Beneficial Properties of Bromelain
Abstract
:1. Introduction
2. Methods
3. Biochemical Characterization
4. Bromelain in Cardiovascular Diseases
4.1. Ischemia/Reperfusion
4.2. Blood Coagulation and Fibrinolysis
5. Antimicrobial Activity
5.1. Diarrhea
5.2. Bromelain in Antibiotic Therapy
5.3. Anthelmintic Efficacy
5.4. Antifungal Properties
5.5. Antibacterial Properties
6. Immunomodulatory Effect of Bromelain
6.1. Lipopolysaccharide Stimulation
6.2. Chronic Inflammatory Bowel Disease
6.3. Modulation of Leukocyte Adhesion and Activation
6.4. Bromelain as an Immunostimulatory Factor
| Target | Experimental Approach | Effect | References |
|---|---|---|---|
| PBMC human peripheral blood mononuclear cells; THP-1 monocytic leukemia cells; U937 human macrophages cells | In vitro bromelain treatment + lipopolysaccharide (LPS) | Anti-inflammatory activity: TNF-α ↓, IL-1β ↓, IL-6 ↓, IL-8 ↓, NF-κB ↓, COX-2 ↓, PGE2 ↓, thromboxane B2 ↓, macrophage inflammatory protein-1α/β (MIP-1α/β) ↓, monocyte chemoattractant protein-1 (MCP-1) ↓ | [113,114] |
| RAW264.7 mouse monocyte macrophage cells; Primary microglial cells from cerebral cortices | In vitro bromelain treatment + LPS | Anti-inflammatory activity: IL-6 ↓, NF-κB ↓, COX-2 ↓, PGE2 ↓, iNOS ↓, Alterations in the expression of MAPK family proteins: p-JNK ↓, p-p38 ↓, p-ERK ½ ↓, c-jun ↓, c-fos ↓ | [115,116,117,118,121] |
| Male Sprague-Dawley (SD) rats; BV-2 mouse microglial cells; Sprague-Dawley male rats | In vivo bromelain treatment + LPS In vitro bromelain treatment + LPS | Anti-inflammatory activity: NF-κB ↓, COX-2 ↓, PGE2 ↓ Alterations in the expression of MAPK family proteins: p-JNK ↓, p-p38 ↓, p-ERK ½ ↓, | [117] |
| IEC-6 rat intestinal epithelial cells with colitis model; Caco2 human colorectal cancer cells | In vivo bromelain treatment + 2,4,6-trinitrobenzene sulfonic acid (TNBS) In vitro bromelain | Anti-inflammatory activity: transmembrane tumor necrosis factor receptors ½ (TNFR1/2) ↓, NF-κB ↓, Bax ↓, Bcl-2 ↑ | [122] |
| C57BL/6 IL-10-deficient mice with spontaneous colitis | In vitro bromelain treatment | Proteolytic degradation of several cell surface molecules: CD44 ↓, CD45R ↓, CD62L ↓, CD8 ↓ Reduction the the clinical and histologic severity of IBD | [123,125] |
| Human cells from endoscopic colon biopsies from patients with ulcerative colitis, Crohn’s disease | In vitro bromelain treatment | Anti-inflammatory activity: granulocyte colony stimulating factor (G-CSF) ↓, granulocyte-macrophage colony stimulating factor (GM-CSF) ↓, IFN-γ ↓, CCL4/macrophage inhibitory protein (MIP)-1β ↓, TNF-α ↓ | [124] |
| PBMC human peripheral blood mononuclear cells | In vitro bromelain treatment In vivo bromelain treatment | Anti-inflammatory activity: CD7 ↓, CD8α ↓, CD14 ↓, CD16 ↓, CD21 ↓, CD25 ↓, CD41 ↓, CD42a ↓, CD44 ↓, CD45RA ↓, CD48 ↓, CD57 ↓, CD62L ↓, CD128a ↓, CD128b ↓, CD128a/CXCR1 ↓, CD128b/CXCR2 ↓ | [127,128,129,130] |
| Ovalbumin (OVA)-induced murine model of allergic airway disease | In vitro bromelain treatment | Inhibition allergic sensitization: CD4+ ↓ CD8+ ↓, CD4+↓, CD25+ ↓, CD44 ↓ | [134,135] |
7. Anticancer Properties of Bromelain
7.1. Apoptosis, Cell Cycle Arrest, and Cell Survival
7.1.1. Breast Cancer
7.1.2. Melanoma and Epidermoid Carcinoma
7.1.3. Chronic Myelogenous Leukemia and Lymphomas
7.1.4. Cancers of the Digestive System—Gastrointestinal and Colon Cancer
7.1.5. Hepatic and Pancreatic Cancer
7.1.6. Other Strategies in Bromelain-Dependent Cancer Treatment
7.2. Autophagy
7.3. Chemosensitizing Effect of a Combination of Bromelain
7.4. Reactive Oxygen Species (ROS)
8. Potential Clinical Applications of Bromelain
8.1. Allergic Sensitization
8.2. Chronic Rhinosinusitis
8.3. Enzymatic Debridement and Pro-Wound Healing Activities
8.4. Osteoarthritis
8.5. Musculoskeletal Function and Surgical Procedures
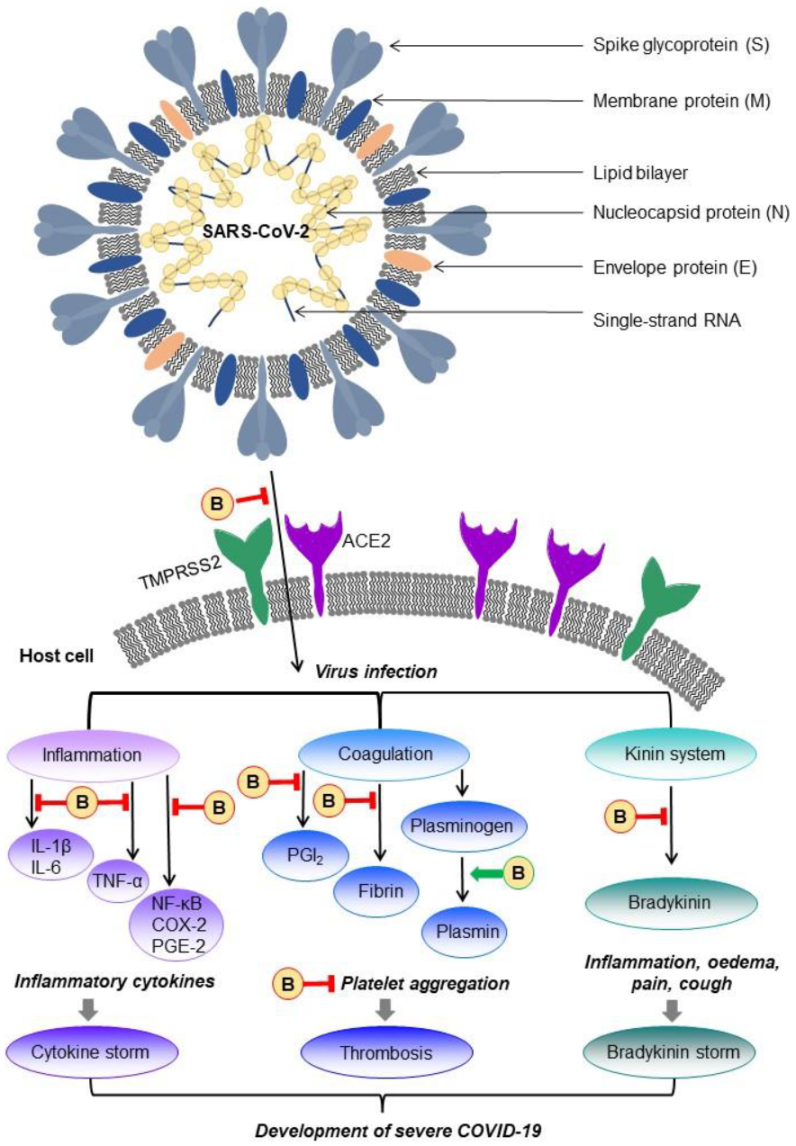
9. Bromelain as a Possible Treatment for COVID-19 Disease
9.1. COVID-19 Disease
9.2. Possible Management of Bromelain in COVID-19 Disease Treatment
10. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Che, C.-T.; Zhang, H. Plant Natural Products for Human Health. Int. J. Mol. Sci. 2019, 20, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Appl. Sci. 2021, 11, 8428. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Manas, N.H.A.; Hamid, A.A.A.; Hamid, H.A.; Illias, R.M. Comparative structural analysis of fruit and stem bromelain from Ananas comosus. Food Chem. 2018, 266, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Ramli, A.N.M.; Aznan, T.N.T.; Illias, R.M. Bromelain: From production to commercialisation. J. Sci. Food Agric. 2016, 97, 1386–1395. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, C.; Geng, L.; Chen, G.; Wang, X.; Chen, W.; Sa, R.; Zhang, J.; Zhang, X. Purification and characterization of bromelain from pineapple (Ananas comosus L.) peel waste. J. Food Sci. 2021, 86, 385–393. [Google Scholar] [CrossRef]
- Lalmanach, G. Proteolytic enzymes: From structures to transport pathways. Biochimie 2008, 90, 191–193. [Google Scholar] [CrossRef]
- Suarez-Puente, X.; Sánchez, L.M.; Overall, C.M.; López-Otín, C. Human and mouse proteases: A comparative genomic approach. Nat. Rev. Genet. 2003, 4, 544–558. [Google Scholar] [CrossRef]
- Paschkowsky, S.; Hsiao, J.M.; Young, J.C.; Munter, L.M. The discovery of proteases and intramembrane proteolysis. Biochem. Cell Biol. 2019, 97, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Gorodkiewicz, E.; Regulska, E. SPR Imaging Biosensor for Aspartyl Cathepsins: Sensor Development and Application for Biological Material. Protein Pept. Lett. 2010, 17, 1148–1154. [Google Scholar] [CrossRef]
- De Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, purification, and applications of bromelain: A review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef]
- Neta, J.L.V.; da Silva Lédo, A.; Lima, A.A.B.; Santana, J.C.C.; Leite, N.S.; Ruzene, D.S.; Silva, D.P.; de Souza, R.R. Bromelain Enzyme from Pineapple: In Vitro Activity Study under Different Micropropagation Conditions. Appl. Biochem. Biotechnol. 2012, 168, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.-I.; Takahashi, K.; Tanokura, M. Bromein, a Bromelain Inhibitor from Pineapple Stem: Structural and Functional Characteristics. Protein Pept. Lett. 2018, 25, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Guo, J.; Miao, Z.; Guo, X. Reverse micellar extraction of bromelain from pineapple peel—Effect of surfactant structure. Food Chem. 2016, 197, 450–456. [Google Scholar] [CrossRef]
- Pang, W.C.; Ramli, A.N.M.; Hamid, A.A.A. Comparative modelling studies of fruit bromelain using molecular dynamics simulation. J. Mol. Model. 2020, 26, 142. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [Green Version]
- Grzonka, Z.; Kasprzykowski, F.; Wiczk, W. Cysteine Proteases. In Industrial Enzymes; Polaina, J., MacCabe, A.P., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 181–195. ISBN 978-1-4020-5376-4. [Google Scholar]
- Kumar, S.; Hemavathi, A.B.; Hebbar, H.U. Affinity based reverse micellar extraction and purification of bromelain from pineapple (Ananas comosus L. Merryl) waste. Process. Biochem. 2011, 46, 1216–1220. [Google Scholar] [CrossRef]
