Pesticide Toxicity Footprints of Australian Dietary Choices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Intake Data
2.2. Diet Quality Analysis
2.3. Pesticide Toxicity Footprint Modelling
2.4. Dietary Patten Model
2.5. Correlation Analysis
3. Results
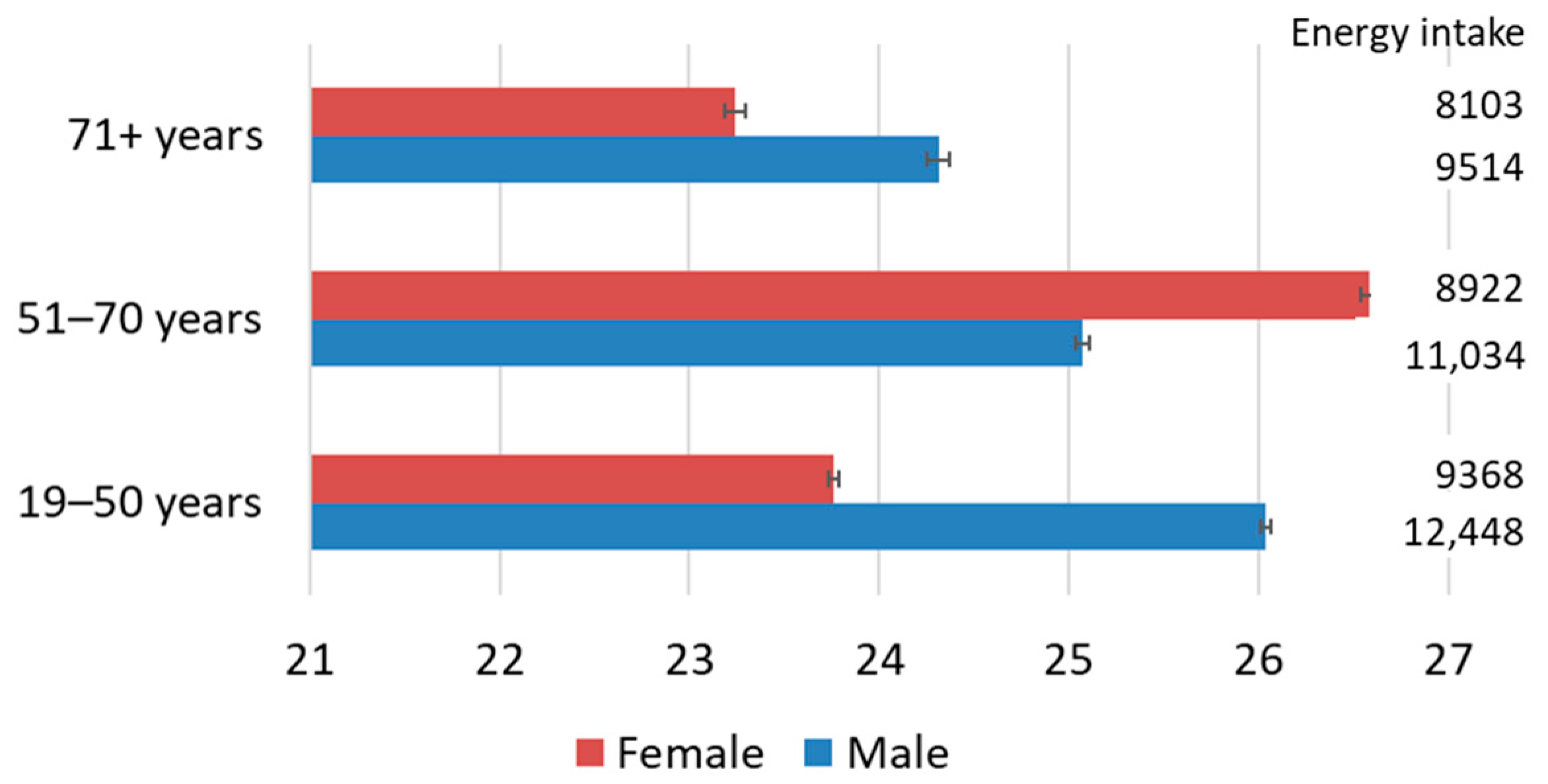
3.1. Pesticide Toxicity Footprint and Energy Intake
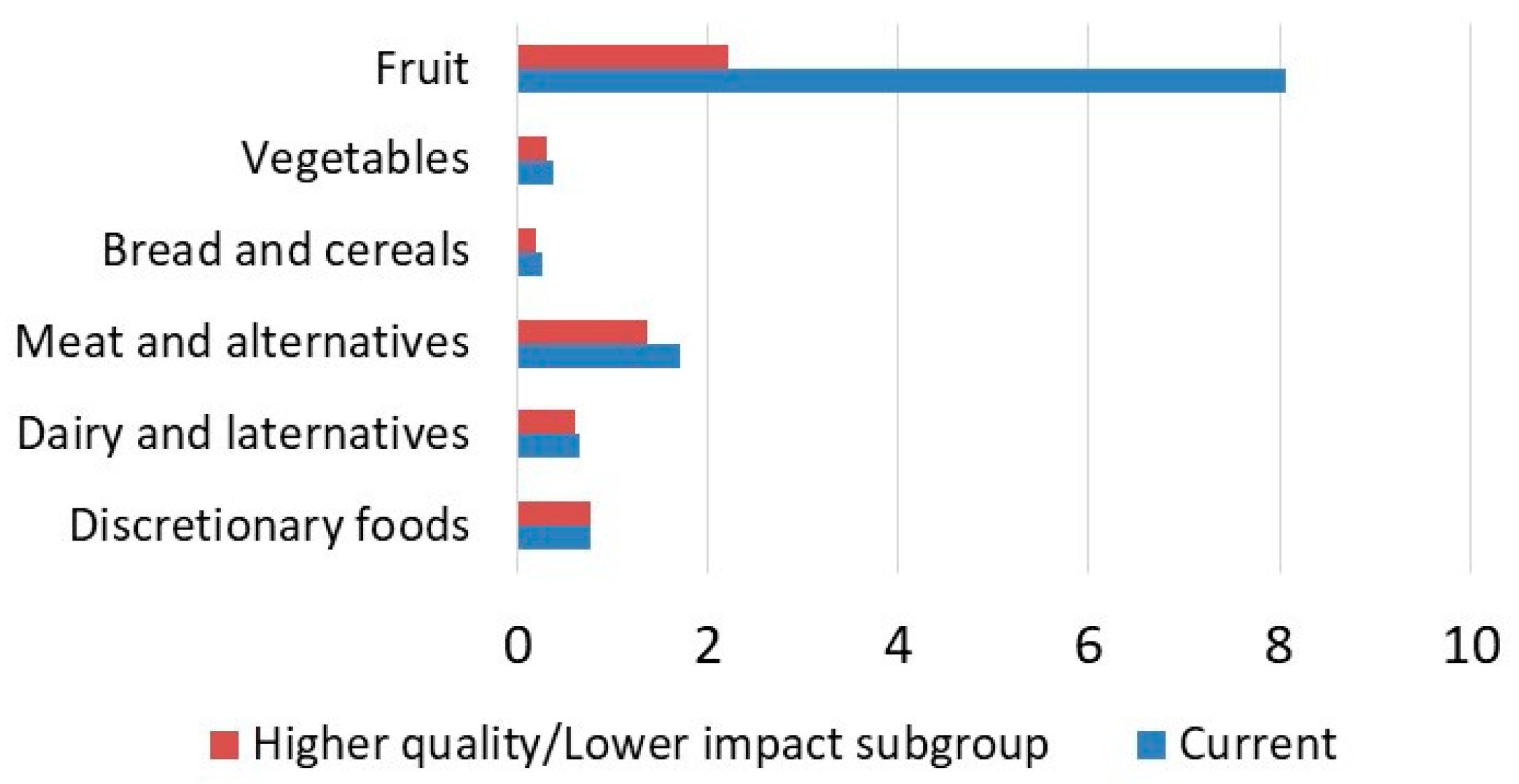
3.2. Contribution Analysis
3.3. Dietary Pattern Analysis
4. Discussion
4.1. Comparison with Other Studies
4.2. Role of Discretionary Foods
4.3. Role of Fruits
4.4. Role of Protein Foods
4.5. Implications for Other Environmental Aspects
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA. Dietary Guidelines for Americans 2020–2025; US Department of Agriculture: Washington, DC, USA, 2020.
- Australia Government. Australian Dietary Guidelines Summary; National Health and Medical Research Council: Canberra, Australia, 2013.
- Fischer, C.G.; Garnett, T. Plates, Pyramids, Planet, Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment; University of Oxford: Oxford, UK, 2016. [Google Scholar]
- Swedish National Food Agency. The Swedish Dietary Guidelines; Swedish National Food Agency: Uppsala, Sweden, 2015.
- Public Health England. The Eatwell Guide; Public Health England: London, UK, 2018.
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012, Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Drewnowski, A.; Finley, J.; Hess, J.M. Towards healthy diets from sustainable food systems. Curr. Dev. Nutr. 2020, 4, nzaa083. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations General Assembly: New York, NY, USA, 2015. [Google Scholar]
- Ridoutt, B.G.; Hendrie, G.A.; Noakes, M. Dietary strategies to reduce environmental impact: A critical review of the evidence base. Adv. Nutr. 2017, 8, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.D.; Hoey, L.; Blesh, J.; Miller, L.; Green, A.; Shapiro, L.F. A systematic review of the measurement of sustainable diets. Adv. Nutr. 2016, 7, 641–664. [Google Scholar] [CrossRef] [Green Version]
- Ridoutt, B.G.; Baird, D.; Anastasiou, K.; Hendrie, G.A. Diet quality and water scarcity: Evidence from a large Australian population health survey. Nutrients 2019, 11, 1846. [Google Scholar] [CrossRef] [Green Version]
- Ridoutt, B.; Anastasiou, K.; Baird, D.; Navarro Garcia, J.; Hendrie, G. Cropland footprints of Australian dietary choices. Nutrients 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ridoutt, B.G.; Huang, J. Environmental relevance—The key to understanding water footprints. Proc. Natl. Acad. Sci. USA 2012, 109, E1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, A.; Brookes, T.M. Land use intensity-specific global characterization factors to assess product biodiversity footprints. Environ. Sci. Technol. 2018, 52, 5094–5104. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.J. Global pesticide use: Profile, trend, cost / benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Choudri, B.S.; Charabi, Y.; Al-Nasiri, N.; Al-Awadhi, T. Pesticides and herbicides. Water Environ. Res. 2020, 92, 1425–1432. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Sánchez-Bayo, F.; van Lexmond, M.B. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Environ. Sci. Pollut. Res. 2021, 28, 11709–11715. [Google Scholar] [CrossRef] [PubMed]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, S.; Henry, M.; Stout, J.C.; White, B. Neonicotinoid residues in honey from urban and rural environments. Environ. Sci. Pollut. Res. 2021, 28, 28179–28190. [Google Scholar] [CrossRef]
- McKnight, U.S.; Rasmussen, J.J.; Kronvang, B.; Binning, P.J.; Bjerg, P.L. Sources, occurrence and predicted aquatic impact of legacy and contemporary pesticides in streams. Environ. Pollut. 2015, 200, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- European Commission, Farm to Fork Targets—Progress. Available online: https://ec.europa.eu/food/plants/pesticides/sustainable-use-pesticides/farm-fork-targets-progress_en (accessed on 1 October 2021).
- Food and Agriculture Organization of the United Nations, Q&A on Pests and Pesticide Management. Available online: http://www.fao.org/news/story/en/item/1398779/icode/ (accessed on 1 October 2021).
- Pesticide Action Network, Pesticides 101. Available online: https://www.panna.org/pesticides-big-picture/pesticides-101 (accessed on 1 October 2021).
- Australian Bureau of Statistics. 4363.0—National Health Survey: Users’ Guide, 2014–2015; Australian Bureau of Statistics: Canberra, Australia, 2017.
