708–712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rivera-Piza, A.; An, Y.J.; Kim, D.K. Protocatechuic acid enhances osteogenesis, but inhibits adipogenesis in C3H10T1/2 and 3T3-L1 cells. J. Med. Food. 2017, 20, 309–319. [Google Scholar] [CrossRef]
- Li, D.; Lin, Z.; Meng, Q.; Wang, K.; Wu, J.; Yan, H. Cannabidiol administration reduces sublesional cancellous bone loss in rats with severe spinal cord injury. Eur. J. Pharmacol. 2017, 809, 13–19. [Google Scholar] [CrossRef]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal–regulated kinase–MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef]
- Han, N.-R.; Kim, H.-Y.; Yang, W.M.; Jeong, H.-J.; Kim, H.-M. Glutamic acid ameliorates estrogen deficiency–induced menopausal-like symptoms in ovariectomized mice. Nutr. Res. 2015, 35, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.-y.; Li, X.-m.; Luo, S.-q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Michaëlsson, K.; Olofsson, H.; Johansson, S.; Melhus, H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001, 288, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kaji, A.; He, Z.; Ma, W.-Y.; Miyamoto, K.-i.; Yang, C.S.; Dong, Z. Inhibitory mechanisms of tea polyphenols on the ultraviolet B-activated phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 2001, 276, 46624–46631. [Google Scholar] [CrossRef]
- Kern, M.; Pahlke, G.; Balavenkatraman, K.K.; Böhmer, F.D.; Marko, D. Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J. Agric. Food Chem. 2007, 55, 4999–5006. [Google Scholar] [CrossRef]
- Larsen, C.A.; Dashwood, R.H.; Bisson, W.H. Tea catechins as inhibitors of receptor tyrosine kinases: Mechanistic insights and human relevance. Pharmacol. Res. 2010, 62, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.H.; Gao, Q.G.; Zhang, Y. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J. Steroid Biochem. Mol. Biol. 2014, 144, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Choudhary, D.; Ahmad, N.; Karvande, A.; Kumar, A.; Banala, V.T.; Mishra, P.R.; Trivedi, R. Dietary flavonoid kaempferol inhibits glucocorticoid-induced bone loss by promoting osteoblast survival. Nutrition 2018, 53, 64–76. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthopaed. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Poundarik, A.A.; Diab, T.; Sroga, G.E.; Ural, A.; Boskey, A.L.; Gundberg, C.M.; Vashishth, D. Dilatational band formation in bone. Proc. Nat. Acad. Sci. USA 2012, 109, 19178–19183. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R.; Gnudi, S.; Nicolini, A.; Carpi, A. L-arginine and L-lysine stimulation on cultured human osteoblasts. Biomed. Pharmacother. 2002, 56, 492–497. [Google Scholar] [CrossRef]
- Huh, J.-E.; Choi, J.-Y.; Shin, Y.-O.; Park, D.-S.; Kang, J.W.; Nam, D.; Choi, D.-Y.; Lee, J.-D. Arginine enhances osteoblastogenesis and inhibits adipogenesis through the regulation of Wnt and NFATc signaling in human mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 13010–13029. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Ralston, S.H. Role of cannabinoids in the regulation of bone remodeling. Front. Endocrinol. 2012, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Scutt, A.; Williamson, E. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB 2 receptors. Calcified Tissue Int. 2007, 80, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.; Levine, M.A. Bones and joints: The effects of cannabinoids on the skeleton. J. Clin. Endocrinol. Metabol. 2019, 104, 4683–4694. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Populo, S.M.; Zhang, J.; Yang, G.; Kodama, H. Effect of Angelica sinensis on the proliferation of human bone cells. Clin. Chim. Acta 2002, 324, 89–97. [Google Scholar] [CrossRef]
- Funck-Brentano, T.; Cohen-Solal, M. Crosstalk between cartilage and bone: When bone cytokines matter. Cytokine Growth Factor Rev. 2011, 22, 91–97. [Google Scholar] [CrossRef] [PubMed]




