Phytoprogestins: Unexplored Food Compounds with Potential Preventive and Therapeutic Effects in Female Diseases
Abstract
1. Introduction
2. Methods
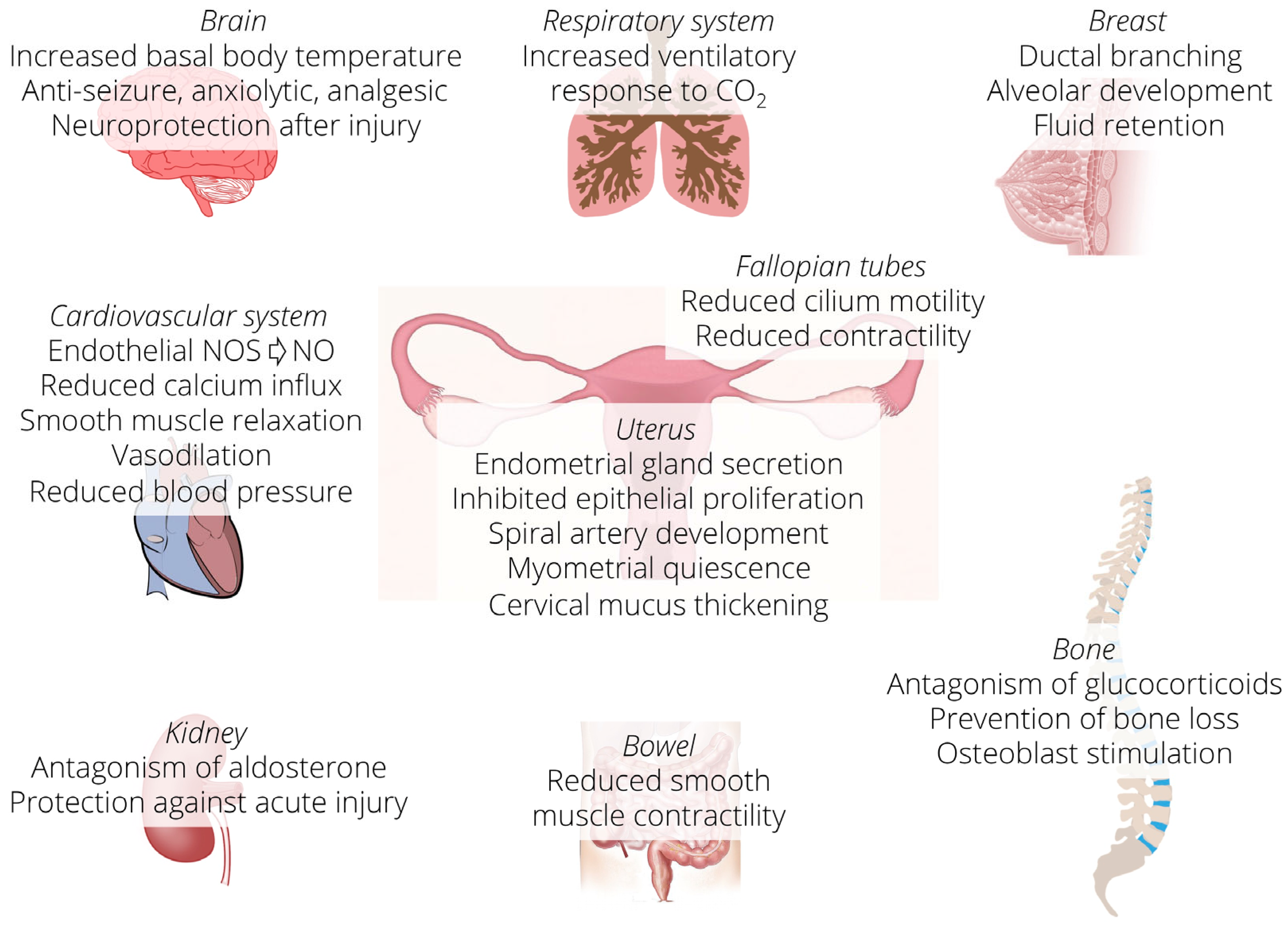
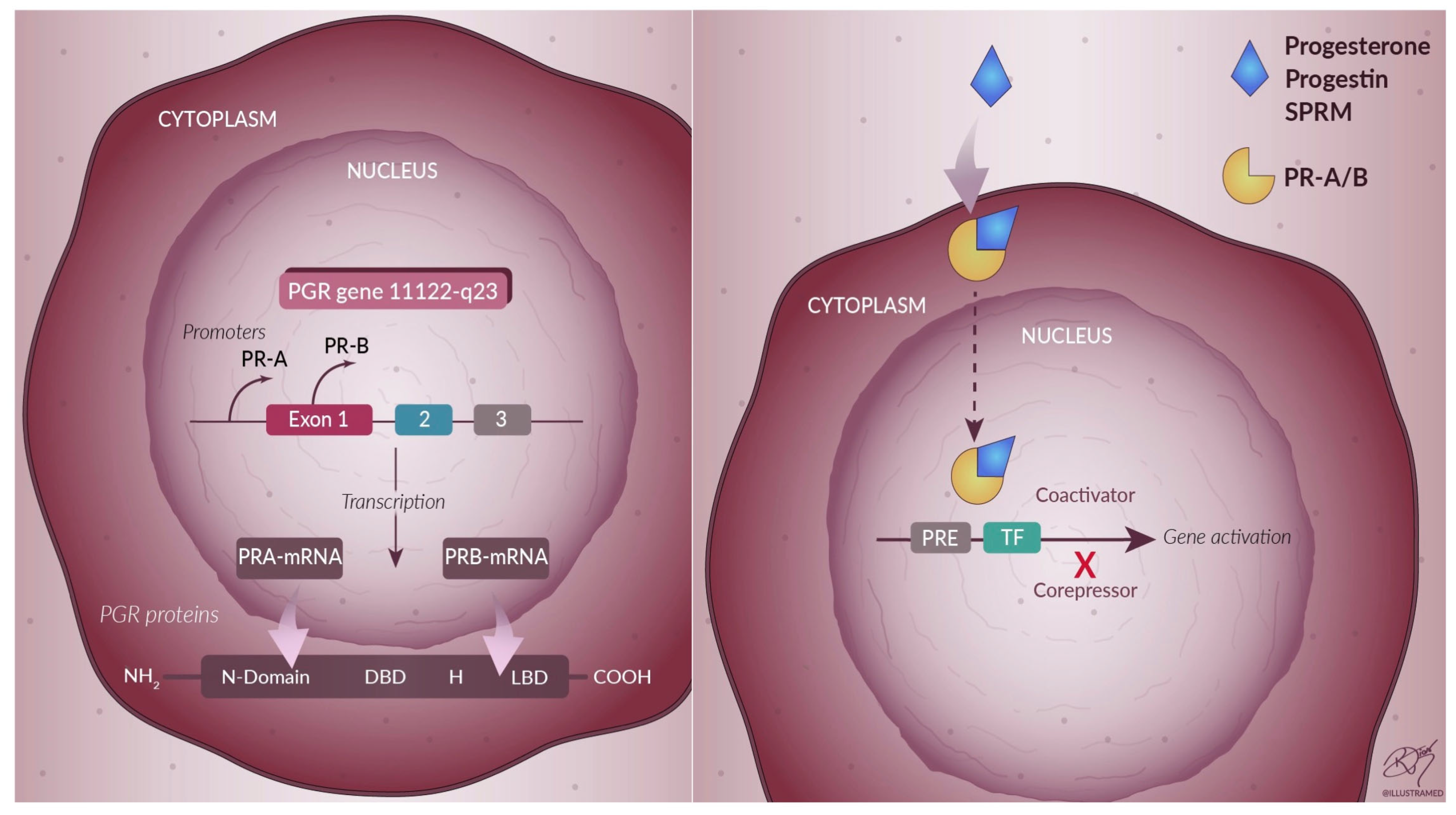
3. Progesterone
4. Progesterone-Based Drug Therapy
5. Phytoprogestins
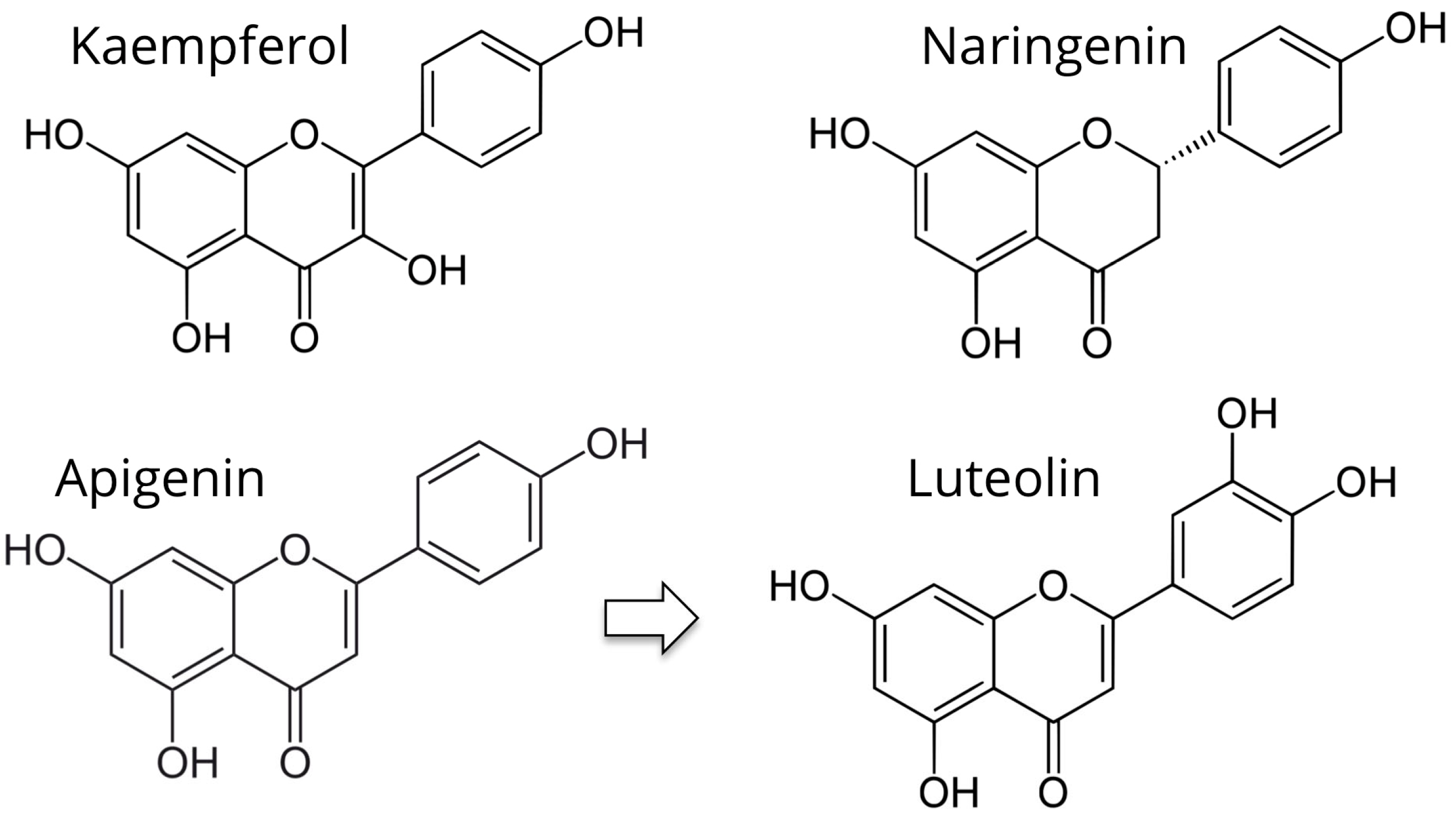
5.1. Kaempferol
5.2. Apigenin and Luteolin
5.3. Naringenin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Islam, M.S.; Greco, S.; Janjusevic, M.; Ciavattini, A.; Giannubilo, S.R.; D’Adderio, A.; Biagini, A.; Fiorini, R.; Castellucci, M.; Ciarmela, P. Growth factors and pathogenesis. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 25–36. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Bloise, E.; Ortiga-Carvalho, T.M. Hormones and pathogenesis of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Group, E.C.W. Hormones and breast cancer. Hum. Reprod. Update 2004, 10, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and Their Bioactive Phytochemicals for Women’s Health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Hajirahimkhan, A.; Dietz, B.M.; Bolton, J.L. Botanical modulation of menopausal symptoms: Mechanisms of action? Planta Med. 2013, 79, 538–553. [Google Scholar] [CrossRef]
- Zava, D.T.; Dollbaum, C.M.; Blen, M. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc. Soc. Exp. Biol. Med. 1998, 217, 369–378. [Google Scholar] [CrossRef]
- Scarpin, K.M.; Graham, J.D.; Mote, P.A.; Clarke, C.L. Progesterone action in human tissues: Regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl. Recept. Signal 2009, 7, e009. [Google Scholar] [CrossRef]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 2015, 94, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef] [PubMed]
- Piette, P.C.M. The pharmacodynamics and safety of progesterone. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 13–29. [Google Scholar] [CrossRef]
- Amadori, A.; Cavallari, C.; Giacomucci, E.; Macrelli, S.; Mastronuzzi, G.; Ucci, N. Fisiologia Della Riproduzione; CLUEB: Bologna, Italy, 1994; pp. 1–92. [Google Scholar]
