Effects of Simultaneous Exposure to a Western Diet and Wheel-Running Training on Brain Energy Metabolism in Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Groups
2.2. Dietary Protocol
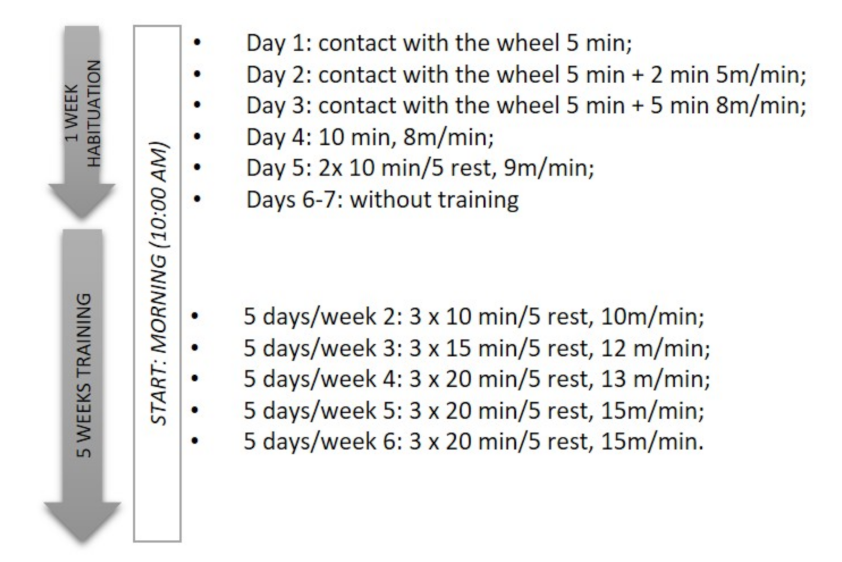
2.3. Wheel-Running Training
2.4. Tissue Collection
2.5. Biochemical and Hormonal Assays
2.6. Global Proteomic Profiling
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Wheel Running Modifies the Biochemical, but Not Phenotypic, Changes Induced by the Consumption of a Western Diet
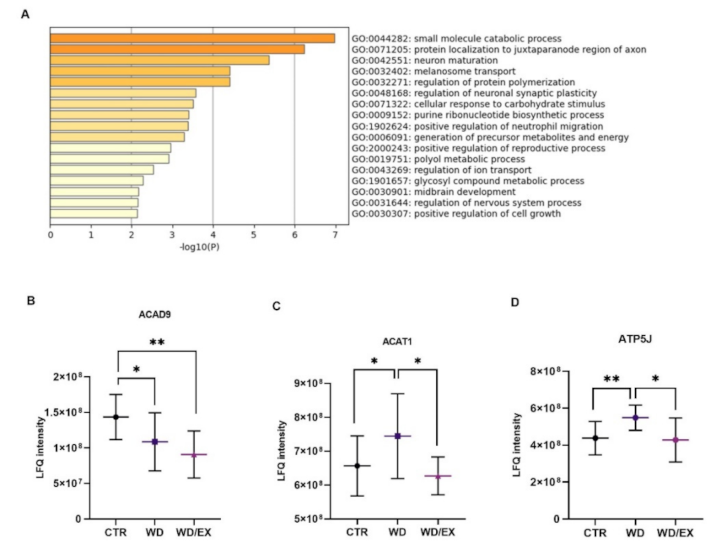
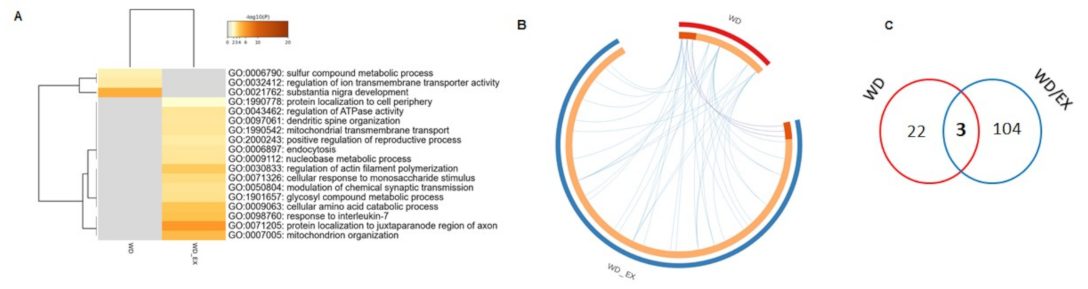
3.2. The Combination of a Western Diet and Wheel Running Alters the Rat Cerebrocortical Proteome Profile
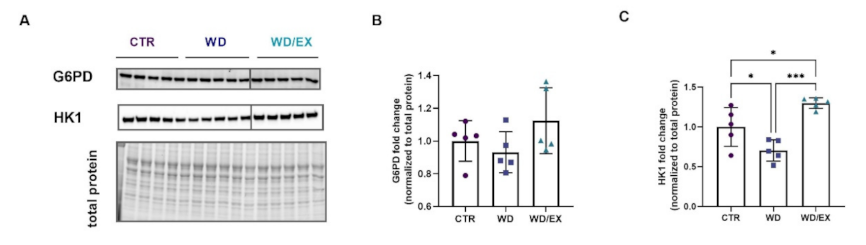
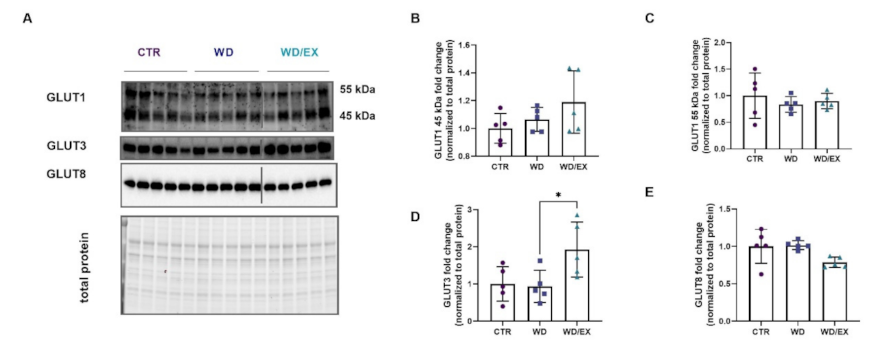
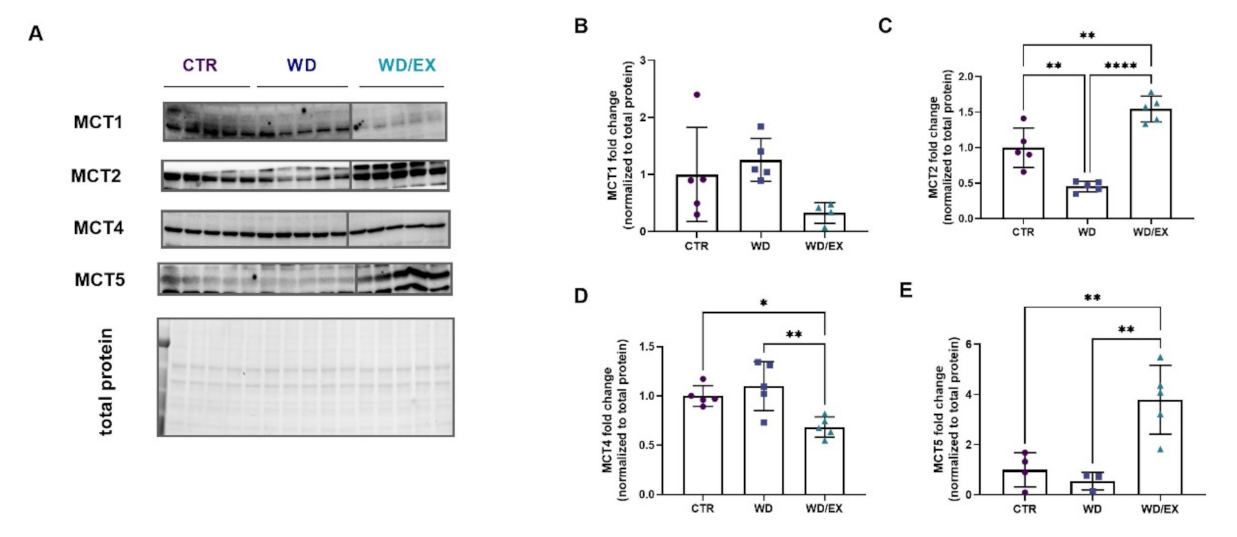
3.3. Simultaneous Exposure to a Western Diet and Wheel Running Induced Changes in the Metabolic Proteins of the Frontal Cortex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Protein Extraction
Appendix A.2. In-Solution Digestion
Appendix A.3. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis of Proteins
Appendix A.4. Data Analysis of Proteins
Appendix B
References
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Mayer, E.A. Food and mood: How do diet and nutrition affect mental wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef]
- Francis, H.; Stevenson, R. The longer-term impacts of Western diet on human cognition and the brain. Appetite 2013, 63, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Hargrave, S.L.; Davidson, T.L.; Lee, T.-J.; Kinzig, K.P. Brain and behavioral perturbations in rats following Western diet access. Appetite 2015, 93, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bruce-Keller, A.J.; Dasuri, K.; Nguyen, A.T.; Liu, Y.; Keller, J.N. Diet-induced metabolic disturbances as modulators of brain homeostasis. Biochim. Biophys. Acta 2009, 1792, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Gildengers, A.G.; Butters, M.A. Physical activity and brain plasticity in late adulthood. Dialogues Clin. Neurosci. 2013, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Fahimi, A. Immune and Neuroprotective effects of physical activity on the brain in depression. Front. Neurosci. 2018, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; Casagrande, A.d.S.; Pedroso, G.d.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Fleshner, M.; Schwartz, M.W.; Mattson, M.P. Exercise, energy intake, glucose homeostasis, and the brain. J. