Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Anthropometric and Biochemical Measurements
2.4. Indirect Calorimetry
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Participants
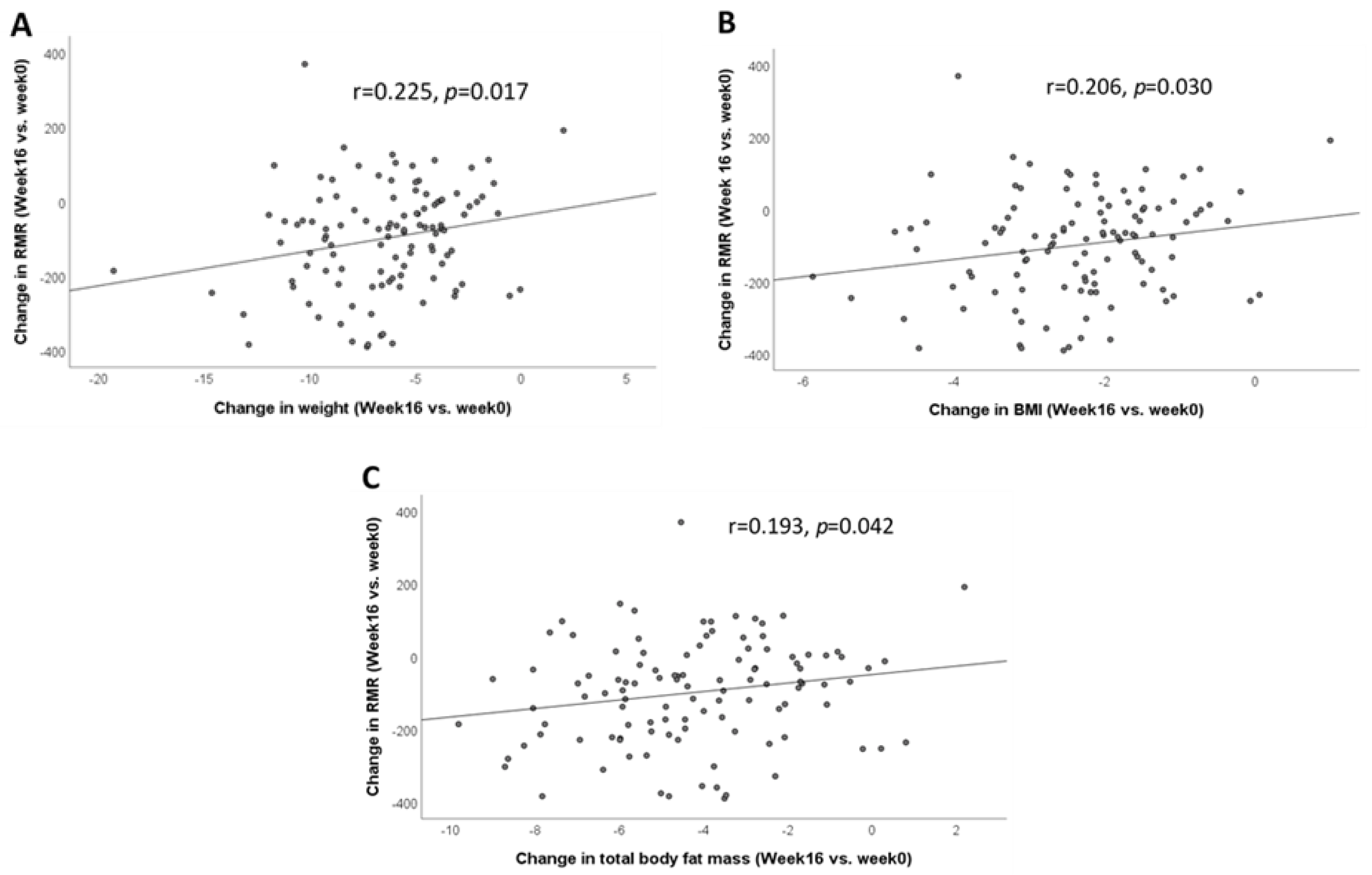
3.2. Correlation between Change in RMR and Change in Metabolic Measures after 16 Weeks of Weight Loss Intervention (Week 16 vs. Week 0)
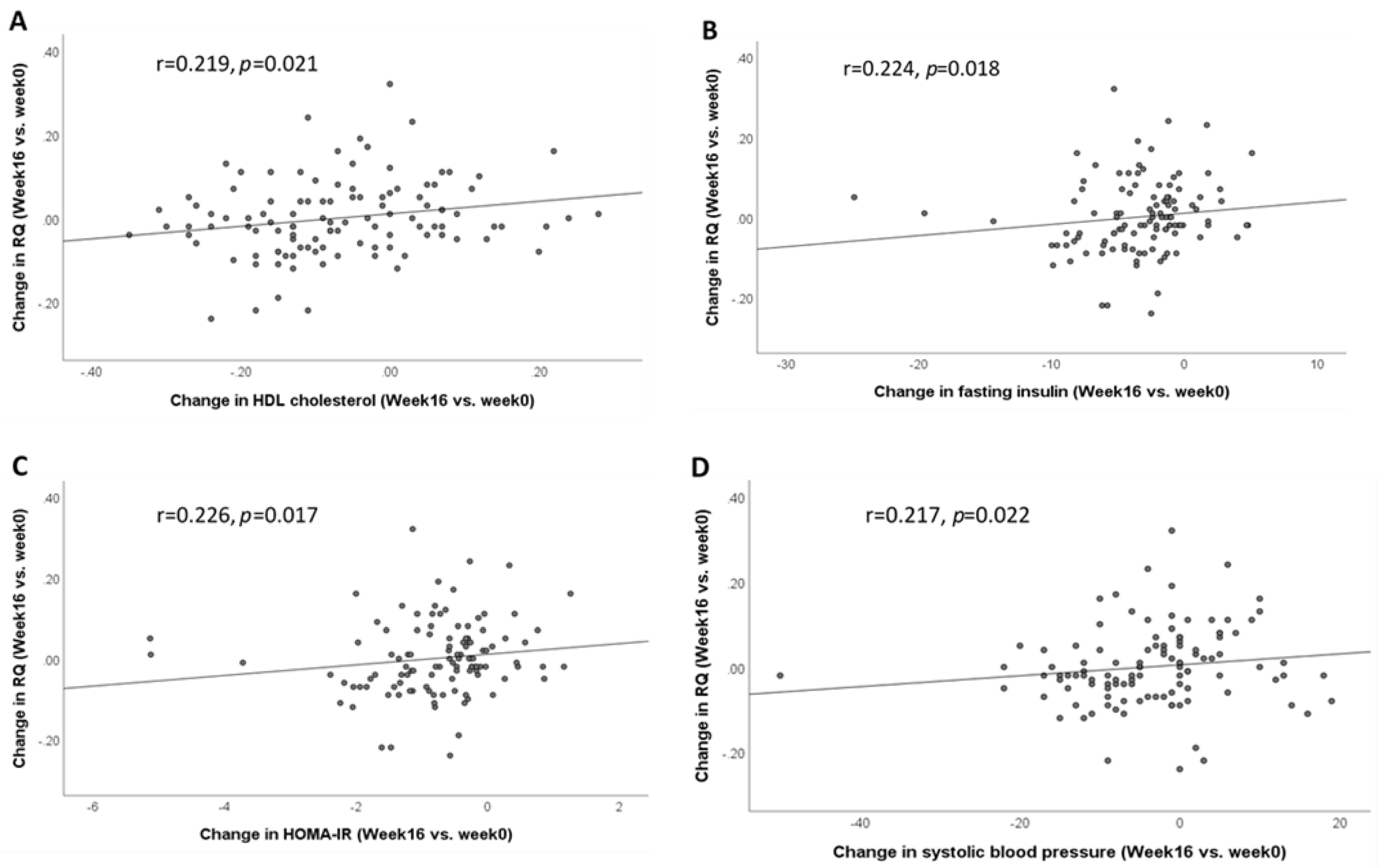
3.3. Correlation between Change in RQ and Change in Metabolic Measures after 16 Weeks of Weight Loss Intervention (Week 16 vs. Week 0)
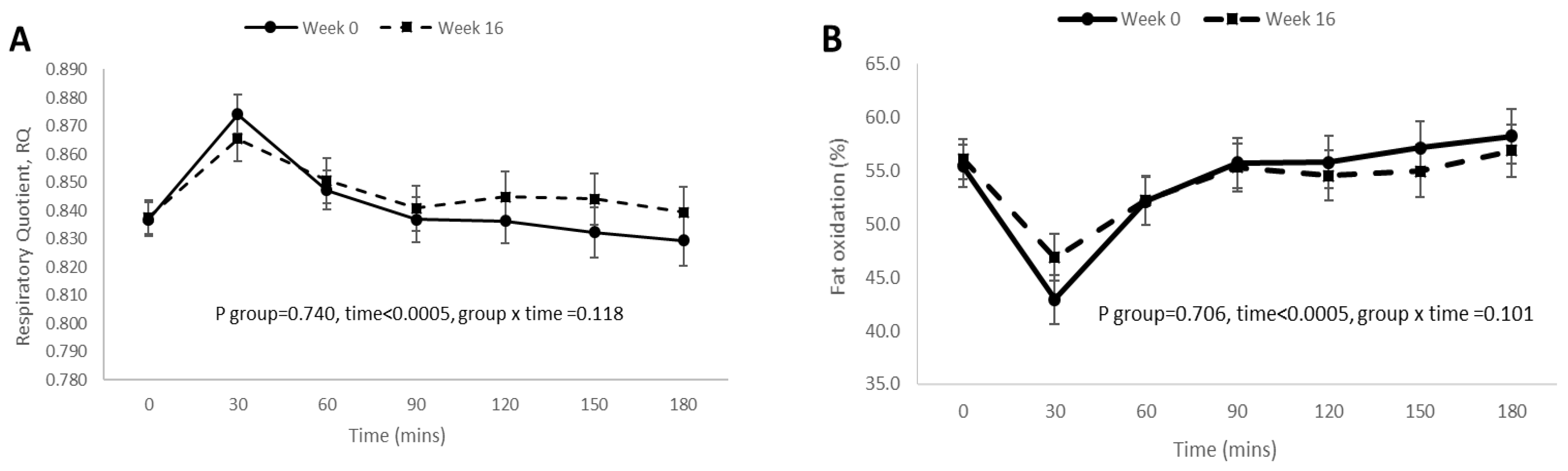
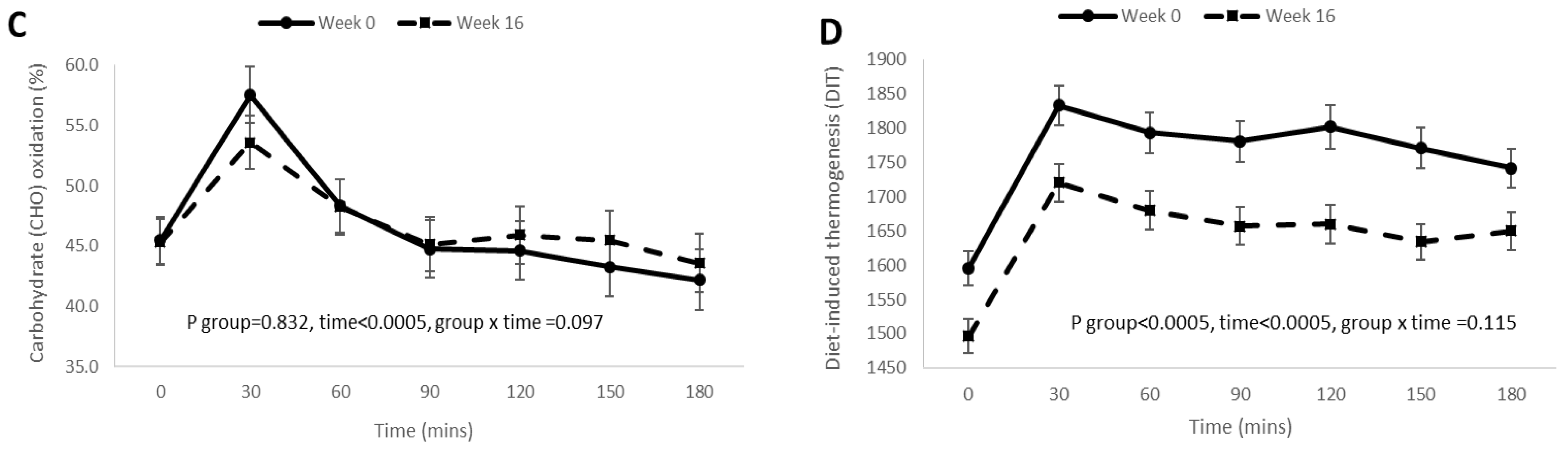
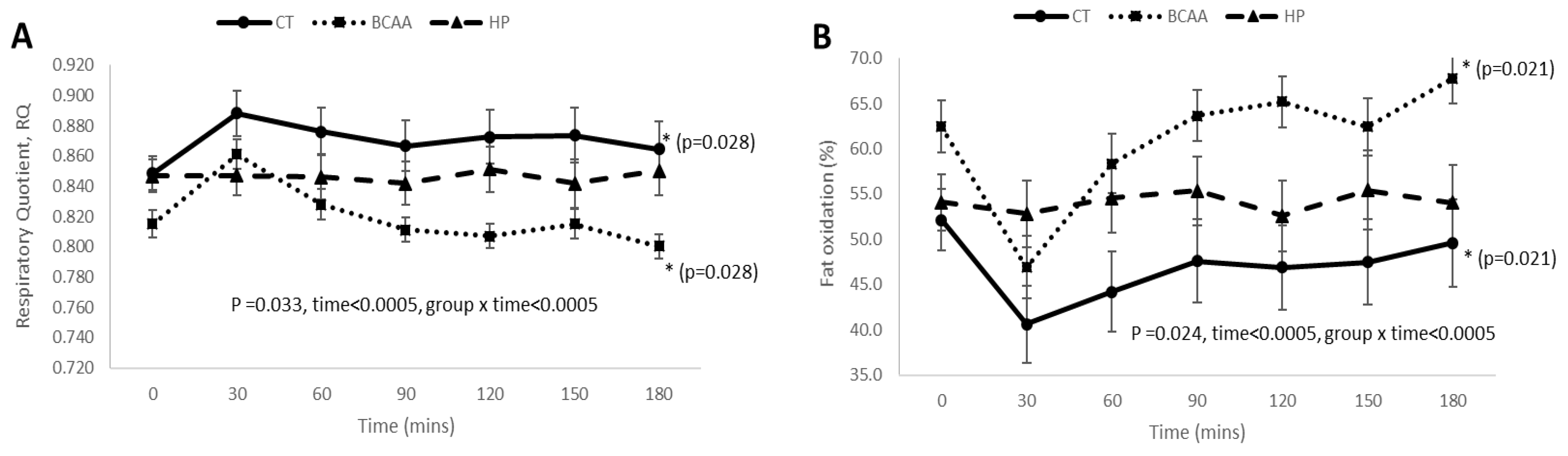
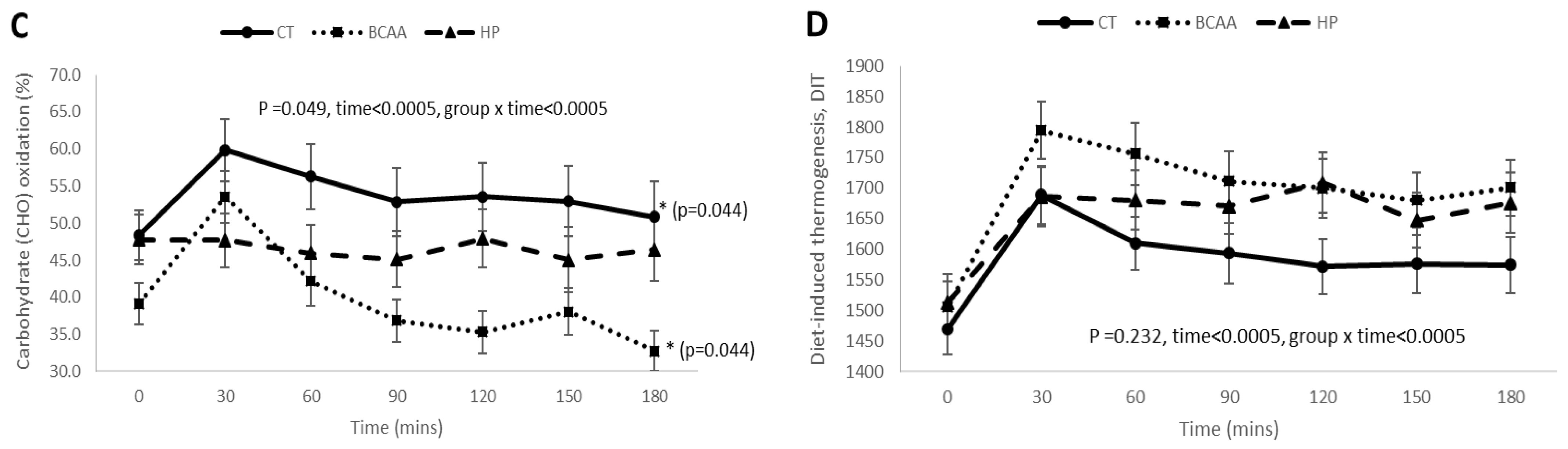
3.4. Postprandial RQ, Fat Oxidation, Carbohydrate Oxidation, and DIT Responses before and after 16 Weeks of Weight Loss Intervention
3.5. Postprandial RQ, Fat Oxidation, Carbohydrate Oxidation, and DIT Responses among the Three Diet Groups at Week 16 of Diet Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravussin, E.; Gautier, J. Metabolic predictors of weight gain. Int. J. Obes. 1999, 23, S37–S41. [Google Scholar] [CrossRef] [Green Version]
- Speakman, J.R.; Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 2003, 62, 621–634. [Google Scholar] [CrossRef]
- Westerterp, K.R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. [Google Scholar] [CrossRef]
- Cunningham, J.J. Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation. Am. J. Clin. Nutr. 1991, 54, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Weinsier, R.L.; Long, C.L.; Schutz, Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am. J. Clin. Nutr. 1992, 56, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Sparti, A.; DeLany, J.; de la Bretonne, J.A.; Sander, G.E.; Bray, G.A. Relationship between resting metabolic rate and the composition of the fat-free mass. Metabolism 1997, 46, 1225–1230. [Google Scholar] [CrossRef]