- Harrach, T.; Eckert, K.; Schulze-Forster, K.; Nuck, R.; Grunow, D.; Maurer, H.R. Isolation and partial characterization of basic proteinases from stem bromelain. Protein J. 1995, 14, 41–52. [Google Scholar] [CrossRef]
- Soares, P.A.; Vaz, A.F.; Correia, M.T.; Pessoa, A.; Carneiro-Da-Cunha, M.G. Purification of bromelain from pineapple wastes by ethanol precipitation. Sep. Purif. Technol. 2012, 98, 389–395. [Google Scholar] [CrossRef]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of Extraction, Purification and Therapeutic Applications. Braz. Arch. Biol. Technol. 2016, 59, e16150010. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Wu, C.-Y.; Branford-White, C.J.; Ning, X.; Nie, H.-L.; Zhu, L.-M. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. J. Mol. Catal. B Enzym. 2010, 63, 188–193. [Google Scholar] [CrossRef]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef]
- Miranda, I.K.S.P.B.; Miranda, A.F.S.; Souza, F.V.D.; Vannier-Santos, M.A.; Pirovani, C.P.; Pepe, I.M.; Rodowanski, I.J.; Ferreira, K.T.D.S.E.; Vaz, L.M.S.; de Assis, S.A. The biochemical characterization, stabilization studies and the antiproliferative effect of bromelain against B16F10 murine melanoma cells. Int. J. Food Sci. Nutr. 2016, 68, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Jutamongkon, R.; Charoenrein, S. Effect of Temperature on the Stability of Fruit Bromelain from Smooth Cayenne Pineapple. Agric. Nat. Resour. 2010, 44, 943–948. [Google Scholar]
- Taussig, S.J.; Yokoyama, M.M.; Chinen, A.; Onari, K.; Yamakido, M. Bromelain: A proteolytic enzyme and its clinical application. A review. Hiroshima J. Med. Sci. 1975, 24, 185–193. [Google Scholar]
- Gutfreund, A.E.; Taussig, S.J.; Morris, A.K. Effect of oral bromelain on blood pressure and heart rate of hypertensive patients. Hawaii Med. J. 1978, 37, 143–146. [Google Scholar]
- Knox, S.; Lang, D.; Hoyt, A. The many flavors of pineapple reactions. Ann. Allergy Asthma Immunol. 2019, 123, 519–521. [Google Scholar] [CrossRef]
- Roehr, C.C.; Edenharter, G.; Reimann, S.; Ehlers, I.; Worm, M.; Zuberbier, T.; Niggemann, B. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin. Exp. Allergy 2004, 34, 1534–1541. [Google Scholar] [CrossRef]
- Reindl, J.; Rihs, H.P.; Scheurer, S.; Wangorsch, A.; Haustein, D.; Vieths, S. IgE reactivity to profilin in pollen-sensitized subjects with adverse reactions to banana and pineapple. Int. Arch. Allergy Immunol. 2002, 128, 105–114. [Google Scholar] [CrossRef]
- McWilliam, V.L.; Koplin, J.J.; Field, M.J.; Sasaki, M.; Dharmage, S.C.; Tang, M.L.; Sawyer, S.M.; Peters, R.L.; Allen, K.J. Self-reported adverse food reactions and anaphylaxis in the SchoolNuts study: A population-based study of adolescents. J. Allergy Clin. Immunol. 2017, 141, 982–990. [Google Scholar] [CrossRef]
- Castell, J.V.; Friedrich, G.; Kuhn, C.S.; Poppe, G.E. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am. J. Physiol. Liver Physiol. 1997, 273, G139–G146. [Google Scholar] [CrossRef]
- White, R.R.; Crawley, F.E.H.; Vellini, M.; Rovati, L.A. Bioavailability of125I bromelain after oral administration to rats. Biopharm. Drug Dispos. 1988, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Setiasih, S.; Adimas, A.C.D.; Dzikria, V.; Hudiyono, S. Stability Test of Partially Purified Bromelain from Pineapple (Ananas comosus (L.) Merr) Core Extract in Artificial Stomach Fluid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 12016. [Google Scholar] [CrossRef]
- Keziah, S.M.; Devi, C.S. Focalization of thrombosis and therapeutic perspectives: A memoir. Orient. Pharm. Exp. Med. 2018, 18, 281–298. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef] [PubMed]
- Vazhappilly, C.G.; Ansari, S.A.; Al-Jaleeli, R.; Al-Azawi, A.M.; Ramadan, W.S.; Menon, V.; Hodeify, R.; Siddiqui, S.S.; Merheb, M.; Matar, R.; et al. Role of flavonoids in thrombotic, cardiovascular, and inflammatory diseases. Inflammopharmacology 2019, 27, 863–869. [Google Scholar] [CrossRef]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, R.; Jain, S.; Kumar, A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibanez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, B. A review on potential properties and therapeutic applications of bromelain. World J. Pharm. Pharm. Sci. 2019, 8, 488–500. [Google Scholar] [CrossRef]
- Ley, C.M. A review of the use of bromelain in cardiovascular diseases. J. Chin. Integr. Med. 2011, 9, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Tochi, B.N.; Wang, Z.; Xu, S.-Y.; Zhang, W. Therapeutic Application of Pineapple Protease (Bromelain): A Review. Pak. J. Nutr. 2008, 7, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Experientia 2001, 58, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Nieper, H.A. Effect of bromelain on coronary heart disease and angina pectoris. Acta Med. Empirica 1978, 5, 274–278. [Google Scholar]
- Seligman, B. Oral Bromelains as Adjuncts in the Treatment of Acute Thrombophlebitis. Angiology 1969, 20, 22–26. [Google Scholar] [CrossRef]
- Juhasz, B.; Thirunavukkarasu, M.; Pant, R.; Zhan, L.; Penumathsa, S.V.; Secor, E.R.; Srivastava, S.; Raychaudhuri, U.; Menon, V.P.; Otani, H.; et al. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am. J. Physiol. Circ. Physiol. 2008, 294, H1365–H1370. [Google Scholar] [CrossRef] [Green Version]
- Penela, P.; Inserte, J.; Ramos, P.; Rodriguez-Sinovas, A.; Garcia-Dorado, D.; Mayor, F. Degradation of GRK2 and AKT is an early and detrimental event in myocardial ischemia/reperfusion. EBioMedicine 2019, 48, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Lu, Z. MicroRNA-144 attenuates cardiac ischemia/reperfusion injury by targeting FOXO1. Exp. Ther. Med. 2019, 17, 2152–2160. [Google Scholar] [CrossRef]
- Bahde, R.; Palmes, D.; Minin, E.; Stratmann, U.; Diller, R.; Haier, J.; Spiegel, H.-U. Bromelain Ameliorates Hepatic Microcirculation After Warm Ischemia. J. Surg. Res. 2007, 139, 88–96. [Google Scholar] [CrossRef]
- Neumayer, C.; Fügl, A.; Nanobashvili, J.; Blumer, R.; Punz, A.; Gruber, H.; Polterauer, P.; Huk, I. Combined Enzymatic and Antioxidative Treatment Reduces Ischemia-Reperfusion Injury in Rabbit Skeletal Muscle. J. Surg. Res. 2006, 133, 150–158. [Google Scholar] [CrossRef]
- Heinicke, R.M.; Van Der Wal, L.; Yokoyama, M. Effect of bromelain (ananase®) on human platelet aggregation. Cell. Mol. Life Sci. 1972, 28, 844–845. [Google Scholar] [CrossRef]
- Gläser, D.; Hilberg, T. The influence of bromelain on platelet count and platelet activity in vitro. Platelets 2006, 17, 37–41. [Google Scholar] [CrossRef]
- Metzig, C.; Grabowska, E.; Eckert, K.; Rehse, K.; Maurer, H.R. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. Vivo 1999, 13, 7–12. [Google Scholar]
- Errasti, M.E.; Prospitti, A.; Viana, C.A.; Gonzalez, M.M.; Ramos, M.V.; Rotelli, A.E.; Caffini, N.O. Effects on fibrinogen, fibrin, and blood coagulation of proteolytic extracts from fruits of Pseudananas macrodontes, Bromelia balansae, and B. hieronymi (Bromeliaceae) in comparison with bromelain. Blood Coagul. Fibrinolysis 2016, 27, 441–449. [Google Scholar] [CrossRef]
