Vitamin D as Modulator of Drug Concentrations: A Study on Two Italian Cohorts of People Living with HIV Administered with Efavirenz
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Efavirenz Plasma Concentrations
2.3. Quantification of 25-Hydroxyvitamin D
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Vitamin D Distribution
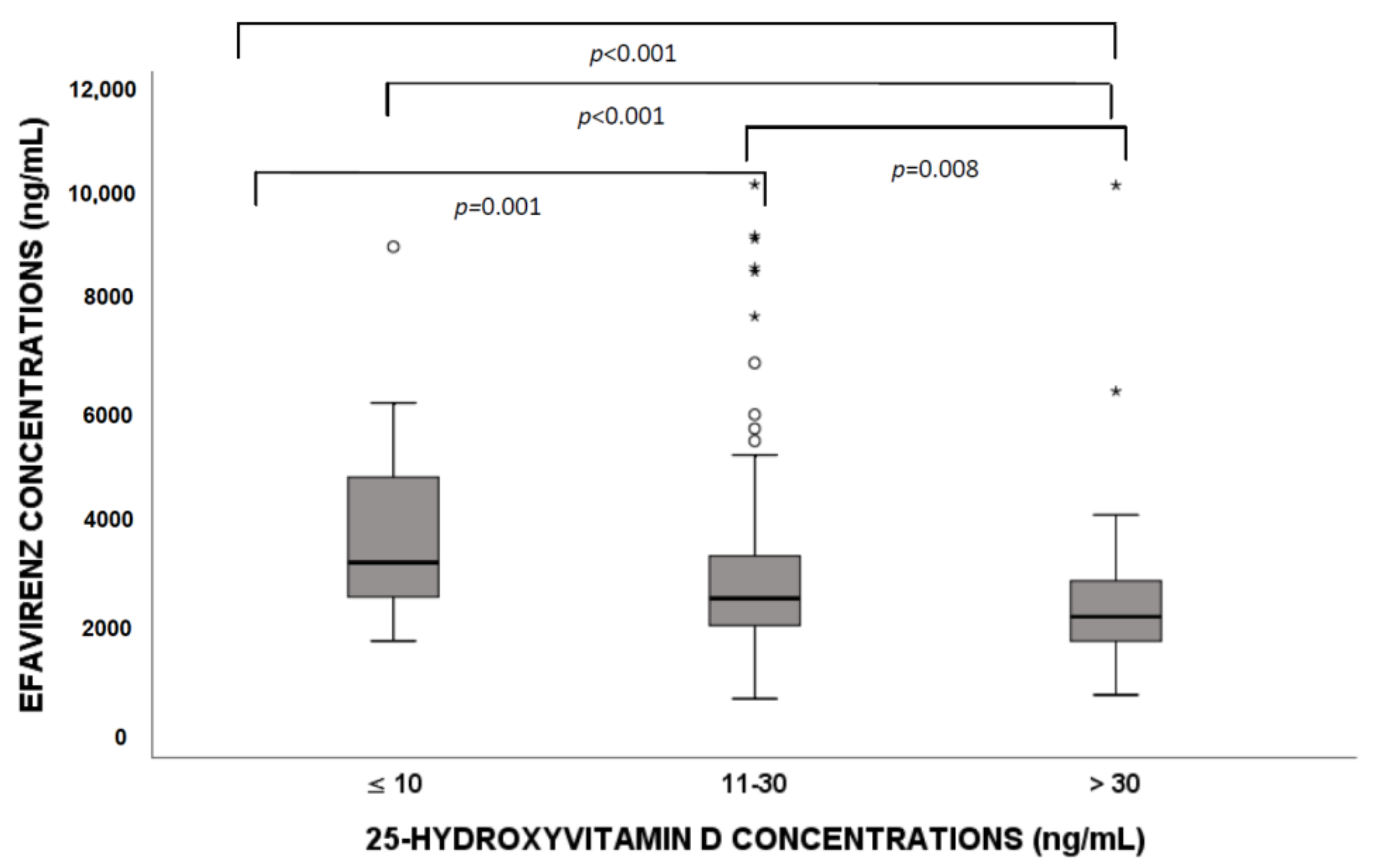
3.3. Efavirenz Distribution According to Vitamin D Levels
3.4. Seasonality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, S.A. The new dietary reference intakes from the Institute of Medicine for calcium and vitamin D. Perspect. Infirm. 2011, 8, 5. [Google Scholar]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [CrossRef]
- National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 8 October 2021).