| Variables | Control (n = 11) | HS Pasta (n = 7) | p-Value |
|---|---|---|---|
| Age (year) | 75 ± 7 | 69 ± 10 | 0.21 |
| VAS (cm) | 6.6 ± 2 | 7.1 ± 2 | 0.64 |
| Weight (Kg) | 80.1 ± 14 | 78.3 ± 14 | 0.79 |
| BMI (Kg/m2) | 31.2 ± 5 | 28.3 ± 3 | 0.17 |
| HG (Kg) | 23.0 ± 7 | 27.2 ± 16 | 0.52 |
| SBP (mmHg) | 130 ± 11 | 135 ± 13 | 0.47 |
| DBP (mmHg) | 82 ± 11 | 78 ± 7 | 0.38 |
| Biochemical evaluation | |||
| Glucose (mg/dL) | 109 ± 22 | 113 ± 25 | 0.73 |
| Creatinine (mg/dL) | 0.83 ± 0.2 | 0.85 ± 0.1 | 0.72 |
| TC (mg/dL) | 173 ± 38 | 174 ± 31 | 0.93 |
| HDL-C (mg/dL) | 45 ± 14 | 47±7 | 0.75 |
| LDL-C (mg/dL) | 104 ± 35 | 100 ± 31 | 0.77 |
| TG (mg/dL) | 117 ± 49 | 138 ± 72 | 0.49 |
| AST (IU/L) | 22 ± 12 | 18 ± 5 | 0.43 |
| ALT (IU/L) | 15 ± 11 | 15 ± 5 | 0.86 |
| Osteocalcin (ng/mL) | 21.7 ± 13 | 19.8 ± 10 | 0.73 |
| BALP (µg/L) | 10.8 ± 4 | 13.2 ± 5 | 0.29 |
| Lymphocyte (×103/µL) | 1.7 ± 0.7 | 2.2 ± 0.7 | 0.16 |
| Monocyte (×103/µL) | 0.38 ± 0.1 | 0.39 ± 0.1 | 0.89 |
| Cytokine evaluation | |||
| IL-1β (pg/mL) | 2.3 ± 0.5 | 2.2 ± 0.4 | 0.86 |
| IL-10 (pg/mL) | 0.69 ± 0.3 | 0.71 ± 0.2 | 0.47 |
| Prevalence | |||
| Gender, Male (%) | 36 | 71 | 0.35 |
| Smokers (%) | 9 | 0 | 1 |
| THR (%) | 36 | 86 | 0.06 |
| Hyperlipidemia (%) | 45 | 57 | 1 |
| Hypertension (%) | 73 | 57 | 0.62 |
| T2D (%) | 36 | 29 | 1 |
| Medications | |||
| NSAIDs (%) | 91 | 86 | 1 |
| Calcium (%) | 0 | 14 | 0.38 |
| Vitamin D (%) | 18 | 14 | 1 |
| Variables | Control (n = 11) | HS Pasta (n = 7) | p-Value |
|---|---|---|---|
| VAS (cm) | −1.3 ± 1.3 | −2.9 ± 1.3 | 0.028 |
| Weight (Kg) | 0.4 ± 1.7 | −0.9 ± 1.9 | 0.17 |
| BMI (Kg/m2) | 0.1 ± 0.7 | −0.3 ± 0.9 | 0.32 |
| HG (Kg) | 0.5 ± 1.8 | 0.2 ± 0.4 | 0.61 |
| SBP (mmHg) | 1 ± 9 | −2 ± 4 | 0.38 |
| DBP (mmHg) | 4 ± 5 | 3 ± 5 | 0.85 |
| Biochemical evaluation | |||
| Glucose (mg/dL) | 8 ± 28 | 0 ± 5 | 0.38 |
| Creatinine (mg/dL) | −0.01 ± 0.1 | 0.01 ± 0.1 | 0.64 |
| TC (mg/dL) | 9 ± 23 | 1 ± 21 | 0.42 |
| HDL-C (mg/dL) | 2 ± 5 | 1 ± 1 | 0.30 |
| LDL-C (mg/dL) | 5 ± 19 | −1 ± 18 | 0.55 |
| TG (mg/dL) | 12 ± 23 | 7 ± 28 | 0.73 |
| AST (IU/L) | −1 ± 4 | −0.7 ± 2 | 0.75 |
| ALT (IU/L) | −1 ± 4 | 1 ± 2 | 0.09 |
| Osteocalcin (ng/mL) | −1.2 ± 8.5 | 2.6 ± 8.4 | 0.38 |
| BALP (µg/L) | 1.1 ± 4.3 | −2.8 ± 3.2 | 0.041 |
| Lymphocyte (×10ˆ3/µL) | −0.03 ± 0.2 | 0.01 ± 0.2 | 0.71 |
| Monocyte (×10ˆ3/µL) | −0.05 ± 0.1 | −0.01 ± 0.1 | 0.40 |
| Cytokine evaluation | |||
| IL-1β (pg/mL) | 0.3 ± 0.2 | 0.5 ± 0.3 | 0.12 |
| IL-10 (pg/mL) | −0.001 ± 0.2 | 0.001 ± 0.2 | 0.65 |
| Molecules | Mean ± SD | % (w/w) |
|---|---|---|
| Proteins (μg/mL) | 5.65 ± 0.01 * 4.67 ± 0.002 δ | 14.1 15.6 |
| Carbohydrates (μg) | 169.35 ± 2.41 | 21.2 |
| Polyphenols (GAE-μg) | 0.0735 ± 0.0008 | 29.4 |
| Others | - | ~33.8 |
| Antioxidant activity (I%) | - | 37.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurotti, S.; Mare, R.; Pujia, R.; Ferro, Y.; Mazza, E.; Romeo, S.; Pujia, A.; Montalcini, T. Hemp Seeds in Post-Arthroplasty Rehabilitation: A Pilot Clinical Study and an In Vitro Investigation. Nutrients 2021, 13, 4330. https://doi.org/10.3390/nu13124330
Maurotti S, Mare R, Pujia R, Ferro Y, Mazza E, Romeo S, Pujia A, Montalcini T. Hemp Seeds in Post-Arthroplasty Rehabilitation: A Pilot Clinical Study and an In Vitro Investigation. Nutrients. 2021; 13(12):4330. https://doi.org/10.3390/nu13124330
Chicago/Turabian StyleMaurotti, Samantha, Rosario Mare, Roberta Pujia, Yvelise Ferro, Elisa Mazza, Stefano Romeo, Arturo Pujia, and Tiziana Montalcini. 2021. "Hemp Seeds in Post-Arthroplasty Rehabilitation: A Pilot Clinical Study and an In Vitro Investigation" Nutrients 13, no. 12: 4330. https://doi.org/10.3390/nu13124330
APA StyleMaurotti, S., Mare, R., Pujia, R., Ferro, Y., Mazza, E., Romeo, S., Pujia, A., & Montalcini, T. (2021). Hemp Seeds in Post-Arthroplasty Rehabilitation: A Pilot Clinical Study and an In Vitro Investigation. Nutrients, 13(12), 4330. https://doi.org/10.3390/nu13124330