- Livio, M.; Bertoni, M.P.; De Gaetano, G. Effect of bromelain on fibrinogen level, prothrombin complex factors and platelet aggregation in the rat: A preliminary report. Drugs Exp. Clin. Res. 1978, 4, 49. [Google Scholar]
- Eckert, K.; Grabowska, E.; Stange, R.; Schneider, U.; Eschmann, K.; Maurer, H.R. Effects of oral bromelain administration on the impaired immunocytotoxicity of mononuclear cells from mammary tumor patients. Oncol. Rep. 1999, 6, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Chobotova, K.; Vernallis, A.B.; Majid, F.A.A. Bromelain’s activity and potential as an anti-cancer agent: Current evidence and perspectives. Cancer Lett. 2010, 290, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Musfiroh, F.F.; Setiasih, S.; Handayani, S.; Hudiyono, S.; Ilyas, N.M. In Vivo antiplatelet activity aggregation assay of bromelain fractionate by ethanol from extract pineapple core (Ananas comosus [l.] merr.). IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 12017. [Google Scholar] [CrossRef]
- Zhi, N.-N.; Zong, K.; Jia, X.-Y.; Wang, L.; Liang, J. Effect of high pressure processing on fibrinolytic activity of fruit bromelain in vivo. J. Food Process. Eng. 2019, 42, e13146. [Google Scholar] [CrossRef]
- Streiff, M.B.; Milentijevic, D.; McCrae, K.; Yannicelli, D.; Fortier, J.; Nelson, W.W.; Laliberté, F.; Crivera, C.; Lefebvre, P.; Schein, J.; et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am. J. Hematol. 2018, 93, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, A.I.; Kankipati, C.S.; Jones, S.; Newman, R.M.; Safrany, S.; Perry, C.J.; Nicholl, I.D. A novel mechanism for the anticancer activity of aspirin and salicylates. Int. J. Oncol. 2019, 54, 1256–1270. [Google Scholar] [CrossRef] [Green Version]
- Lichtenberger, L.M.; Vijayan, K.V. Are Platelets the Primary Target of Aspirin’s Remarkable Anticancer Activity? Cancer Res. 2019, 79, 3820–3823. [Google Scholar] [CrossRef] [Green Version]
- Ranger, G.S.; McKinley-Brown, C.; Rogerson, E.; Schimp-Manuel, K. Aspirin Use, Compliance, and Knowledge of Anticancer Effect in the Community. Perm. J. 2020, 24, 116. [Google Scholar] [CrossRef] [Green Version]
- Gaspani, L.; Limiroli, E.; Ferrario, P.; Bianchi, M. In vivo and in vitro Effects of Bromelain on PGE2 and SP Concentrations in the Inflammatory Exudate in Rats. Pharmacology 2002, 65, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Vellini, M.; Desideri, D.; Milanese, A.; Omini, C.; Daffonchio, L.; Hernandez, A.; Brunelli, G. Possible involvement of eicosanoids in the pharmacological action of bromelain. Arzneimittelforschung 1986, 36, 110–112. [Google Scholar] [PubMed]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef] [Green Version]
- Selendy, J.M.H. Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 9781119416210. [Google Scholar]
- Roselli, M.; Britti, M.S.; Le Huërou-Luron, I.; Marfaing, H.; Zhu, W.Y.; Mengheri, E. Effect of different plant extracts and natural substances (PENS) against membrane damage induced by enterotoxigenic Escherichia coli K88 in pig intestinal cells. Toxicol. Vitr. 2007, 21, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Mynott, T.L.; Guandalini, S.; Raimondi, F.; Fasano, A. Bromelain prevents secretion caused by Vibrio cholerae and Escherichia coli enterotoxins in rabbit ileum in vitro. Gastroenterology 1997, 113, 175–184. [Google Scholar] [CrossRef]
- Mynott, T.L.; Chandler, D.S.; Luke, R.K. Efficacy of enteric-coated protease in preventing attachment of enterotoxigenic Escherichia coli and diarrheal disease in the RITARD model. Infect. Immun. 1991, 59, 3708–3714. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, R.A. A plant protease for potentiation of and possible replacement of antibiotics. Exp. Med. Surg. 1961, 19, 143–160. [Google Scholar] [PubMed]
- Ryan, R.E. A double-blind clinical evaluation of bromelains in the treatment of acute sinusitis. Headache 1967, 7, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.N.; Ffrazier, C.V.; Martin, G.J. Bromelains. The pharmacology of the enzymes. Arch. Int. Pharmacodyn. Ther. 1963, 145, 166–189. [Google Scholar] [PubMed]
- Tinozzi, S.; Venegoni, A. Effect of bromelain on serum and tissue levels of amoxicillin. Drugs Exp. Clin. Res. 1978, 4, 39–44. [Google Scholar]
- Luerti, M.; Vignali, M.L. Influence of bromelain on penetration of antibiotics in uterus, salpinx and ovary. Drugs Exp. Clin. Res. 1978, 4, 45–48. [Google Scholar]
- Shahid, S.K.; Turakhia, N.H.; Kundra, M.; Shanbag, P.; Daftary, G.V.; Schiess, W. Efficacy and safety of phlogenzym—A protease formulation, in sepsis in children. J. Assoc. Physicians India 2002, 50, 527–531. [Google Scholar] [PubMed]
- Cai, T.; Tiscione, D.; Gallelli, L.; Verze, P.; Palmieri, A.; Mirone, V.; Bartoletti, R.; Malossini, G. Serenoa repens associated with selenium and lycopene extract and bromelain and methylsulfonylmethane extract are able to improve the efficacy of levofloxacin in chronic bacterial prostatitis patients. Arch. Ital. Urol. Androl. 2016, 88, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Rizvi, S.M.D.; Avaish, M.; Arshad, M.; Bagga, P.; Khan, M.S. A novel process for size controlled biosynthesis of gold nanoparticles using bromelain. Mater. Lett. 2015, 159, 373–376. [Google Scholar] [CrossRef]
- Bagga, P.; Ansari, T.M.; Siddiqui, H.H.; Syed, A.; Bahkali, A.H.; Rahman, A.; Khan, M.S. Bromelain capped gold nanoparticles as the novel drug delivery carriers to aggrandize effect of the antibiotic levofloxacin. EXCLI J. 2016, 15, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Hussain, T.; Alshammari, T.M.; Ahmad, W.; Tabrez, S.; Al-Qahtani, M.H.; Abuzenadah, A.M. Synthesis and Characterization of Cefotaxime Conjugated Gold Nanoparticles and Their Use to Target Drug-Resistant CTX-M-Producing Bacterial Pathogens. J. Cell. Biochem. 2017, 118, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, V.; Bernkop-Schnürch, A. Improvement of the intestinal membrane permeability of low molecular weight heparin by complexation with stem bromelain. Int. J. Pharm. 2006, 326, 153–159. [Google Scholar] [CrossRef]
- Banerjee, S.; Arora, A.; Vijayaraghavan, R.; Patti, A.F. Extraction and crosslinking of bromelain aggregates for improved stability and reusability from pineapple processing waste. Int. J. Biol. Macromol. 2020, 158, 318–326. [Google Scholar] [CrossRef]
- Stepek, G.; Lowe, A.E.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology 2006, 132, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.D.S.; Wanderley, L.F.; Junior, L.M.C. The potential of plant and fungal proteins in the control of gastrointestinal nematodes from animals. Rev. Bras. Parasitol. Vet. 2019, 28, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Stepek, G.; Buttle, D.J.; Duce, I.R.; Lowe, A.; Behnke, J.M. Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology 2004, 130, 203–211. [Google Scholar] [CrossRef]