- Horie, K.; Takakura, K.; Fujiwara, H.; Suginami, H.; Liao, S.; Mori, T. Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Hum. Reprod. 1992, 7, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, J.H. Progesterone receptors in the human uterus and their possible role in parturition. J. Steroid Biochem. Mol. Biol. 2005, 97, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Gokral, J.; Maitra, A.; Meherji, P.; Kadam, S.; Pires, E.; Modi, D. Altered expression of progesterone receptors in testis of infertile men. Reprod. Biomed. Online 2008, 17, 175–184. [Google Scholar] [CrossRef]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef]
- Doglioni, C.; Gambacorta, M.; Zamboni, G.; Coggi, G.; Viale, G. Immunocytochemical localization of progesterone receptors in endocrine cells of the human pancreas. Am. J. Pathol. 1990, 137, 999–1005. [Google Scholar]
- Bland, R. Steroid hormone receptor expression and action in bone. Clin. Sci. 2000, 98, 217–240. [Google Scholar] [CrossRef]
- Branchini, G.; Schneider, L.; Cericatto, R.; Capp, E.; Brum, I.S. Progesterone receptors A and B and estrogen receptor alpha expression in normal breast tissue and fibroadenomas. Endocrine 2009, 35, 459–466. [Google Scholar] [CrossRef]
- Batra, S.C.; Iosif, C.S. Progesterone receptors in the female lower urinary tract. J. Urol. 1987, 138, 1301–1304. [Google Scholar] [CrossRef]
- Lonard, D.M.; Lanz, R.B.; O'Malley, B.W. Nuclear receptor coregulators and human disease. Endocr. Rev. 2007, 28, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Faber, L.E.; Toft, D.O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J. Biol. Chem. 1990, 265, 3996–4003. [Google Scholar] [CrossRef]
- Pratt, W.B.; Galigniana, M.D.; Morishima, Y.; Murphy, P.J. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Tata, J.R. Signalling through nuclear receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, H.; Meyer, M.E.; Bocquel, M.T.; Kastner, P.; Turcotte, B.; Chambon, P. Progestin receptors: Isoforms and antihormone action. J. Steroid. Biochem. Mol. Biol. 1991, 40, 271–278. [Google Scholar] [CrossRef]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef]
- Losel, R.M.; Besong, D.; Peluso, J.J.; Wehling, M. Progesterone receptor membrane component 1--many tasks for a versatile protein. Steroids 2008, 73, 929–934. [Google Scholar] [CrossRef]
- Kowalik, M.K.; Rekawiecki, R.; Kotwica, J. The putative roles of nuclear and membrane-bound progesterone receptors in the female reproductive tract. Reprod. Biol. 2013, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.J.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744–9749. [Google Scholar] [CrossRef]