- Maggio, D.; Cherubini, A.; Lauretani, F.; Russo, R.C.; Bartali, B.; Pierandrei, M.; Ruggiero, C.; Macchiarulo, M.C.; Giorgino, R.; Minisola, S.; et al. 25(OH)D Serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1414–1419. [Google Scholar] [CrossRef]
- Carnevale, V.; Modoni, S.; Pileri, M.; Di Giorgio, A.; Chiodini, I.; Minisola, S.; Vieth, R.; Scillitani, A. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: Seasonal and gender differences. Osteoporos. Int. 2001, 12, 1026–1030. [Google Scholar] [CrossRef]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolo, A.; Lucchini, R.; Keller, F.; Cantu, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Huff, H.; Merchant, A.T.; Lonn, E.; Pullenayegum, E.; Smaill, F.; Smieja, M. Vitamin D and progression of carotid intima-media thickness in HIV-positive Canadians. HIV Med. 2017, 19, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Tin, A.; Zhang, L.; Estrella, M.M.; Hoofnagle, A.; Rebholz, C.M.; Brown, T.T.; Palella, F.J., Jr.; Witt, M.D.; Jacobson, L.P.; Kingsley, L.A.; et al. Vitamin D Status and Kidney Function Decline in HIV-Infected Men: A Longitudinal Study in the Multicenter AIDS Cohort Study. AIDS Res. Hum. Retrovir. 2017, 33, 1140–1148. [Google Scholar] [CrossRef]
- Noe, S.; Oldenbuettel, C.; Heldwein, S.; Wiese, C.; von Krosigk, A.; Pascucci, R.; Ruecker, K.; Jaeger, H.; Wolf, E. Secondary Hyperparathyroidism in HIV-Infected Patients in Central Europe. Horm. Metab. Res. 2018, 50, 317–324. [Google Scholar] [CrossRef]
- Dadabhai, A.S.; Saberi, B.; Lobner, K.; Shinohara, R.T.; Mullin, G.E. Influence of vitamin D on liver fibrosis in chronic hepatitis C: A systematic review and meta-analysis of the pooled clinical trials data. World J. Hepatol. 2017, 9, 278–287. [Google Scholar] [CrossRef]
- Jao, J.; Freimanis, L.; Mussi-Pinhata, M.M.; Cohen, R.A.; Monteiro, J.P.; Cruz, M.L.; Branch, A.; Sperling, R.S.; Siberry, G.K. Severe Vitamin D Deficiency in Human Immunodeficiency Virus-Infected Pregnant Women is Associated with Preterm Birth. Am. J. Perinatol. 2016, 34, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Sousa, M.A.; Martinez, I.; Medrano, L.M.; Fernandez-Rodriguez, A.; Resino, S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front. Immunol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Lindh, J.D.; Bjorkhem-Bergman, L.; Eliasson, E. Vitamin D and drug-metabolising enzymes. Photochem. Photobiol. Sci. 2012, 11, 1797–1801. [Google Scholar] [CrossRef]
- Drocourt, L.; Ourlin, J.C.; Pascussi, J.M.; Maurel, P.; Vilarem, M.J. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J. Biol. Chem. 2002, 277, 25125–25132. [Google Scholar] [CrossRef] [Green Version]
- Lindh, J.D.; Andersson, M.L.; Eliasson, E.; Bjorkhem-Bergman, L. Seasonal variation in blood drug concentrations and a potential relationship to vitamin D. Drug Metab. Dispos. 2011, 39, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Guenthner, T.; Gan, L.S.; Humphreys, W.G. CYP3A4 induction by xenobiotics: Biochemistry, experimental methods and impact on drug discovery and development. Curr. Drug Metab. 2004, 5, 483–505. [Google Scholar] [CrossRef]
- Cozzolino, M.; Vidal, M.; Arcidiacono, M.V.; Tebas, P.; Yarasheski, K.E.; Dusso, A.S. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. Aids 2003, 17, 513–520. [Google Scholar] [CrossRef]
- Burger, D.; van der Heiden, I.; la Porte, C.; van der Ende, M.; Groeneveld, P.; Richter, C.; Koopmans, P.; Kroon, F.; Sprenger, H.; Lindemans, J.; et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: The effect of gender, race, and CYP2B6 polymorphism. Br. J. Clin. Pharmacol. 2006, 61, 148–154. [Google Scholar] [CrossRef]
- D’Avolio, A.; Baietto, L.; Siccardi, M.; Sciandra, M.; Simiele, M.; Oddone, V.; Bonora, S.; Di Perri, G. An HPLC-PDA method for the simultaneous quantification of the HIV integrase inhibitor raltegravir, the new nonnucleoside reverse transcriptase inhibitor etravirine, and 11 other antiretroviral agents in the plasma of HIV-infected patients. Ther. Drug Monit. 2008, 30, 662–669. [Google Scholar] [CrossRef]
- Marzolini, C.; Telenti, A.; Decosterd, L.A.; Greub, G.; Biollaz, J.; Buclin, T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. Aids 2001, 15, 71–75. [Google Scholar] [CrossRef]
- FDA. Health Based Reference Values LIAISON® Vitamin D Total Assay. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/K071480.pdf (accessed on 8 October 2021).