- Stepek, G.; Lowe, A.E.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. Anthelmintic action of plant cysteine proteinases against the rodent stomach nematode, Protospirura muricola, in vitroandin vivo. Parasitology 2006, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Stepek, G.; Lowe, A.E.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. In vitro anthelmintic effects of cysteine proteinases from plants against intestinal helminths of rodents. J. Helminthol. 2007, 81, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wasso, S.; Maina, N.; Kagira, J. Toxicity and anthelmintic efficacy of chitosan encapsulated bromelain against gastrointestinal strongyles in Small East African goats in Kenya. Veter World 2020, 13, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Domingues, L.F.; Giglioti, R.; Feitosa, K.A.; Fantatto, R.R.; Rabelo, M.D.; Oliveira, M.C.D.S.; Bechara, G.H.; De Oliveira, G.P.; Junior, W.B.; Chagas, A.C.D.S. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Veter Parasitol. 2013, 197, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Luoga, W.; Mansur, F.; Buttle, D.J.; Duce, I.R.; Garnett, M.C.; Lowe, A.; Behnke, J.M. The relative anthelmintic efficacy of plant-derived cysteine proteinases on intestinal nematodes. J. Helminthol. 2013, 89, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Nwagu, T.N.; Ugwuodo, C.J. Stabilizing bromelain for therapeutic applications by adsorption immobilization on spores of probiotic Bacillus. Int. J. Biol. Macromol. 2019, 127, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Brakebusch, M.; Wintergerst, U.; Petropoulou, T.; Notheis, G.; Husfeld, L.; Belohradsky, B.H.; Adam, D. Bromelain is an accelerator of phagocytosis, respiratory burst and Killing of Candida albicans by human granulocytes and monocytes. Eur. J. Med. Res. 2001, 6, 193–200. [Google Scholar] [PubMed]
- López-García, B.; Hernández, M.; Segundo, B.S. Bromelain, a cysteine protease from pineapple (Ananas comosus) stem, is an inhibitor of fungal plant pathogens. Lett. Appl. Microbiol. 2012, 55, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Milala, M.A.; Gulani, I.A. Antimicrobial Effects of Crude Bromelain Extracted from Pineapple Fruit (Ananas comosus (Linn.) Merr.). Adv. Biochem. 2015, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Zharfan, R.S.; Purwono, P.B.; Mustika, A. Antimicrobial activity of pineapple (Ananas comosus l. merr) extract against multidrug-resistant of pseudomonas aeruginosa: An in vitro study. Indones. J. Trop. Infect. Dis. 2017, 6, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Widyarman, A.S.; Liliany, D.; Erfan, E.; Sudiono, J.; Djamil, M.S.; Sadono, J. Enzymatic activity of bromelain isolated pineapple (Ananas comosus) hump and its antibacterial effect on Enterococcus faecalis. Sci. Dent. J. 2018, 2, 39–52. [Google Scholar] [CrossRef]
- Ahamed, T.S.; Priya, V.V.; Gayathri, R.; Geetha, R.V. Evaluation of Anti Microbial Activity of Pineapple Extract against Selected Oral Pathogen. J. Pharm. Sci. Res. 2016, 8, 491–492. [Google Scholar]
- Goudarzi, M.; Mehdipour, M.; Hajikhani, B.; Sadeghinejad, S.; Sadeghi-Nejad, B. Antibacterial Properties of Citrus limon and Pineapple Extracts on Oral Pathogenic Bacteria (Streptococcus mutans and Streptococcus sanguis). Int. J. Enteric Pathog. 2019, 7, 99–103. [Google Scholar] [CrossRef]
- Rahmi, H.; Widayanti, A.; Hanif, A. Utilization of Bromelain Enzyme from Pineapple Peel Waste on Mouthwash Formula AgainstStreptococcus mutans. IOP Conf. Ser. Earth Environ. Sci. 2019, 217, 012036. [Google Scholar] [CrossRef]
- Praveen, N.C.; Rajesh, A.; Madan, M.; Chaurasia, V.R.; Hiremath, N.V.; Sharma, A.M. In vitro Evaluation of Antibacterial Efficacy of Pineapple Extract (Bromelain) on Periodontal Pathogens. J. Int. Oral Health 2014, 6, 96–98. [Google Scholar] [PubMed]
- Loon, Y.K.; Satari, M.H.; Dewi, W. Antibacterial effect of pineapple (Ananas comosus) extract towards Staphylococcus aureus. Padjadjaran J. Dent. 2018, 30, 16099. [Google Scholar] [CrossRef]
- Anjos, M.M.; Endo, E.H.; Leimann, F.V.; Gonçalves, O.H.; Dias-Filho, B.P.; Filho, B.A.D.A. Preservation of the antibacterial activity of enzymes against Alicyclobacillus spp. through microencapsulation. LWT 2018, 88, 18–25. [Google Scholar] [CrossRef]
- dos Anjos, M.M.; da Silva, A.A.; de Pascoli, I.C.; Mikcha, J.M.G.; Machinski, M.; Peralta, R.M.; Filho, B.A.A. Antibacterial activity of papain and bromelain on Alicyclobacillus spp. Int. J. Food Microbiol. 2016, 216, 121–126. [Google Scholar] [CrossRef]
- Ataide, J.A.; De Carvalho, N.M.; Rebelo, M.D.A.; Chaud, M.V.; Grotto, D.; Gerenutti, M.; Rai, M.; Mazzola, P.; Jozala, A.F. Bacterial Nanocellulose Loaded with Bromelain: Assessment of Antimicrobial, Antioxidant and Physical-Chemical Properties. Sci. Rep. 2017, 7, 18031. [Google Scholar] [CrossRef]
- Amini, N.; Setiasih, S.; Handayani, S.; Hudiyono, S.; Saepudin, E. Potential antibacterial activity of partial purified bromelain from pineapple core extracts using acetone and ammonium sulphate against dental caries-causing bacteria. AIP Conf. Proc. 2018, 2023, 020071. [Google Scholar] [CrossRef]
- Hidayat, Y.; Hermawati, E.; Setiasih, S.; Hudiyono, S.; Saepudin, E. Antibacterial activity test of the partially purified bromelain from pineapple core extract (Ananas comosus [L.] Merr) by fractionation using ammonium sulfate acetone. In Proceedings of the 3rd International Symposium on Current Progress in Mathematics and Sciences 2017 (ISCPMS2017), Bali, Indonesia, 26–27 July 2017; p. 20067. [Google Scholar]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Mandal, S.K. Current developments on anti-inflammatory natural medicines. Asian J. Pharm. Clin. Res. 2018, 11, 61–65. [Google Scholar] [CrossRef]
- Huang, J.-R.; Wu, C.-C.; Hou, R.C.-W.; Jeng, K.-C. Bromelain Inhibits Lipopolysaccharide-Induced Cytokine Production in Human THP-1 Monocytes via the Removal of CD14. Immunol. Investig. 2008, 37, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Kasemsuk, T.; Vivithanaporn, P.; Unchern, S. Anti-inflammatory Effects of Bromelain in Lps-induced Human U937 Macrophages. Chiang Mai J. Sci. 2018, 45, 299–307. [Google Scholar]
- Insuan, O.; Janchai, P.; Thongchuai, B.; Chaiwongsa, R.; Khamchun, S.; Saoin, S.; Insuan, W.; Pothacharoen, P.; Apiwatanapiwat, W.; Boondaeng, A.; et al. Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Curr. Issues Mol. Biol. 2021, 43, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J.-B.; Lee, J.-T.; Park, H.-R.; Kim, J.-B. Medicinal Effects of Bromelain (Ananas comosus) Targeting Oral Environment as an Anti-oxidant and Anti-inflammatory Agent. J. Food Nutr. Res. 2018, 6, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Hou, R.C.-W.; Chen, Y.-S.; Huang, J.-R.; Jeng, K.-C.G. Cross-Linked Bromelain Inhibits Lipopolysaccharide-Induced Cytokine Production Involving Cellular Signaling Suppression in Rats. J. Agric. Food Chem. 2006, 54, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Habashi, S.A.; Sabouni, F.; Moghimi, A.; Majd, S.A. Modulation of Lipopolysaccharide Stimulated Nuclear Factor kappa B Mediated iNOS/NO Production by Bromelain in Rat Primary Microglial Cells. Iran. Biomed. J. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Somasundaram, V.; Basudhar, D.; Bharadwaj, G.; No, J.H.; Ridnour, L.A.; Cheng, R.Y.S.; Fujita, M.; Thomas, D.D.; Anderson, S.K.; McVicar, D.W.; et al. Molecular Mechanisms of Nitric Oxide in Cancer Progression, Signal Transduction, and Metabolism. Antioxid. Redox Signal. 2019, 30, 1124–1143. [Google Scholar] [CrossRef] [PubMed]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Wen, S.; Huang, T.H.W.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Bromelain improves decrease in defecation in postoperative rats: Modulation of colonic gene expression of inducible nitric oxide synthase. Life Sci. 2006, 78, 995–1002. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Feng, P.; Yin, L.; Wang, C.; Zhi, S.; Dong, J.; Wang, J.; Lin, Y.; Chen, D.; et al. Inhibition of Epithelial TNF-α Receptors by Purified Fruit Bromelain Ameliorates Intestinal Inflammation and Barrier Dysfunction in Colitis. Front. Immunol. 2017, 8, 1468. [Google Scholar] [CrossRef] [Green Version]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; Gottfried, M.R. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin. Immunol. 2005, 116, 135–142. [Google Scholar] [CrossRef]