- Ravussin, E.; Burnand, B.; Schutz, Y.; Jéquier, E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am. J. Clin. Nutr. 1982, 35, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Weinsier, R.L.; Hunter, G.R.; Zuckerman, P.A.; Redden, D.T.; Darnell, B.E.; Larson, D.E.; Newcomer, B.R.; Goran, M.I. Energy expenditure and free-living physical activity in black and white women: Comparison before and after weight loss. Am. J. Clin. Nutr. 2000, 71, 1138–1146. [Google Scholar] [CrossRef] [Green Version]
- Goele, K.; Bosy-Westphal, A.; Rümcker, B.; Lagerpusch, M.; Müller, M.J. Influence of changes in body composition and adaptive thermogenesis on the difference between measured and predicted weight loss in obese women. Obes. Facts 2009, 2, 105–109. [Google Scholar] [CrossRef]
- Tremblay, A.; Royer, M.-M.; Chaput, J.-P.; Doucet, E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int. J. Obes. 2013, 37, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Doucet, E.; St-Pierre, S.; Alméras, N.; Després, J.-P.; Bouchard, C.; Tremblay, A. Evidence for the existence of adaptive thermogenesis during weight loss. Br. J. Nutr. 2001, 85, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.J.; Enderle, J.; Bosy-Westphal, A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr. Obes. Rep. 2016, 5, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trexler, E.T.; Smith-Ryan, A.E.; Norton, L.E. Metabolic adaptation to weight loss: Implications for the athlete. J. Int. Soc. Sports Nutr. 2014, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravussin, E.; Lillioja, S.; Knowler, W.C.; Christin, L.; Freymond, D.; Abbott, W.G.; Boyce, V.; Howard, B.V.; Bogardus, C. Reduced Rate of Energy Expenditure as a Risk Factor for Body-Weight Gain. N. Engl. J. Med. 1988, 318, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Verga, S.; Caimi, G.; Cerasola, G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int. J. Obes. 