- Mulac-Jericevic, B.; Mullinax, R.A.; De Mayo, F.J.; Lydon, J.P.; Conneely, O.M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000, 289, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Mote, P.A.; Balleine, R.L.; McGowan, E.M.; Clarke, C.L. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 1999, 84, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Mote, P.A.; Bartow, S.; Tran, N.; Clarke, C.L. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res. Treat. 2002, 72, 163–172. [Google Scholar] [CrossRef]
- Graham, J.D.; Yeates, C.; Balleine, R.L.; Harvey, S.S.; Milliken, J.S.; Bilous, A.M.; Clarke, C.L. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995, 55, 5063–5068. [Google Scholar] [PubMed]
- Arnett-Mansfield, R.L.; deFazio, A.; Wain, G.V.; Jaworski, R.C.; Byth, K.; Mote, P.A.; Clarke, C.L. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001, 61, 4576–4582. [Google Scholar]
- Taylor, A.H.; McParland, P.C.; Taylor, D.J.; Bell, S.C. The cytoplasmic 60 kDa progesterone receptor isoform predominates in the human amniochorion and placenta at term. Reprod. Biol. Endocrinol. 2009, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Hawkins, P.; Baker, C.; Norris, B.; Sheridan, P.L.; Quinn, P.G. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol. Endocrinol. 1996, 10, 1379–1387. [Google Scholar] [CrossRef][Green Version]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Key, T.J.; Pike, M.C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: Its central role in explaining and predicting endometrial cancer risk. Br. J. Cancer 1988, 57, 205–212. [Google Scholar] [CrossRef]
- Siiteri, P.K. Steroid hormones and endometrial cancer. Cancer Res. 1978, 38, 4360–4366. [Google Scholar]
- Doherty, J.A.; Weiss, N.S.; Fish, S.; Fan, W.; Loomis, M.M.; Sakoda, L.C.; Rossing, M.A.; Zhao, L.P.; Chen, C. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.; Ravindernath, A.; Suzuki, N.; Terashima, I.; Sugarman, S.M.; Grollman, A.P.; Pearl, M.L. Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis 2000, 21, 1461–1467. [Google Scholar] [CrossRef]