- Onken, J.E.; Greer, P.K.; Calingaert, B.; Hale, L.P. Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clin. Immunol. 2008, 126, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Hale, L.P.; Chichlowski, M.; Trinh, C.T.; Greer, P.K. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm. Bowel Dis. 2010, 16, 2012–2021. [Google Scholar] [CrossRef]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.P.; Haynes, B.F. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J. Immunol. 1992, 149, 3809–3816. [Google Scholar] [PubMed]
- Haleabc, L.P.; Greer, P.K.; Sempowskicd, G.D. Bromelain Treatment Alters Leukocyte Expression of Cell Surface Molecules Involved in Cellular Adhesion and Activation. Clin. Immunol. 2002, 104, 183–190. [Google Scholar] [CrossRef]
- Fitzhugh, D.J.; Shan, S.; Dewhirst, M.W.; Hale, L.P. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secor, E.R.; Singh, A.; Guernsey, L.A.; McNamara, J.T.; Zhan, L.; Maulik, N.; Thrall, R.S. Bromelain treatment reduces CD25 expression on activated CD4+ T cells in vitro. Int. Immunopharmacol. 2009, 9, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engwerda, C.R.; Andrew, D.; Ladhams, A.; Mynott, T.L. Bromelain Modulates T Cell and B Cell Immune Responses in Vitro and in Vivo. Cell. Immunol. 2001, 210, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, H.; Guseo, A.; Klein, R. In vitro study on the immunological effect of bromelain and trypsin on mononuclear cells from humans. Eur. J. Med. Res. 2005, 10, 325–331. [Google Scholar]
- Desser, L.; Rehberger, A.; Kokron, E.; Paukovits, W. Cytokine Synthesis in Human Peripheral Blood Mononuclear Cells after Oral Administration of Polyenzyme Preparations. Oncology 1993, 50, 403–407. [Google Scholar] [CrossRef]
- Secor, E.R.; Szczepanek, S.M.; Castater, C.A.; Adami, A.J.; Matson, A.P.; Rafti, E.T.; Guernsey, L.; Natarajan, P.; McNamara, J.T.; Schramm, C.M.; et al. Bromelain Inhibits Allergic Sensitization and Murine Asthma via Modulation of Dendritic Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 702196. [Google Scholar] [CrossRef]
- Secor, E.R.; Carson, W.F.; Cloutier, M.M.; Guernsey, L.A.; Schramm, C.M.; Wu, C.A.; Thrall, R.S. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell. Immunol. 2005, 237, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Dhandayuthapani, S.; Perez, H.D.; Paroulek, A.; Chinnakkannu, P.; Kandalam, U.; Jaffe, M.; Rathinavelu, A. Bromelain-Induced Apoptosis in GI-101A Breast Cancer Cells. J. Med. Food 2012, 15, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, F.; Raeisi, E.; Heidarian, E.; Shahbazi-Gahroui, D.; Lemoigne, Y. Bromelain Inhibitory Effect on Colony Formation: An In vitro Study on Human AGS, PC3, and MCF7 Cancer Cells. J. Med. Signals Sens. 2019, 9, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bhui, K.; Tyagi, S.; Prakash, B.; Shukla, Y. Pineapple bromelain induces autophagy, facilitating apoptotic response in mammary carcinoma cells. BioFactors 2010, 36, 474–482. [Google Scholar] [CrossRef]
- Bhui, K.; Prasad, S.; George, J.; Shukla, Y. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009, 282, 167–176. [Google Scholar] [CrossRef]
- Bhui, K.; Tyagi, S.; Srivastava, A.K.; Singh, M.; Roy, P.; Singh, R.; Shukla, Y. Bromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G2/M arrest to apoptosis. Mol. Carcinog. 2011, 51, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Kalra, N.; Bhui, K.; Roy, P.; Srivastava, S.; George, J.; Prasad, S.; Shukla, Y. Regulation of p53, nuclear factor κB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin. Toxicol. Appl. Pharmacol. 2008, 226, 30–37. [Google Scholar] [CrossRef]
- Morris, D.L.; Ehteda, A.; Moghaddam, S.M.; Akhter, J.; Pillai, K. Cytotoxic effects of bromelain in human gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21). OncoTargets Ther. 2013, 6, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Morris, D.L. Bromelain and N-acetylcysteine inhibit proliferation and survival of gastrointestinal cancer cells in vitro: Significance of combination therapy. J. Exp. Clin. Cancer Res. 2014, 33, 92. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Barat, S.; Chen, X.; Bui, K.C.; Bozko, P.; Malek, N.P.; Plentz, R.R. Comparative study of antitumor effects of bromelain and papain in human cholangiocarcinoma cell lines. Int. J. Oncol. 2016, 48, 2025–2034. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-C.; Wei, P.-L.; Makondi, P.T.; Chen, W.-T.; Huang, C.-Y.; Chang, Y.-J. Bromelain inhibits the ability of colorectal cancer cells to proliferate via activation of ROS production and autophagy. PLoS ONE 2019, 14, e0210274. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.; Kim, M.; Jin, E.-J. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim. Cells Syst. 2018, 22, 334–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, B.; Fasolino, I.; Pagano, E.; Capasso, R.; Pace, S.; De Rosa, G.; Milic, N.; Orlando, P.; Izzo, A.A.; Borrelli, F. The chemopreventive action of bromelain, from pineapple stem (Ananas comosus L.), on colon carcinogenesis is related to antiproliferative and proapoptotic effects. Mol. Nutr. Food Res. 2013, 58, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Debnath, R.; Chatterjee, N.; Das, S.; Mishra, S.; Bose, D.; Banerjee, S.; Das, S.; Das Saha, K.; Ghosh, D.; Maiti, D. Bromelain with peroxidase from pineapple are more potent to target leukemia growth inhibition—A comparison with only bromelain. Toxicol. Vitr. 2018, 55, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Debnath, R.; Majumder, D.; Nath, P.; Ghosh, D.; Maiti, D. Bromelain plus peroxidase reduces non-Hodgkin lymphoma progression in invivo via up-regulation of antioxidant enzymes and modulating apoptotic protein expression. Nutr. Cancer 2019, 72, 1200–1210. [Google Scholar] [CrossRef]
- Debnath, R.; Majumder, D.; Singha, A.K.; Ghosh, D.; Maiti, D. Bromelain Plus Peroxidase from Pineapple induces apoptosis Via mitochondrial dependent apoptosis pathway in lymphoma cells. Int. J. Pharm. Sci. Res. 2018, 9, 4610–4618. [Google Scholar]