2005, 29, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, M.; Leibel, R.L. Adaptive thermogenesis in humans. Int. J. Obes. 2010, 34, S47–S55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyer, C.; Pratley, R.E.; Salbe, A.D.; Bogardus, C.; Ravussin, E.; Tataranni, P.A. Energy Expenditure, Fat Oxidation, and Body Weight Regulation: A Study of Metabolic Adaptation to Long- Term Weight Change. J. Clin. Endocrinol. Metab. 2000, 85, 1087–1094. [Google Scholar] [CrossRef]
- Shook, R.P.; Hand, G.A.; Paluch, A.E.; Wang, X.; Moran, R.G.; Hebert, J.R.; Jakicic, J.M.; Blair, S.N. High respiratory quotient is associated with increases in body weight and fat mass in young adults. Eur. J. Clin. Nutr. 2016, 70, 1197–1202. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Smith-Ryan, A.E.; Blue, M.N.; Mock, M.G.; Trexler, E.T. Influence of segmental body composition and adiposity hormones on resting metabolic rate and substrate utilization in overweight and obese adults. J. Endocrinol. Investig. 2017, 40, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lightowler, H.; Schweitzer, L.; Theis, S.; Henry, C.J. Changes in Weight and Substrate Oxidation in Overweight Adults Following Isomaltulose Intake During a 12-Week Weight Loss Intervention: A Randomized, Double-Blind, Controlled Trial. Nutrients 2019, 11, 2367. [Google Scholar] [CrossRef] [Green Version]
- Carbone, J.W.; Pasiakos, S.M. Dietary Protein and Muscle Mass: Translating Science to Application and Health Benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef] [Green Version]
- Sahni, S.; Mangano, K.; Hannan, M.T.; Kiel, D.; McLean, R.R. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, E.; Luscombe, N.D.; Noakes, M.; Wittert, G.; Argyiou, E.; Clifton, P.M. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am. J. Clin. Nutr. 2003, 78, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Verreijen, A.M.; Verlaan, S.; Engberink, M.F.; Swinkels, S.; Bosch, J.D.V.