- Gompel, A. Progesterone and endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.L.; Desautels, D.N.; Amir, E.; MacKay, H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Vierikko, P.; Kauppila, A.; Ronnberg, L.; Vihko, R. Steroidal regulation of endometriosis tissue: Lack of induction of 17 beta-hydroxysteroid dehydrogenase activity by progesterone, medroxyprogesterone acetate, or danazol. Fertil. Steril. 1985, 43, 218–224. [Google Scholar] [CrossRef]
- Brandon, D.D.; Erickson, T.E.; Keenan, E.J.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Warner, C.; Clinton, G.M. Estrogen receptor gene expression in human uterine leiomyomata. J. Clin. Endocrinol. Metab. 1995, 80, 1876–1881. [Google Scholar] [CrossRef]
- Soper, J.T.; McCarty, K.S., Jr.; Creasman, W.T.; Clarke-Pearson, D.L. Induction of cytoplasmic progesterone receptor in human endometrial carcinoma transplanted into nude mice. Am. J. Obstet. Gynecol. 1984, 150, 437–439. [Google Scholar] [CrossRef]
- Murji, A.; Biberoglu, K.; Leng, J.; Mueller, M.D.; Romer, T.; Vignali, M.; Yarmolinskaya, M. Use of dienogest in endometriosis: A narrative literature review and expert commentary. Curr. Med. Res. Opin. 2020, 36, 895–907. [Google Scholar] [CrossRef]
- Goodman, A.L. Progesterone therapy in uterine fibromyoma. J. Clin. Endocrinol. Metab. 1946, 6, 402–408. [Google Scholar] [CrossRef]
- Lumbiganon, P.; Rugpao, S.; Phandhu-fung, S.; Laopaiboon, M.; Vudhikamraksa, N.; Werawatakul, Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: A multicentre case-control study. BJOG Int. J. Obstet. Gynaecol. 1996, 103, 909–914. [Google Scholar] [CrossRef]
- Schindler, A.E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J.R.; Schweppe, K.W.; Thijssen, J.H. Classification and pharmacology of progestins. Maturitas 2008, 61, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ichigo, S.; Takagi, H.; Matsunami, K.; Suzuki, N.; Imai, A. Beneficial effects of dienogest on uterine myoma volume: A retrospective controlled study comparing with gonadotropin-releasing hormone agonist. Arch. Gynecol. Obstet. 2011, 284, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Rott, H. Thrombotic risks of oral contraceptives. Curr. Opin. Obstet. Gynecol. 2012, 24, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Combined hormonal contraception and the risk of venous thromboembolism: A guideline. Fertil. Steril. 2017, 107, 43–51. [Google Scholar] [CrossRef]
- DeMarzo, A.M.; Beck, C.A.; Onate, S.A.; Edwards, D.P. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc. Natl. Acad. Sci. USA 1991, 88, 72–76. [Google Scholar] [CrossRef]