- Cusato, J.; Genova, C.; Tomasello, C.; Carrega, P.; Ottonello, S.; Pietra, G.; Mingari, M.C.; Cossu, I.; Rijavec, E.; Leggieri, A.; et al. Influence of Vitamin D in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab. Cancers 2019, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Cusato, J.; De Nicolo, A.; Boglione, L.; Favata, F.; Ariaudo, A.; Pinna, S.M.; Carcieri, C.; Guido, F.; Cariti, G.; Di Perri, G.; et al. Vitamin D pathway gene polymorphisms affecting daclatasvir plasma concentration at 2 weeks and 1 month of therapy. Pharmacogenomics 2018, 19, 701–707. [Google Scholar] [CrossRef]
- Cusato, J.; De Nicolo, A.; Boglione, L.; Favata, F.; Ariaudo, A.; Mornese Pinna, S.; Carcieri, C.; Guido, F.; Avataneo, V.; Cariti, G.; et al. Vitamin D pathway genetic variants are able to influence sofosbuvir and its main metabolite pharmacokinetics in HCV mono-infected patients. Infect. Genet. Evol. 2018, 60, 42–47. [Google Scholar] [CrossRef]
- Allegra, S.; Cusato, J.; De Francia, S.; Longo, F.; Pirro, E.; Massano, D.; Piga, A.; D’Avolio, A. Effect of pharmacogenetic markers of vitamin D pathway on deferasirox pharmacokinetics in children. Pharmacogenet. Genom. 2017, 28, 17–22. [Google Scholar] [CrossRef]
- Cusato, J.; Boglione, L.; De Nicolo, A.; Favata, F.; Ariaudo, A.; Mornese Pinna, S.; Guido, F.; Avataneo, V.; Cantu, M.; Carcieri, C.; et al. Vitamin D pathway gene polymorphisms and hepatocellular carcinoma in chronic hepatitis C-affected patients treated with new drugs. Cancer Chemother. Pharmacol. 2018, 81, 615–620. [Google Scholar] [CrossRef]
- Cusato, J.; Boglione, L.; De Nicolo, A.; Imbornone, R.; Cardellino, C.S.; Ghisetti, V.; Carcieri, C.; Cariti, G.; Di Perri, G.; D’Avolio, A. Association of vitamin D pathway SNPs and clinical response to interferon in a cohort of HBeAg-negative patients. Pharmacogenomics 2017, 18, 651–661. [Google Scholar] [CrossRef]
- Cusato, J.; Boglione, L.; De Nicolo, A.; Cardellino, C.S.; Carcieri, C.; Cariti, G.; Di Perri, G.; D’Avolio, A. Vitamin D pathway gene polymorphisms as predictors of hepatitis C virus-related mixed cryoglobulinemia. Pharmacogenet. Genom. 2016, 26, 307–310. [Google Scholar] [CrossRef]
- Cusato, J.; Allegra, S.; Boglione, L.; De Nicolo, A.; Baietto, L.; Cariti, G.; Di Perri, G.; D’Avolio, A. Vitamin D pathway gene variants and HCV-2/3 therapy outcomes. Antivir. Ther. 2014, 20, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Cusato, J.; Mula, J.; Palermiti, A.; Manca, A.; Antonucci, M.; Avataneo, V.; De Vivo, E.D.; Ianniello, A.; Calcagno, A.; Di Perri, G.; et al. Seasonal variation of antiretroviral drug exposure during the year: The experience of 10 years of therapeutic drug monitoring. Biomedicines 2021, 9, 1202. [Google Scholar] [CrossRef]
- Cervero, M.; Agud, J.L.; Garcia-Lacalle, C.; Alcazar, V.; Torres, R.; Jusdado, J.J.; Moreno Guillen, S. Prevalence of vitamin D deficiency and its related risk factor in a Spanish cohort of adult HIV-infected patients: Effects of antiretroviral therapy. AIDS Res. Hum. Retrovir. 2012, 28, 963–971. [Google Scholar] [CrossRef]