- Fouz, N.; Amid, A.; Hashim, Y.Z.H.-Y. Gene Expression Analysis in MCF-7 Breast Cancer Cells Treated with Recombinant Bromelain. Appl. Biochem. Biotechnol. 2014, 173, 1618–1639. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Patnaik, S.; Srivastava, A.K.; Mudiam, M.K.R.; Shukla, Y.; Panda, A.K.; Pant, A.B.; Kumar, P.; Gupta, K.C. Anti-cancer activity of bromelain nanoparticles by oral administration. J. Biomed. Nanotechnol. 2014, 10, 3558–3575. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. Anticancer Property of Bromelain With Therapeutic Potential in Malignant Peritoneal Mesothelioma. Cancer Investig. 2013, 31, 241–250. [Google Scholar] [CrossRef]
- Higashi, T.; Kogo, T.; Sato, N.; Hirotsu, T.; Misumi, S.; Nakamura, H.; Iohara, D.; Onodera, R.; Motoyama, K.; Arima, H. Efficient Anticancer Drug Delivery for Pancreatic Cancer Treatment Utilizing Supramolecular Polyethylene-Glycosylated Bromelain. ACS Appl. Bio Mater. 2020, 3, 3005–3014. [Google Scholar] [CrossRef]
- Pillai, K.; Mekkawy, A.H.; Akhter, J.; Badar, S.; Dong, L.; Liu, A.I.; Morris, D.L. Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine–an in vitro study with pancreatic and hepatic cancer cells. Am. J. Transl. Res. 2020, 12, 7404–7419. [Google Scholar]
- Dong, L.; Badar, S.; Pillai, K.; Akhter, J.; Mekkawy, A.H.; Morris, D.L. Bromelain and N-acetylcysteine as therapeutic agents for soft tissue sarcoma. Int. J. Clin. Exp. Med. 2019, 12, 13311–13324. [Google Scholar]
- Lee, J.-H.; Lee, J.-T.; Park, H.R.; Kim, J.-B. The potential use of bromelain as a natural oral medicine having anticarcinogenic activities. Food Sci. Nutr. 2019, 7, 1656–1667. [Google Scholar] [CrossRef] [Green Version]
- Mekkawy, M.H.; Fahmy, H.A.; Nada, A.S.; Ali, O.S. Study of the Radiosensitizing and Radioprotective Efficacy of Bromelain (a Pineapple Extract): In Vitro and In Vivo. Integr. Cancer Ther. 2020, 19, 1534735420950468. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations. J. Cell Biol. 2020, 219, 219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Chong, Q.; Wang, L.; Cher, G.B.; Soo, R.A. Different treatment efficacies and side effects of cytotoxic chemotherapy. J. Thorac. Dis. 2020, 12, 3785–3795. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taşkın, A.; Tarakçıoğlu, M.; Ulusal, H.; Örkmez, M.; Taysi, S. Idarubicin-bromelain combination sensitizes cancer cells to conventional chemotherapy. Iran. J. Basic Med. Sci. 2019, 22, 1172–1178. [Google Scholar] [CrossRef]
- Mohamad, N.E.; Abu, N.; Yeap, S.K.; Alitheen, N.B. Bromelain Enhances the Anti-tumor Effects of Cisplatin on 4T1 Breast Tumor Model In Vivo. Integr. Cancer Ther. 2019, 18, 1534735419880258. [Google Scholar] [CrossRef] [Green Version]
- Pauzi, A.Z.M.; Yeap, S.K.; ABU, N.; Lim, K.L.; Omar, A.R.; Aziz, S.A.; Chow, A.L.T.; Subramani, T.; Tan, S.G.; Alitheen, N.B. Combination of cisplatin and bromelain exerts synergistic cytotoxic effects against breast cancer cell line MDA-MB-231 in vitro. Chin. Med. 2016, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeisi, E.; Aazami, M.H.; Aghamiri, S.M.R.; Satari, A.; Hosseinzadeh, S.; Lemoigne, Y.; Heidarian, E. Bromelain-based chemo-herbal combination effect on human cancer cells: In-vitro study on AGS and MCF7 proliferation and apoptosis. Curr. Issues Pharm. Med. Sci. 2020, 33, 155–161. [Google Scholar] [CrossRef]
- Chermahini, F.A.; Raeisi, E.; Aazami, M.H.; Mirzaei, A.; Heidarian, E.; Lemoigne, Y. Does Bromelain-Cisplatin Combination Afford In-Vitro Synergistic Anticancer Effects on Human Prostatic Carcinoma Cell Line, PC3? Galen Med. J. 2020, 9, 1749. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Ehteda, A.; Akhter, J.; Chua, T.C.; Morris, D.L. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anti-Cancer Drugs 2014, 25, 150–160. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Xu, X.; Fang, Q.; Tang, R. pH-sensitive bromelain nanoparticles by ortho ester crosslinkage for enhanced doxorubicin penetration in solid tumor. Mater. Sci. Eng. C 2020, 113, 111004. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Marverti, G.; Lauriola, A.; D’Arca, D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxidative Med. Cell. Longev. 2018, 2018, 2389523. [Google Scholar] [CrossRef] [PubMed]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [Green Version]
- Cordero, C.M.; León-González, A.J.; Montaño, J.M.C.; Morón, E.B.; Lopez-Lazaro, M. Pro-Oxidant Natural Products as Anticancer Agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef]
- Zavadova, E.; Desser, L.; Mohr, T. Stimulation of Reactive Oxygen Species Production and Cytotoxicity in Human Neutrophilsin vitroand after Oral Administration of a Polyenzyme Preparation. Cancer Biother. 1995, 10, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoggard, M.; MacKenzie, B.W.; Jain, R.; Taylor, M.W.; Biswas, K.; Douglas, R.G. Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease. Clin. Microbiol. Rev. 2016, 30, 321–348. [Google Scholar] [CrossRef] [Green Version]
- Helms, S.; Miller, A.L. Natural treatment of chronic rhinosinusitis. Altern. Med. Rev. 2006, 11, 196–207. [Google Scholar] [PubMed]
- Griffin, A.S.; Cabot, P.; Wallwork, B.; Panizza, B. Alternative therapies for chronic rhinosinusitis: A review. Ear Nose Throat J. 2020, 3, E25–E33. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Cantery, P.H.; Ernst, E. Herbal Medicines for the Treatment of Rhinosinusitis: A Systematic Review. Otolaryngol. Neck Surg. 2006, 135, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Matschke, R.; Zeman, F.; Huppertz, G.; Koller, M.; Meiser, P. Therapeutic Applications and Benefits from Postsurgical Use of the Phytotherapeutic Bromelain in Otorhinolaryngology: A Non-Interventional Study. Otolaryngology 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Passali, D.; Passali, G.C.; Bellussi, L.M.; Sarafoleanu, C.; Loglisci, M.; Manea, C.; Iosif, C.; Passali, F.M. Bromelain’s penetration into the blood and sinonasal mucosa in patients with chronic rhinosinusitis. Acta Otorhinolaryngol. Ital. 2018, 38, 225–228. [Google Scholar] [CrossRef]
- Singer, A.J.; Toussaint, J.; Chung, W.T.; McClain, S.A.; Clark, R.A.F.; Asculai, E.; Geblinger, D.; Rosenberg, L. Development of a contaminated ischemic porcine wound model and the evaluation of bromelain based enzymatic debridement. Burns 2018, 44, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Jones, C.D.; Widdowson, D.; Bahia, H. Bromelain-based enzymatic debridement of e-cigarette burns: A single unit experience. J. Wound Care 2019, 28, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Wickham, N.; Alexander, K.S.; Fletcher, A.; O’Boyle, C. Successful treatment of mixed depth flame burns using enzymatic debridement with Nexobrid™ in a patient with aggressive systemic sclerosis (scleroderma). Scars Burn. Health 2019, 5, 2059513118821563. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Almeland, S.K.; Dheansa, B.; Fuchs, P.; Governa, M.; Hoeksema, H.; Korzeniowski, T.; Lumenta, D.B.; Marinescu, S.; Martinez-Mendez, J.R.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns 2020, 46, 782–796. [Google Scholar] [CrossRef]