- Smith, C.L.; O'Malley, B.W. Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004, 25, 45–71. [Google Scholar] [CrossRef]
- Bouchard, P.; Chabbert-Buffet, N.; Fauser, B.C. Selective progesterone receptor modulators in reproductive medicine: Pharmacology, clinical efficacy and safety. Fertil. Steril. 2011, 96, 1175–1189. [Google Scholar] [CrossRef]
- Wilkens, J.; Male, V.; Ghazal, P.; Forster, T.; Gibson, D.A.; Williams, A.R.; Brito-Mutunayagam, S.L.; Craigon, M.; Lourenco, P.; Cameron, I.T.; et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J. Immunol. 2013, 191, 2226–2235. [Google Scholar] [CrossRef]
- Donnez, J. Uterine Fibroids and Progestogen Treatment: Lack of Evidence of Its Efficacy: A Review. J. Clin. Med. 2020, 9, 3948. [Google Scholar] [CrossRef]
- Bressler, L.H.; Bernardi, L.A.; Snyder, M.A.; Wei, J.J.; Bulun, S. Treatment of endometriosis-related chronic pelvic pain with Ulipristal Acetate and associated endometrial changes. HSOA J. Reprod. Med. Gynaecol. Obstet. 2017, 2. [Google Scholar] [CrossRef]
- Lee, O.; Sullivan, M.E.; Xu, Y.; Rogers, C.; Muzzio, M.; Helenowski, I.; Shidfar, A.; Zeng, Z.; Singhal, H.; Jovanovic, B.; et al. Selective Progesterone Receptor Modulators in Early-Stage Breast Cancer: A Randomized, Placebo-Controlled Phase II Window-of-Opportunity Trial Using Telapristone Acetate. Clin. Cancer Res. 2020, 26, 25–34. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Pharmacokinetics, toxicological and clinical aspects of ulipristal acetate: Insights into the mechanisms implicated in the hepatic toxicity. Drug Metab. Rev. 2021, 1–9. [Google Scholar] [CrossRef]
- Islam, M.S.; Afrin, S.; Jones, S.I.; Segars, J. Selective Progesterone Receptor Modulators-Mechanisms and Therapeutic Utility. Endocr. Rev. 2020, 41, 643–694. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Feng, X.L.; Zhan, X.X.; Zuo, L.S.; Mo, X.F.; Zhang, X.; Liu, K.Y.; Li, L.; Zhang, C.X. Associations between serum concentration of flavonoids and breast cancer risk among Chinese women. Eur. J. Nutr. 2021, 60, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kupeli, E.; Terzioglu, S.; Yesilada, E. Bioassay-guided isolation of kaempferol-3-O-beta-D-galactoside with anti-inflammatory and antinociceptive activity from the aerial part of Calluna vulgaris L. J. Ethnopharmacol. 