- Grigsby, I.F.; Pham, L.; Mansky, L.M.; Gopalakrishnan, R.; Mansky, K.C. Tenofovir-associated bone density loss. Ther. Clin. Risk Manag. 2010, 6, 41–47. [Google Scholar]
- Havens, P.L.; Kiser, J.J.; Stephensen, C.B.; Hazra, R.; Flynn, P.M.; Wilson, C.M.; Rutledge, B.; Bethel, J.; Pan, C.G.; Woodhouse, L.R.; et al. Association of higher plasma vitamin D binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: Cause of a functional vitamin D deficiency? Antimicrob. Agents Chemother. 2013, 57, 5619–5628. [Google Scholar] [CrossRef] [Green Version]
- Kimlin, M.G. Geographic location and vitamin D synthesis. Mol. Asp. Med. 2008, 29, 453–461. [Google Scholar] [CrossRef]
- Mormile, R. Vitamin D Intake and its Protective Role in Multiple Sclerosis: The Checkmate to Survivin? Iran J. Pharm. Res. 2016, 15, 383–384. [Google Scholar]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Klassen, K.M.; Fairley, C.K.; Kimlin, M.G.; Hocking, J.; Kelsall, L.; Ebeling, P.R. Vitamin D deficiency is common in HIV-infected southern Australian adults. Antivir. Ther. 2015, 21, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Vaida, F.; Wong, J.; Sanders, C.A.; Kao, Y.T.; Croteau, D.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.M.; et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J. Neurovirol. 2015, 22, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Ciccarelli, N.; Fabbiani, M.; Di Giambenedetto, S.; Fanti, I.; Baldonero, E.; Bracciale, L.; Tamburrini, E.; Cauda, R.; De Luca, A.; Silveri, M.C. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 2011, 76, 1403–1409. [Google Scholar] [CrossRef]
- Vergori, A.; Pinnetti, C.; Lorenzini, P.; Brita, A.; Libertone, R.; Mastrorosa, I.; Cicalini, S.; Antinori, A.; Ammassari, A. Vitamin D deficiency is associated with neurocognitive impairment in HIV-infected subjects. Infection 2019, 47, 929–935. [Google Scholar] [CrossRef]
| Characteristics | Turin Cohort | Rome Cohort | Total | p-Value |
|---|---|---|---|---|
| n° patients | 227 | 89 | 316 | |
| Age (year), median (IQR) | 46 (39–51) | 45 (37.5–53) | 44 (37.5–49) | 0.867 |
| Caucasian ethnicity, n (%) | 184 (81.1) | 72 (80.9) | 256 (81) | 0.003 |
| Male sex, n (%) | 177 (78) | 85 (95.5) | 262 (82.9) | <0.001 |
| Viral load (copies/mL), median (IQR) | 75.5 (28.8–34.8) | / | 75.5 (28.8–34.8) | / |
| CD4 (cells/mL), median (IQR) | 717 (553.3–870.0) | 546 (408.5–685.5) | 584 (450–746) | <0.001 |
| Vitamin D levels (ng/mL), median (IQR) | 22.3 (15.1–31.2) | 21.9 (16.1–28.8) | 22.3 (15.5–30.3) | 0.657 |
| Deficiency (≤10 ng/mL), n (%) | 23 (10.1) | 11 (12.4) | 34 (10.8) | 0.565 |
| Insufficiency (11–30 ng/mL), n (%) | 143 (63) | 61 (68.5) | 204 (64.6) | 0.333 |
| Sufficiency (>30 ng/mL), n (%) | 61 (26.9) | 17 (19.1) | 78 (24.7) | 0.339 |
| Vitamin D supplementation, n (%) | 17 (7.85) | / | 17 (7.5) | / |
| Efavirenz < 4000 ng/mL n (%) | Efavirenz ≥ 4000 ng/mL n (%) | Total n (%) | |||
|---|---|---|---|---|---|
| Turin | Deficiency (≤10) | 16 (69.6) | 7 (30.4) | 23 (100) | p = 0.017 |