-V.D.; Weijs, P.J.M. A high whey protein–, leucine-, and vitamin D–enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Edmonds, R.; He, F.; Ward, E.; Gumpricht, E.; Mohr, A.; Ormsbee, M.J.; Astrup, A. Protein-Pacing Caloric-Restriction Enhances Body Composition Similarly in Obese Men and Women during Weight Loss and Sustains Efficacy during Long-Term Weight Maintenance. Nutrients 2016, 8, 476. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, L.; Scalera, G.; Pilone, V.; de Sena, G.; Quagliariello, V.; Iannelli, A.; Barbarisi, A. A Comparative Study Examining the Impact of a Protein-Enriched Vs Normal Protein Postoperative Diet on Body Composition and Resting Metabolic Rate in Obese Patients after Sleeve Gastrectomy. Obes. Surg. 2017, 27, 881–888. [Google Scholar] [CrossRef]
- Spillane, M.; Schwarz, N.; Willoughby, D.S. Heavy resistance training and peri-exercise ingestion of a multi-ingredient ergogenic nutritional supplement in males: Effects on body composition, muscle performance and markers of muscle protein synthesis. J. Sports Sci. Med. 2014, 13, 894–903. [Google Scholar]
- AbuMoh’d, M.F.; Matalqah, L.; Al-Abdulla, Z. Effects of Oral Branched-Chain Amino Acids (BCAAs) Intake on Muscular and Central Fatigue During an Incremental Exercise. J. Hum. Kinet. 2020, 72, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K.; et al. Branched-Chain Amino Acid Supplementation Before Squat Exercise and Delayed-Onset Muscle Soreness. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, W.D.; Kelley, E.P.; Scheett, T.P. In a single-blind, matched group design: Branched-chain amino acid supplementation and resistance training maintains lean body mass during a caloric restricted diet. J. Int. Soc. Sports Nutr. 2016, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mourier, A.; Bigard, A.X.; de Kerviler, E.; Roger, B.; Legrand, H.; Guezennec, C.Y. Combined Effects of Caloric Restriction and Branched-Chain Amino Acid Supplementation on Body Composition and Exercise Performance in Elite Wrestlers. Int. J. Sports Med. 1997, 18, 47–55. [Google Scholar] [CrossRef]