2007, 114, 32–37. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, E.K.; Heo, H.S.; Kim, M.S.; Sung, B.; Kim, M.K.; Lee, J.; Kim, N.D.; Anton, S.; Choi, J.S.; et al. The anti-inflammatory effect of kaempferol in aged kidney tissues: The involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinase pathways. J. Med. Food 2009, 12, 351–358. [Google Scholar] [CrossRef]
- Chuwa, A.H.; Sone, K.; Oda, K.; Tanikawa, M.; Kukita, A.; Kojima, M.; Oki, S.; Fukuda, T.; Takeuchi, M.; Miyasaka, A.; et al. Kaempferol, a natural dietary flavonoid, suppresses 17beta-estradiol-induced survivin expression and causes apoptotic cell death in endometrial cancer. Oncol. Lett. 2018, 16, 6195–6201. [Google Scholar] [CrossRef]
- Hu, G.; Liu, H.; Wang, M.; Peng, W. IQ Motif Containing GTPase-Activating Protein 3 (IQGAP3) Inhibits Kaempferol-Induced Apoptosis in Breast Cancer Cells by Extracellular Signal-Regulated Kinases 1/2 (ERK1/2) Signaling Activation. Med. Sci. Monit. 2019, 25, 7666–7674. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Z.; Wu, C. Mechanistic Study of the Inhibitory Effect of Kaempferol on Uterine Fibroids In Vitro. Med. Sci. Monit. 2016, 22, 4803–4808. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Ther. 2006, 5, 945–951. [Google Scholar] [CrossRef]
- Mafuvadze, B.; Benakanakere, I.; Hyder, S.M. Apigenin blocks induction of vascular endothelial growth factor mRNA and protein in progestin-treated human breast cancer cells. Menopause 2010, 17, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Goyette, S.; Mafuvadze, B.; Hyder, S.M. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus 2015, 4, 444. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer 2017, 9, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the NO-cGMP-PKG-KATP Channel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yoon, S.; Moon, J.O. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol. Pharm. Bull. 2004, 27, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Mafuvadze, B.; Benakanakere, I.; Lopez Perez, F.R.; Besch-Williford, C.; Ellersieck, M.R.; Hyder, S.M. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev. Res. 2011, 4, 1316–1324. [Google Scholar] [CrossRef]