- Ridoutt, B.; Baird, D.; Hendrie, G.A. Diets within environmental limits: The climate impact of current and recommended Australian diets. Nutrients 2021, 13, 1122. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. 4364.0.55.007—Australian Health Survey: Nutrition First Results—Foods and Nutrients, 2011–2012; Australian Bureau of Statistics: Canberra, Australia, 2014.
- Food Standards Australia New Zealand. Australian Food Composition Database. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd/Pages/default.aspx (accessed on 1 October 2020).
- Macdiarmid, J.L.; Douglas, F.; Campbell, J. Eating like there’s no tomorrow: Public awareness of the environmental impact of food and reluctance to eat less meat as part of a sustainable diet. Appetite 2016, 96, 487–493. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Scott, S.E.; Duarte, C. Responding to food, environment and health challenges by changing meat consumption behaviours in consumers. Nutr. Bull. 2018, 43, 125–134. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Lal, A.; Peeters, A.; Brown, V.; Nguyen, P.; Tran, H.N.Q.; Nguyen, T.; Tonmukayakul, U.; Sacks, G.; Calache, H.; Martin, J.; et al. The modelled population obesity-related health benefits of reducing consumption of discretionary foods in Australia. Nutrients 2020, 12, 649. [Google Scholar] [CrossRef] [Green Version]
- Fayet-Moore, F.; McConnell, A.; Cassettari, T.; Petocz, P. Breakfast choice is associated with nutrient, food group and discretionary intakes in Australian adults at both breakfast and the rest of the day. Nutrients 2019, 11, 175. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Rangan, A.; Meertens, B.; Wu, J.H.Y. Changes in typical portion sizes of commonly consumed discretionary foods among Australian adults from 1995 to 2011–2012. Nutrients 2017, 9, 577. [Google Scholar] [CrossRef] [Green Version]
- Golley, R.K.; Hendrie, G.A. The Dietary Guidelines Index for children and adolescents: What is the impact of the new dietary guidelines? Nutr. Diet. 2014, 71, 210–212. [Google Scholar] [CrossRef]
- Navarro, J.; Hadjikakou, M.; Ridoutt, B.; Parry, H.; Bryan, B.A. Pesticide toxicity hazard of agriculture: Regional and commodity hotspots in Australia. Environ. Sci. Technol. 2021, 55, 1290–1300. [Google Scholar] [CrossRef]
- Rosenbaum, R.K.; Bachmann, T.; Gold, L.S.; Huijbregts, M.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox–the UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Life Cycle Initiative. Available online: https://www.lifecycleinitiative.org/ (accessed on 5 October 2021).
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef]
- Ridoutt, B.; Baird, D.; Bastiaans, K.; Darnell, R.; Hendrie, G.; Riley, M.; Sanguansri, P.; Syrette, J.; Noakes, M.; Keating, B. Australia’s nutritional food balance: Situation, outlook and policy implications. Food Sec. 2017, 9, 211–226. [Google Scholar] [CrossRef]
- The Prime Minister’s Science, Engineering and Innovation Council. Australia and Food Security in a Changing World; The Prime Minister’s Science, Engineering and Innovation Council: Canberra, Australia, 2010. [Google Scholar]
- Crenna, E.; Secchi, M.; Benini, L.; Sala, S. Global environmental impacts: Data sources and methodological choices for calculating normalization factors for LCA. Int. J. Life Cycle Assess. 2019, 24, 1851–1877. [Google Scholar] [CrossRef] [Green Version]