- Novin, Z.S.; Ghavamzadeh, S.; Mehdizadeh, A. The Weight Loss Effects of Branched Chain Amino Acids and Vitamin B6: A Randomized Controlled Trial on Obese and Overweight Women. Int. J. Vitam. Nutr. Res. 2018, 88, 80–89. [Google Scholar] [CrossRef]
- Ooi, D.S.Q.; Ling, J.Q.R.; Sadananthan, S.A.; Velan, S.S.; Ong, F.Y.; Khoo, C.M.; Tai, E.S.; Henry, C.J.; Leow, M.K.S.; Khoo, E.Y.H.; et al. Branched-Chain Amino Acid Supplementation Does Not Preserve Lean Mass or Affect Metabolic Profile in Adults with Overweight or Obesity in a Randomized Controlled Weight Loss Intervention. J. Nutr. 2021, 151, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, E.J.; Smith-Ryan, A.E. Effects of Branched-chain Amino Acids on Resting Metabolic Rate, Body Composition, And Satiety in Females. Med. Sci. Sports Exerc. 2017, 49, 81. [Google Scholar] [CrossRef]
- Tamburo, S.; Sponsiello, N. Short–term effects of BCAA, Arginine and CLA supplementation on rest energy expenditure and body composition. In Proceedings of the 16th Annual Congress of the European College of Sport Science–Ecss, Liverpool, UK, 6–9 July 2011; Volume 18, pp. 47–55. [Google Scholar]
- She, P.; Reid, T.M.; Bronson, S.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of BCATm in Mice Leads to Increased Energy Expenditure Associated with the Activation of a Futile Protein Turnover Cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarrah, R.W.; Zhang, G.-F.; Christopher, B.A.; Deleye, Y.; Walejko, J.M.; Page, S.; Ilkayeva, O.; White, P.J.; Newgard, C.B. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am. J. Physiol. Metab. 2020, 318, E216–E223. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Bozza, T.; Campos-Ferraz, P.; Roschel, H.; Costa, A.; Marquezi, M.; Benatti, F.; Lancha, A., Jr. Branched chain amino acids supplementation enhance exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J. Sports Med. Phys. Fit. 2011, 51, 82–88. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Fouré, A.; Nosaka, K.; Gastaldi, M.; Mattei, J.-P.; Boudinet, H.; Guye, M.; Vilmen, C.; Le Fur, Y.; Bendahan, D.; Gondin, J. Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: A randomized placebo-controlled trial. Clin. Nutr. 2016, 35, 83–94. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kobayashi, H.; Mawatari, K.; Akita, K.; Inaguma, A.; Watanabe, S.; Bajotto, G.; Sato, J. Effects of squat exercise and branched-chain amino acid supplementation on plasma free amino acid concentrations in young women. J. Nutr. Sci. Vitaminol. 2009, 55, 288–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2017, 174, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Luscombe, N.D.; Tsopelas, C.; Bellon, M.; Clifton, P.M.; Kirkwood, I.; Wittert, G.A. Use of [14C]-sodium bicarbonate/urea to measure total energy expenditure in overweight men and women before and after low calorie diet induced weight loss. Asia Pac. J. Clin. Nutr. 2006, 15, 307–316. [Google Scholar]
- Henry, J.; Lightowler, H.J.; Marchini, J. Intra-individual variation in resting metabolic rate during the menstrual cycle. Br. J. Nutr. 2003, 89, 811–817. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 1990, 6, 213–221. [Google Scholar]
- Zurlo, F.; Lillioja, S.; Puente, A.E.-D.; Nyomba, B.L.G.; Raz, I.; Saad, M.F.; Swinburn, B.A.; Knowler, W.C.; Bogardus, C.; Ravussin, E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. Metab. 1990, 259, E650–E657. [Google Scholar] [CrossRef] [PubMed]
- Heshka, S.; Yang, M.U.; Wang, J.; Burt, P.; Pi-Sunyer, F.X. Weight loss and change in resting metabolic rate. Am. J. Clin. Nutr. 1990, 52, 981–986. [Google Scholar] [CrossRef]