- Mafuvadze, B.; Liang, Y.; Besch-Williford, C.; Zhang, X.; Hyder, S.M. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm. Cancer 2012, 3, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, K.B.; Sartorius, C.A. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: A hypothesis. J. Clin. Endocrinol. Metab. 2008, 93, 3295–3298. [Google Scholar] [CrossRef]
- Yin, F.; Giuliano, A.E.; Law, R.E.; Van Herle, A.J. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001, 21, 413–420. [Google Scholar] [PubMed]
- Lee, H.H.; Jung, J.; Moon, A.; Kang, H.; Cho, H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma through Suppression of IL-6 Expression. Int. J. Mol. Sci. 2019, 20, 3143. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.M. Sex-steroid regulation of vascular endothelial growth factor in breast cancer. Endocr. Relat. Cancer 2006, 13, 667–687. [Google Scholar] [CrossRef]
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann. Nutr. Metab. 2006, 50, 167–172. [Google Scholar] [CrossRef]
- Chen, D.; Landis-Piwowar, K.R.; Chen, M.S.; Dou, Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007, 9, R80. [Google Scholar] [CrossRef]
- Dean, M.; Austin, J.; Jinhong, R.; Johnson, M.E.; Lantvit, D.D.; Burdette, J.E. The Flavonoid Apigenin Is a Progesterone Receptor Modulator with In Vivo Activity in the Uterus. Horm. Cancer 2018, 9, 265–277. [Google Scholar] [CrossRef]
- Fidelis, Q.C.; Faraone, I.; Russo, D.; Aragao Catunda, F.E., Jr.; Vignola, L.; de Carvalho, M.G.; de Tommasi, N.; Milella, L. Chemical and Biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2019, 33, 1500–1503. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J. Nutr. Biochem. 2019, 67, 161–172. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; van der Hooft, J.; Clifford, M.N.; Del Rio, D.; Lean, M.E.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange juice (poly)phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A., Jr. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Hu, H.; Tang, N.; Zhang, C.; Liang, W.; Wang, M. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-beta1 in cultured rat hepatic stellate cells. Pharm. Res. 2006, 23, 82–89. [Google Scholar] [CrossRef]
- Rosenberg, R.S.; Grass, L.; Jenkins, D.J.; Kendall, C.W.; Diamandis, E.P. Modulation of androgen and progesterone receptors by phytochemicals in breast cancer cell lines. Biochem. Biophys. Res. Commun. 1998, 248, 935–939. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef] [PubMed]