- Sala, S.; Benini, L.; Mancini, L.; Pant, R. Integrated assessment of environmental impact of Europe in 2010: Data sources and extrapolation strategies for calculating normalisation factors. Int. J. Life Cycle Assess. 2015, 20, 1568–1585. [Google Scholar] [CrossRef] [Green Version]
- Castellani, V.; Sala, S.; Benini, L. Hotspots analysis and critical interpretation of food life cycle assessment studies for selecting eco-innovation options and for policy support. J. Clean. Prod. 2017, 140, 556–568. [Google Scholar] [CrossRef]
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst. Environ. 2002, 93, 379–392. [Google Scholar] [CrossRef]
- Tidåker, P.; Potter, H.K.; Carlsson, G.; Röös, E. Towards sustainable consumption of legumes: How origin, processing and transport affect the environmental impact of pulses. Sustain. Prod. Consum. 2021, 27, 496–508. [Google Scholar] [CrossRef]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Castellani, V.; Sala, S. Environmental impacts of food consumption in Europe. J. Clean. Prod. 2017, 140, 753–765. [Google Scholar] [CrossRef]
- Moberg, E.; Säll, S.; Hansson, P.-A.; Röös, E. Taxing food consumption to reduce environmental impacts—Identification of synergies and goal conflicts. Food Policy 2021, 101, 102090. [Google Scholar] [CrossRef]
- Beylot, A.; Secchi, M.; Cerutti, A.; Merciai, S.; Schmidt, J.; Sala, S. Assessing the environmental impacts of EU consumption at macro-scale. J. Clean. Prod. 2019, 216, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Marlow, H.J.; Harwatt, H.; Soret, S.; Sabaté, J. Comparing the water, energy, pesticide and fertilizer usage for the production of foods consumed by different dietary types in California. Public Health Nutr. 2015, 18, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Soheilifard, F.; Marzban, A.; Raini, M.G.; Taki, M.; van Zelm, R. Chemical footprint of pesticides used in citrus orchards based on canopy deposition and off-target losses. Sci. Total Environ. 2020, 732, 139118. [Google Scholar] [CrossRef]
- Soode-Schimonsky, E.; Richter, K.; Weber-Blaschke, G. Product environmental footprint of strawberries: Case studies in Estonia and Germany. J. Environ. Manag. 2017, 203, 564–577. [Google Scholar] [CrossRef]
- Romero-Gámez, M.; Suárez-Rey, E.M. Environmental footprint of cultivating strawberry in Spain. Int. J. Life Cycle Assess. 2020, 25, 719–732. [Google Scholar] [CrossRef]
- Tassielli, G.; Notarnicola, B.; Renzulli, P.A.; Arcese, G. Environmental life cycle assessment of fresh and processed sweet cherries in southern Italy. J. Clean. Prod. 2018, 171, 184–197. [Google Scholar] [CrossRef]
- Ingwersen, W.W. Life cycle assessment of fresh pineapple from Costa Rica. J. Clean. Prod. 2012, 35, 152–163. [Google Scholar] [CrossRef]
- Peña, N.; Knudsen, M.T.; Fantke, P.; Antón, A.; Hermansen, J.E. Freshwater ecotoxicity assessment of pesticide use in crop production: Testing the influence of modeling choices. J. Clean. Prod. 2019, 209, 1332–1341. [Google Scholar] [CrossRef]
- Hallström, E.; Håkansson, N.; Åkesson, A.; Wolk, A.; Sonesson, U. Climate impact of alcohol consumption in Sweden. J. Clean. Prod. 2018, 201, 287–294. [Google Scholar] [CrossRef]