- Pujia, A.; Mazza, E.; Ferro, Y.; Gazzaruso, C.; Coppola, A.; Doldo, P.; Grembiale, R.D.; Pujia, R.; Romeo, S.; Montalcini, T. Lipid Oxidation Assessed by Indirect Calorimetry Predicts Metabolic Syndrome and Type 2 Diabetes. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Liu, H.; Li, Y.; Liu, Y.; Kong, X.; Zhang, Y.; Deng, D.; Tang, Y.; Feng, Z.; et al. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front. Biosci. 2015, 20, 796–813. [Google Scholar] [CrossRef] [Green Version]
- Evangeliou, A.; Spilioti, M.; Doulioglou, V.; Kalaidopoulou, P.; Ilias, A.; Skarpalezou, A.; Katsanika, I.; Kalamitsou, S.; Vasilaki, K.; Chatziioanidis, I.; et al. Branched Chain Amino Acids as Adjunctive Therapy to Ketogenic Diet in Epilepsy: Pilot Study and Hypothesis. J. Child Neurol. 2009, 24, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Bendahan, D. Is Branched-Chain Amino Acids Supplementation an Efficient Nutritional Strategy to Alleviate Skeletal Muscle Damage? A Systematic Review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters | CT (n = 37) | BCAA (n = 35) | HP (n = 39) | p |
|---|---|---|---|---|
| Weight (kg) | −6.62 ± 3.44 | −6.39 ± 3.99 | −6.35 ± 2.37 | 0.931 |
| BMI (kg/m2) | −2.45 ± 1.36 | −2.26 ± 1.33 | −2.33 ± 0.811 | 0.780 |
| Waist circumference (cm) | −6.32 ± 3.69 | −7.43 ± 4.55 | −7.18 ± 3.12 | 0.427 |
| Body fat percentage (%) | −2.34 ± 1.95 | −2.37 ± 2.41 | −2.75 ± 1.92 | 0.631 |
| Total body fat mass (kg) | −4.23 ± 2.24 | −4.09 ± 2.88 | −4.34 ± 1.91 | 0.902 |
| Total body lean mass (kg) | −2.39 ± 1.50 | −2.15 ± 1.74 | −1.80 ± 1.52 | 0.272 |
| RMR (kcal/day) | −98.6 ± 165 | −108 ± 119 | −90.8 ± 140 | 0.874 |
| Fat (%) | −2.35 ± 27.0 | 4.06 ± 23.4 | −0.95 ± 32.6 | 0.599 |
| CHO (%) | 2.35 ± 26.9 | −4.09 ± 23.4 | 0.95 ± 32.5 | 0.595 |
| RQ | 0.008 ± 0.09 | −0.012 ± 0.07 | 0.006 ± 0.11 | 0.599 |