- Da Elisa Silva López, R. Debridement Applications of Bromelain: A Complex of Cysteine Proteases from Pineapple. Adv. Biotechnol. Microbiol. 2017, 3, 555624. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hu, W.; Zhang, B.; Liu, S.; Wang, J.-M.; Wang, A.-M. Bromelain Ameliorates the Wound Microenvironment and Improves the Healing of Firearm Wounds. J. Surg. Res. 2012, 176, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Cucchi, A.; Bonaccorso, A.; Ferroni, L.; Gardin, C.; Mortellaro, C.; Zavan, B. In Vitro Effect of Bromelain on the Regenerative Properties of Mesenchymal Stem Cells. J. Craniofacial Surg. 2019, 30, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Sulastri, E.; Iwo, M.I.; Safitri, D.; Rahma, A. Bromelain Encapsulated in Self Assembly Nanoemulsion Exhibits Better Debridement Effect in Animal Model of Burned Skin. J. Nano Res. 2016, 40, 158–166. [Google Scholar] [CrossRef]
- Schulz, A.; Fuchs, P.C.; Oplaender, C.; Valdez, L.B.; Schiefer, J.L. Effect of Bromelain-Based Enzymatic Debridement on Skin Cells. J. Burn. Care Res. 2018, 39, 527–535. [Google Scholar] [CrossRef]
- Berner, J.E.; Keckes, D.; Pywell, M.; Dheansa, B. Limitations to the use of bromelain-based enzymatic debridement (NexoBrid®) for treating diabetic foot burns: A case series of disappointing results. Scars Burn. Health 2018, 4, 2059513118816534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, F.; Walker, C. Osteoarthritis and inflammation: A serious disease with overlapping phenotypic patterns. Postgrad. Med. 2020, 132, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.; Lewith, G.; Walker, A.; Hicks, S.M.; Middleton, D. Bromelain as a Treatment for Osteoarthritis: A Review of Clinical Studies. Evid.-Based Complement. Altern. Med. 2004, 1, 251–257. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Saengpetch, N.; Sibmooh, N.; Unchern, S. Improved WOMAC score following 16-week treatment with bromelain for knee osteoarthritis. Clin. Rheumatol. 2016, 35, 2531–2540. [Google Scholar] [CrossRef]
- Jayachandran, S.; Khobre, P. Efficacy of Bromelain along with Trypsin, Rutoside Trihydrate Enzymes and Diclofenac Sodium Combination Therapy for the treatment of TMJ Osteoarthritis—A Randomised Clinical Trial. J. Clin. Diagn. Res. 2017, 11, ZC09–ZC11. [Google Scholar] [CrossRef]
- Italiano, G.; Raimondo, M.; Giannetti, G.; Gargiulo, A. Benefits of a Food Supplement Containing Boswellia serrata and Bromelain for Improving the Quality of Life in Patients with Osteoarthritis: A Pilot Study. J. Altern. Complement. Med. 2020, 26, 123–129. [Google Scholar] [CrossRef]
- Conrozier, T.; Mathieu, P.; Bonjean, M.; Marc, J.-F.; Renevier, J.-L.; Balblanc, J.-C. A complex of three natural anti-inflammatory agents provides relief of osteoarthritis pain. Altern. Ther. Health Med. 2014, 20, 32–37. [Google Scholar]
- Dighe, N.S.; Pattan, S.R.; Merekar, A.N.; Laware, R.B.; Bhawar, S.B.; Nirmal, S.N.; Gaware, V.M.; Hole, M.B.; Musmade, D.S. Bromelain a wonder supplement: A review. Pharmacologyonline 2010, 1, 11–18. [Google Scholar]
- Aiyegbusi, A.I.; Duru, F.I.O.; Anunobi, C.C.; Noronha, C.C.; Okanlawon, A.O. Bromelain in the early phase of healing in acute crush Achilles tendon injury. Phytother. Res. 2010, 25, 49–52. [Google Scholar] [CrossRef]
- Buford, T.W.; Cooke, M.B.; Redd, L.L.; Hudson, G.M.; Shelmadine, B.D.; Willoughby, D.S. Protease Supplementation Improves Muscle Function after Eccentric Exercise. Med. Sci. Sports Exerc. 2009, 41, 1908–1914. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.C.; Bailey, S.P.; Barnes, M.E.; Derr, S.J.; Hall, E.E. The effects of protease supplementation on skeletal muscle function and DOMS following downhill running. J. Sports Sci. 2004, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Chen, C.-S.; Chan, Y.-J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 24 October 2021).
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Global Coronavirus COVID-19 Clinical Trial Tracker. Available online: https://www.covid-trials.org (accessed on 24 October 2021).
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 6, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Solnier, J.; Fladerer, J.-P. Flavonoids: A complementary approach to conventional therapy of COVID-19? Phytochem. Rev. 2020, 20, 773–795. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Shaikh, A.; Singh, R.; Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, M.; Donelli, D.; Maggini, V.; Firenzuoli, F. Phytotherapic compounds against coronaviruses: Possible streams for future research. Phytother. Res. 2020, 34, 1469–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Z.; Ye, K.; He, X.; Sun, B.; Qin, Z.; Yu, J.; Yao, J.; Wu, Q.; Bao, Z.; et al. SARS-CoV-2: Characteristics and current advances in research. Virol. J. 2020, 17, 117. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.-Y.; Zhao, R.; Gao, L.-J.; Gao, X.-F.; Wang, D.-P.; Cao, J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, J.; Xiao, T.; Peng, H.; Sterling, S.M.; Walsh, R.M.; Rawson, S.; Rits-Volloch, S.; Chen, B. Distinct conformational states of SARS-CoV-2 spike protein. Science 2020, 369, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.A.; Guzman, H.V.; Boopathi, S.; Baker, J.L.; Poma, A.B. Characterization of Structural and Energetic Differences between Conformations of the SARS-CoV-2 Spike Protein. Materials 2020, 13, 5362. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-Ray Structures Reveal a Large Hinge-bending Motion Important for Inhibitor Binding and Catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef] [Green Version]
- Sagar, S.; Rathinavel, A.K.; Lutz, W.E.; Struble, L.R.; Khurana, S.; Schnaubelt, A.T.; Mishra, N.K.; Guda, C.; Palermo, N.Y.; Broadhurst, M.J.; et al. Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and spike protein. Clin. Transl. Med. 2021, 11, e281. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Bhattacharyya, S. Impact of Thiol–Disulfide Balance on the Binding of Covid-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor. ACS Omega 2020, 5, 16292–16298. [Google Scholar] [CrossRef]
- Akhter, J.; Quéromès, G.; Pillai, K.; Kepenekian, V.; Badar, S.; Mekkawy, A.H.; Frobert, E.; Valle, S.J.; Morris, D.L. The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2. Viruses 2021, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Fatimawali, A.Y.; Idroes, R.; Kusumawaty, D.; Emran, T.B.; Yesiloglu, T.Z.; Sippl, W.; Mahmud, S.; Alqahtani, T.; Alqahtani, A.M.; et al. An Analysis Based on Molecular Docking and Molecular Dynamics Simulation Study of Bromelain as Anti-SARS-CoV-2 Variants. Front. Pharmacol. 2021, 12, 717757. [Google Scholar] [CrossRef]
- Greig, A.S.; Bouillant, A.M. Binding effects of concanavalin A on a coronavirus. Can. J. Comp. Med. Rev. Can. Med. Comp. 1977, 41, 122–126. [Google Scholar]
- Schlegel, A.; Schaller, J.; Jentsch, P.; Kempf, C. Semliki forest virus core protein fragmentation: Its possible role in nucleocapsid disassembly. Biosci. Rep. 1993, 13, 333–347. [Google Scholar] [CrossRef]