- Perignon, M.; Sinfort, C.; El Ati, J.; Traissac, P.; Drogué, S.; Darmon, N.; Amiot, M.J.; Achir, N.; Alouane, L.; Bellagha, S.; et al. How to meet nutritional recommendations and reduce diet environmental impact in the Mediterranean region? An optimization study to identify more sustainable diets in Tunisia. Glob. Food Sec. 2019, 23, 227–235. [Google Scholar] [CrossRef]
- Dogbe, W.; Revoredo-Giha, C. Nutritional and environmental assessment of increasing the content of fruit and vegetables in the UK diet. Sustainability 2021, 13, 1076. [Google Scholar] [CrossRef]
- Goldstein, B.; Hansen, S.F.; Gjerris, M.; Laurent, A.; Birkved, M. Ethical aspects of life cycle assessments of diets. Food Policy 2016, 59, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Colombo, P.E.; Milner, J.; Scheelbeek, P.F.D.; Taylor, A.; Parlesak, A.; Kastner, T.; Nicholas, O.; Elinder, L.S.; Dangour, A.D.; Green, R. Pathways to “5-a-day”: Modeling the health impacts and environmental footprints of meeting the target for fruit and vegetable intake in the United Kingdom. Am. J. Clin. Nutr. 2021, 114, 530–539. [Google Scholar]
- Garnett, T. Plating up solutions. Science 2016, 353, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrowicz, L.; Green, R.; Joy, E.J.M.; Smith, P.; Haines, A. The impacts of dietary change on greenhouse gas emissions, land use, water use, and health: A systematic review. PLoS ONE 2016, 11, e0165797. [Google Scholar] [CrossRef] [Green Version]
- Nordborg, M.; Davis, J.; Cederberg, C.; Woodhouse, A. Freshwater ecotoxicity impacts from pesticide use in animal and vegetable foods produced in Sweden. Sci. Total Environ. 2017, 581–582, 448–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridoutt, B.; Navarro Garcia, J. Cropland footprints from the perspective of productive land scarcity, malnutrition-related health impacts and biodiversity loss. J. Clean. Prod. 2020, 260, 121150. [Google Scholar] [CrossRef]
- Ridoutt, B.G.; Baird, D.; Hendrie, G.A. Diets within planetary boundaries: What is the potential of dietary change alone? Sustain. Prod. Consum. 2021, 28, 802–810. [Google Scholar] [CrossRef]
- Fantke, P.; Chiu, W.A.; Aylward, L.; Judson, R.; Huang, L.; Jang, S.; Gouin, T.; Rhomberg, L.; Aurisano, N.; McKone, T.; et al. Exposure and toxicity characterization of chemical emissions and chemicals in products: Global recommendations and implementation in USEtox. Int. J. Life Cycle Assess. 2021, 26, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Ernstoff, A.; Niero, M.; Muncke, J.; Trier, X.; Rosenbaum, R.K.; Hauschild, M.; Fantke, P. Challenges of including human exposure to chemicals in food packaging as a new exposure pathway in life cycle impact assessment. Int. J. Life Cycle Assess. 2019, 24, 543–552. [Google Scholar] [CrossRef] [Green Version]

| Food | FE | HT-c | HT-nc | PTF |
|---|---|---|---|---|
| Fruit | 29.2 | 9.3 | 19.6 | 28.3 |
| Vegetables | 5.5 | 8.6 | 4.8 | 5.5 |
| Breads and cereals | 6.0 | 11.6 | 12.9 | 6.6 |
| Meat and alternatives | 17.6 | 29.6 | 21.8 | 18.3 |
| Seafood | (0.3) | (0.9) | (0.5) | (0.3) |
| Red Meat | (5.2) | (9.6) | (6.7) | (5.4) |
| Poultry | (6.3) | (11.2) | (8.1) | (6.6) |
| Vegetarian alternatives (eggs, etc.) | (4.4) | (5.2) | (4.3) | (4.4) |
| Reptiles, offal | (<0.1) | (<0.1) | (<0.1) | (<0.1) |
| Pork | (1.4) | (2.5) | (2.2) | (1.5) |