| Total cholesterol (mmol/L) | −0.32 ± 0.60 | −0.25 ± 0.51 | −0.36 ± 0.48 | 0.648 |
| Triglycerides (mmol/L) | −0.13 ± 0.38 | −0.17 ± 0.54 | −0.23 ± 0.31 | 0.581 |
| HDL cholesterol (mmol/L) | −0.08 ± 0.13 | −0.07 ± 0.14 | −0.05 ± 0.12 | 0.469 |
| LDL cholesterol (mmol/L) | −0.17 ± 0.47 | −0.10 ± 0.49 | −0.21 ± 0.40 | 0.585 |
| Fasting glucose (mmol/L) | −0.19 ± 0.32 | −0.15 ± 0.25 | −0.16 ± 0.31 | 0.874 |
| Fasting insulin (mU/L) | −3.54 ± 4.21 | −3.60 ± 4.96 | −2.96 ± 3.77 | 0.779 |
| HOMA-IR | −0.84 ± 1.06 | −0.79 ± 1.03 | −0.68 ± 0.91 | 0.768 |
| Systolic blood pressure (mmHg) | −3 ± 8 | −4 ± 9 | −3 ± 12 | 0.932 |
| Diastolic blood pressure (mmHg) | −3 ± 9 | −6 ± 6 | −2 ± 11 | 0.138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, D.S.Q.; Ling, J.Q.R.; Ong, F.Y.; Tai, E.S.; Henry, C.J.; Leow, M.K.S.; Khoo, E.Y.H.; Tan, C.S.; Chong, M.F.F.; Khoo, C.M.; et al. Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention. Nutrients 2021, 13, 4245. https://doi.org/10.3390/nu13124245
Ooi DSQ, Ling JQR, Ong FY, Tai ES, Henry CJ, Leow MKS, Khoo EYH, Tan CS, Chong MFF, Khoo CM, et al. Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention. Nutrients. 2021; 13(12):4245. https://doi.org/10.3390/nu13124245
Chicago/Turabian StyleOoi, Delicia Shu Qin, Jennifer Qiu Rong Ling, Fang Yi Ong, E Shyong Tai, Christiani Jeyakumar Henry, Melvin Khee Shing Leow, Eric Yin Hao Khoo, Chuen Seng Tan, Mary Foong Fong Chong, Chin Meng Khoo, and et al. 2021. "Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention" Nutrients 13, no. 12: 4245. https://doi.org/10.3390/nu13124245
APA StyleOoi, D. S. Q., Ling, J. Q. R., Ong, F. Y., Tai, E. S., Henry, C. J., Leow, M. K. S., Khoo, E. Y. H., Tan, C. S., Chong, M. F. F., Khoo, C. M., & Lee, Y. S. (2021). Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention. Nutrients, 13(12), 4245. https://doi.org/10.3390/nu13124245