| Substance | Study Design | Effects | Significance | References |
|---|---|---|---|---|
| Kaempferol | Experiments in mice and rats | Anti-inflammatory | Could be useful to treat chronic pelvic pain and its causes | [69,70] |
| In vitro culture of human neutrophils | Antioxidant | Another potential therapeutic mechanism to treat endometriosis | [68] | |
| In vitro culture of endometrial cancer cells | Growth inhibition and apoptosis | Could be effective against endometrial hyperplasia and cancer | [71] | |
| Apigenin | In vitro culture of human cancer cell lines | Growth inhibition and apoptosis VEGF inhibition | Could be effective against endometrial hyperplasia and cancer | [74,75] |
| Luteolin | Human breast tumor xenografts in nude mice | Inhibition of tumor growth and angiogenesis | Could be an adjuvant therapy of breast cancer | [76,77] |
| Naringenin | Mouse model in vivo | Analgesic, anti-inflammatory and antioxidant | Could be useful to treat chronic pelvic pain and its causes | [78] |
| Rat model of hepatic injury in vivo | Antifibrotic | Could be effective to treat uterine fibroids | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, S.; Pellegrino, P.; Zannotti, A.; Delli Carpini, G.; Ciavattini, A.; Reis, F.M.; Ciarmela, P. Phytoprogestins: Unexplored Food Compounds with Potential Preventive and Therapeutic Effects in Female Diseases. Nutrients 2021, 13, 4326. https://doi.org/10.3390/nu13124326
Greco S, Pellegrino P, Zannotti A, Delli Carpini G, Ciavattini A, Reis FM, Ciarmela P. Phytoprogestins: Unexplored Food Compounds with Potential Preventive and Therapeutic Effects in Female Diseases. Nutrients. 2021; 13(12):4326. https://doi.org/10.3390/nu13124326
Chicago/Turabian StyleGreco, Stefania, Pamela Pellegrino, Alessandro Zannotti, Giovanni Delli Carpini, Andrea Ciavattini, Fernando M. Reis, and Pasquapina Ciarmela. 2021. "Phytoprogestins: Unexplored Food Compounds with Potential Preventive and Therapeutic Effects in Female Diseases" Nutrients 13, no. 12: 4326. https://doi.org/10.3390/nu13124326
APA StyleGreco, S., Pellegrino, P., Zannotti, A., Delli Carpini, G., Ciavattini, A., Reis, F. M., & Ciarmela, P. (2021). Phytoprogestins: Unexplored Food Compounds with Potential Preventive and Therapeutic Effects in Female Diseases. Nutrients, 13(12), 4326. https://doi.org/10.3390/nu13124326