| Insufficiency (11–30) | 123 (86.0) | 20 (14.0) | 143 (100) | ||
| Sufficiency (>30) | 57 (93.4) | 4 (6.6) | 61 (100) | ||
| Total | 196 (86.3) | 31 (13.7) | 227 (100) | ||
| Rome | Deficiency (≤10) | 3 (27.3) | 8 (72.7) | 11 (100) | p < 0.001 |
| Insufficiency (11–30) | 56 (91.8) | 5 (8.2) | 61 (100) | ||
| Sufficiency (>30) | 17 (100) | 17 (100) | |||
| Total | 76 (85.4) | 13 (14.6) | 89 (100) | ||
| Total | Deficiency (≤10) | 19 (55.9) | 15 (44.1) | 34 (100) | p < 0.001 |
| Insufficiency (11–30) | 179 (87.7) | 25 (12.3) | 204 (100) | ||
| Sufficiency (>30) | 74 (94.9) | 4 (5.1) | 78 (100) | ||
| Total | 272 (86.1) | 44 (13.9) | 316 (100) |
| Efv Levels < 1000 ng/mL | Efv Levels 1000–4000 ng/mL | Efv Levels > 4000 ng/mL | Total | |||
|---|---|---|---|---|---|---|
| No vitamin D supplementation | Deficient (≤10) | 16 (69.6) | 7 (30.4) | 23 (100) | ||
| Insufficient (11–30) | 5 (3.8) | 110 (83.3) | 17 (12.9) | 132 (100) | p = 0.042 | |
| Normal (>30) | 3 (5.5) | 49 (89.1) | 3 (5.5) | 55 (100) | ||
| Total | 8 (3.8) | 175 (83.3) | 27 (12.9) | 210 (100) | ||
| Vitamin D supplementation | Insufficient (11–30) | 8 (72.7) | 3 (27.3) | 11 (100) | ||
| Normal (>30) | 5 (83.3) | 1 (16.7) | 6 (100) | p = 0.622 | ||
| Total | 13 (76.5) | 4 (23.5) | 17 (100) | |||
| All | Deficient (≤10) | 16 (69.6) | 7 (30.4) | 23 (100) | ||
| Insufficient (11–30) | 5 (3.5) | 118 (82.5) | 20 (14.0) | 143 (100) | p = 0.064 | |
| Normal (>30) | 3 (4.9) | 54 (88.5) | 4 (6.6) | 61 (100) | ||
| Total | 8 (3.5) | 188 (82.8) | 31 (13.7) | 227 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusato, J.; Tempestilli, M.; Calcagno, A.; Vergori, A.; Piselli, P.; Antonucci, M.; Avataneo, V.; Palermiti, A.; Notari, S.; Antinori, A.; et al. Vitamin D as Modulator of Drug Concentrations: A Study on Two Italian Cohorts of People Living with HIV Administered with Efavirenz. Nutrients 2021, 13, 3571. https://doi.org/10.3390/nu13103571
Cusato J, Tempestilli M, Calcagno A, Vergori A, Piselli P, Antonucci M, Avataneo V, Palermiti A, Notari S, Antinori A, et al. Vitamin D as Modulator of Drug Concentrations: A Study on Two Italian Cohorts of People Living with HIV Administered with Efavirenz. Nutrients. 2021; 13(10):3571. https://doi.org/10.3390/nu13103571
Chicago/Turabian StyleCusato, Jessica, Massimo Tempestilli, Andrea Calcagno, Alessandra Vergori, Pierluca Piselli, Miriam Antonucci, Valeria Avataneo, Alice Palermiti, Stefania Notari, Andrea Antinori, and et al. 2021. "Vitamin D as Modulator of Drug Concentrations: A Study on Two Italian Cohorts of People Living with HIV Administered with Efavirenz" Nutrients 13, no. 10: 3571. https://doi.org/10.3390/nu13103571
APA StyleCusato, J., Tempestilli, M., Calcagno, A., Vergori, A., Piselli, P., Antonucci, M., Avataneo, V., Palermiti, A., Notari, S., Antinori, A., Di Perri, G., Agrati, C., & D’Avolio, A. (2021). Vitamin D as Modulator of Drug Concentrations: A Study on Two Italian Cohorts of People Living with HIV Administered with Efavirenz. Nutrients, 13(10), 3571. https://doi.org/10.3390/nu13103571