- Cueno, M.E.; Imai, K. Structural Comparison of the SARS-CoV 2 Spike Protein Relative to Other Human-Infecting Coronaviruses. Front. Med. 2021, 7, 594439. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Meini, S.; Zanichelli, A.; Sbrojavacca, R.; Iuri, F.; Roberts, A.T.; Suffritti, C.; Tascini, C. Understanding the Pathophysiology of COVID-19: Could the Contact System Be the Key? Front. Immunol. 2020, 11, 2014. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Gunupuru, R. Targeting cyclooxygenase enzyme for the adjuvantCOVID-19 therapy. Drug Dev. Res. 2021, 82, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.I. An Overview of the Crystallized Structures of the SARS-CoV-2. Protein J. 2020, 39, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Leaf, R.S.K.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef]
- Jose, R.J.P.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Bakare, A.O.; Ologe, M.O. Bromelain: A Review on its Potential as a Therapy for the Management of COVID-19. Niger. J. Physiol. Sci. 2020, 35, 10–19. [Google Scholar] [PubMed]
- Van De Veerdonk, F.L.; Netea, M.G.; Van Deuren, M.; Van Der Meer, J.W.; De Mast, Q.; Brüggemann, R.J.; Van Der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H.; et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef]
- Tolouian, R.; Vahed, S.Z.; Ghiyasvand, S.; Tolouian, A.; Ardalan, M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J. Ren. Inj. Prev. 2020, 9, e19. [Google Scholar] [CrossRef]
- Lotz-Winter, H. On the Pharmacology of Bromelain: An Update with Special Regard to Animal Studies on Dose-Dependent Effects. Planta Med. 1990, 56, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Iii, C.V.H.; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
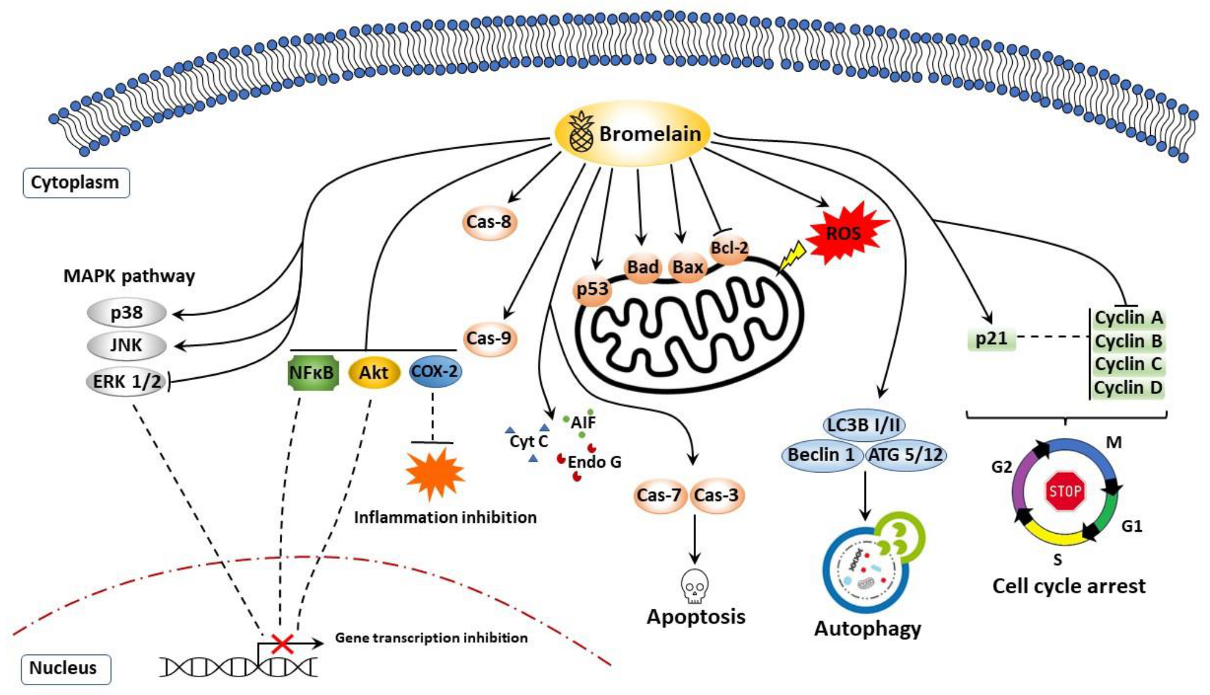
| Target | Experimental Approach | Effect | References |
|---|---|---|---|
| GI-101A human breast carcinoma cells | In vitro bromelain treatment | PCD induction: Caspase-3 ↑, Caspase-9 ↑ | [136] |
| AGS human gastric carcinoma cells; MCF7 human breast Adenocarcinoma cells; PC3 human prostate carcinoma cells | In vitro bromelain treatment | Cell proliferative and colony formation inhibition | [137] |
| MCF7 human breast adenocarcinoma cells; MDA-MB231 human breast cells’ adenocarcinoma triple-negative breast cells | In vitro bromelain treatment | PCD induction: Increase in the population of Sub-G1 cells, alterations in the expression of MAPK family proteins: JNK ↑, p38 ↑, ERK ½ ↓ Autophagy induction: LC3BII ↑, beclin 1 ↑ | [138] |
| HeLa human cervical cancer cells; MCF7 human breast adenocarcinoma cells; A549 human lung carcinoma cells; Caco-2 human epithelial colorectal adenocarcinoma cells; Male, Swiss albino mice | In vitro and in vivo (oral administration) bromelain nanoparticles treatment | PCD induction: Increase in the population of Sub-G1 cells, p53 ↑, Bax ↑, Bcl-2 ↓, ROS ↑ Cell cycle arrest: p21 ↑ | [152] |
| Female, Swiss albino mice—skin tumorigenesis model; A431 human epidermoid carcinoma cells; A375 human melanoma cells; | In vivo (oral administration) bromelain treatment In vitro bromelain treatment | PCD induction: Increase in the population of Sub-G1 cells, p53 ↑, Bax ↑, Bcl-2 ↓, Caspase-3 ↑, Caspase-9 ↑, COX-2 ↓, NF-κB ↓, ERK ½ ↓, p-Akt ↓, ROS ↑ | [139,140,141] |
| K562 human chronic myelogenous leukemia cells; HepG2 human hepatocellular carcinoma cells; HCT 116 human colorectal carcinoma cells; Sarcoma 180 murine sarcoma cells; B16F10 musculus skin melanoma cells; DLA Dalton’s lymphoma ascites cells; Swiss albino mice—Dalton’s lymphoma cells; | In vitro bromelain + peroxidase treatment In vivo (oral administration) bromelain + peroxidase treatment | PCD induction: p53 ↑, Bad ↑, Bax ↑, Bcl-2 ↓, ROS ↑, Caspase-3 ↑, cytochrome c ↑, NF-κB ↓ | [148,149,150] |
| MKN45, KATO-III gastrointestinal carcinoma cells; HT29-5F12, HT29-5M21, LS174T colon adenocarcinoma cells; | In vitro bromelain or bromelain + N-acetylcysteine treatment | PCD induction: Caspase-3 ↑, Caspase-7 ↑, Caspase-8 ↑, Caspase-9 ↑, cytochrome c ↑, cleaved PARP ↑, Bcl-2 ↓, p-Akt ↓, MUC1 ↓, p53 ↑ Cell cycle arrest: cyclins A, B, D and E ↓ Autophagy induction: LC3BII ↑, beclin 1 ↑ | [142,143] |
| FK-1, SZ-1 cholangiocarcinoma (CC) cells | In vitro bromelain treatment | Decrease in the proliferation, invasion, and migration of CC cells PCD induction: cleaved PARP ↑, p-Akt ↓, NF-κB ↓, alterations in the expression of MAPK family proteins: ERK ½ ↓, STAT-3 ↓, Changes in epithelial-mesenchymal transformation: E-cadherin ↑, N-cadherin ↓ | [144] |
| DLD-1, HT-29, HCT116 human colorectal cancer cells | In vitro bromelain treatment | PCD induction: Caspase-3 ↑, Caspase-8 ↑, Caspase-9 ↑, apoptosis inducing factor (AIF) ↑, endonuclease G (Endo G) ↑, cleaved PARP ↑, ROS ↑, Autophagy induction: LC3BI/II ↑, beclin 1 ↑, p62 ↑, ATG5/12 ↑, | [145] |
| Caco2, CT116, G13D human colorectal cancer cells; KRASG12D mutant heterozygous mice | In vitro and in vivo bromelain treatment | Ferroptosis induction: accumulation of lipid-based ROS, Long-chain-fatty-acid—CoA ligase 4 (ACSL4) ↑ | [146] |
| DLD-1, Caco2 human colorectal cancer cells; Male imprinting control region mice | In vitro and in vivo bromelain treatment | PCD induction: p-Akt ↓, Caspase-3 ↑, Caspase-7 ↑, ROS ↑, alterations in the expression of MAPK family proteins: ERK ½ ↓, Reduction in the development of aberrant crypt foci, polyps | [147] |
| FPAC, ASPC1, HEP3B, HEPG2 human pancreatic cells; AGS human gastric carcinoma cells; PC3 human prostate carcinoma cells; MCF7 human breast Adenocarcinoma cells; Mice challenged with 4T1 triple-negative breast cancer cells; MDA-MB231 human breast adenocarcinoma triple-negative breast cells; PET, YOU malignant peritoneal mesothelioma cells; | In vitro bromelain/bromelain + N-acetylcysteine with combination with Gemcitabine/5-fluorouracil/Oxaliplatin/Doxorubicin treatment In vivo bromelain + cisplatine treatment In vivo bromelain + cisplatine treatment In vitro bromelain + cisplatine/5-FU | The synergistic action of the mixture: cell proliferative and colony formation inhibition, reduction the doses of chemotherapeutic agents The synergistic action of the mixture: modulation the tumor environmental Inflammation—Gremlin ↓, IL-1β ↓, IL-4 ↓, NF-κB ↓ Bcl-2 ↓, Bax ↑ The synergistic action of the mixture: Caspase-3 ↑, Caspase-7 ↑, Caspase-8 ↑, Caspase-9 ↑, cytochrome c ↑, Bcl-2 ↓, cleaved PARP ↑, p-Akt ↓, MUC1 ↓, LC3BI/II ↑, NF-κB ↓, | [155,163,164,165,166,167] |
| HT1080, SW872, VA-ES-BJ, SW982 human sarcoma cells; | In vitro bromelain + N-acetylcysteine treatment | PCD induction: Caspase-3 ↑, Caspase-8 ↑, cleaved PARP ↑, Bcl-2 ↓, Bax ↑ Autophagy induction: LC3BI/II ↑ | [156] |
| SCC25 human oral squamous carcinoma cells; Ca9-22 human oral squamous carcinoma cells | In vitro bromelain treatment | PCD induction: Increase in the population of Sub-G1 cells, p53 ↑, Caspase-3 ↑, Caspase-7 ↑, Caspase-9 ↑ cleaved PARP ↑, Bcl-2 ↓, Bax ↑, Lamin A/C ↓, cytochrome c ↑, AIF ↑ | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. https://doi.org/10.3390/nu13124313
Hikisz P, Bernasinska-Slomczewska J. Beneficial Properties of Bromelain. Nutrients. 2021; 13(12):4313. https://doi.org/10.3390/nu13124313
Chicago/Turabian StyleHikisz, Pawel, and Joanna Bernasinska-Slomczewska. 2021. "Beneficial Properties of Bromelain" Nutrients 13, no. 12: 4313. https://doi.org/10.3390/nu13124313
APA StyleHikisz, P., & Bernasinska-Slomczewska, J. (2021). Beneficial Properties of Bromelain. Nutrients, 13(12), 4313. https://doi.org/10.3390/nu13124313