| Dairy and alternatives | 5.6 | 10.2 | 5.3 | 5.7 |
| Beverages | 3.2 | 0.6 | 0.3 | 2.9 |
| Discretionary foods | 29.8 | 28.2 | 33.4 | 29.7 |
| Sugar-sweetened beverages | (2.4) | (0.3) | (1.4) | (2.2) |
| Savory and sweet biscuits, cakes, and waffles | (2.9) | (2.9) | (2.9) | (2.9) |
| Sweet and savory pastries, pies | (1.4) | (2.5) | (1.7) | (1.5) |
| Processed meat, burgers, tacos, pizza, and hot dogs | (6.7) | (12.1) | (9.6) | (7.1) |
| Dairy based desserts, cream, and butter | (1.4) | (2.0) | (1.1) | (1.4) |
| Fried potato products and extruded snacks | (2.0) | (2.4) | (1.8) | (2.0) |
| Muesli bars, confectionary, and chocolate | (4.0) | (1.5) | (2.7) | (3.8) |
| Alcohol | (7.2) | (2.5) | (10.5) | (7.2) |
| Other | (1.8) | (2.0) | (1.6) | (1.8) |
| Healthy fats and oils | 2.7 | 1.1 | 1.6 | 2.6 |
| Miscellaneous | 0.4 | 0.6 | 0.4 | 0.4 |
| Food | Current | Higher Diet Quality and Lower Impact Subgroup | ||
|---|---|---|---|---|
| Servings | PTF | Servings | PTF | |
| Fruit | 1.38 | 11.14 | 1.22 | 2.72 |
| Vegetables | 2.47 | 0.92 | 3.61 | 1.09 |
| Breads and cereals | 4.57 | 1.17 | 5.98 | 1.09 |
| Meat and alternatives | 2.32 | 3.98 | 2.55 | 3.45 |
| Seafood | (0.22) | (0.06) | (0.32) | (0.06) |
| Red Meat | (0.66) | (0.87) | (0.76) | (0.99) |
| Poultry | (0.74) | (1.44) | (0.78) | (1.47) |
| Vegetarian alternatives | (0.51) | (1.29) | (0.49) | (0.60) |
| Reptiles, offal | (<0.01) | (<0.01) | (<0.01) | (<0.01) |
| Pork | (0.18) | (0.32) | (0.19) | (0.33) |
| Dairy and alternatives | 1.46 | 0.97 | 1.50 | 0.89 |
| Discretionary foods | 7.42 | 5.66 | 2.31 | 1.76 |
| Miscellaneous | 1.08 | 0.73 | ||
| Total | 24.93 | 11.74 | ||
| Food | Recommended Diet Based on Average Food Choices | Recommended Diet Based on Lower Pesticide Toxicity Footprint Food Choices | ||
|---|---|---|---|---|
| Servings | PTF | Servings | PTF | |
| Fruit | 2.0 | 16.12 | 2.0 | 4.44 |
| Vegetables | 5.5 | 2.05 | 5.5 | 1.66 |
| Breads and cereals | 6.0 | 1.54 | 6.0 | 1.10 |
| Meat and alternatives | 2.8 | 4.81 | 2.8 | 3.79 |
| Seafood | (0.27) | (0.07) | (0.35) | (0.07) |
| Red Meat | (0.79) | (1.05) | (0.83) | (1.09) |
| Poultry | (0.90) | (1.74) | (0.86) | (1.62) |
| Vegetarian alternatives | (0.61) | (1.56) | (0.54) | (0.66) |
| Reptiles, offal | (<0.01) | (<0.01) | (<0.01) | (<0.01)) |
| Pork | (0.22) | (0.38) | (0.21) | (0.36) |
| Dairy and alternatives | 2.5 | 1.66 | 2.5 | 1.49 |
| Discretionary foods | 2.8 | 2.14 | 2.8 | 2.14 |
| Miscellaneous | 1.08 | 0.73 | ||
| Total | 29.40 | 15.35 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridoutt, B.; Baird, D.; Navarro, J.; Hendrie, G.A. Pesticide Toxicity Footprints of Australian Dietary Choices. Nutrients 2021, 13, 4314. https://doi.org/10.3390/nu13124314
Ridoutt B, Baird D, Navarro J, Hendrie GA. Pesticide Toxicity Footprints of Australian Dietary Choices. Nutrients. 2021; 13(12):4314. https://doi.org/10.3390/nu13124314
Chicago/Turabian StyleRidoutt, Bradley, Danielle Baird, Javier Navarro, and Gilly A. Hendrie. 2021. "Pesticide Toxicity Footprints of Australian Dietary Choices" Nutrients 13, no. 12: 4314. https://doi.org/10.3390/nu13124314
APA StyleRidoutt, B., Baird, D., Navarro, J., & Hendrie, G. A. (2021). Pesticide Toxicity Footprints of Australian Dietary Choices. Nutrients, 13(12), 4314. https://doi.org/10.3390/nu13124314







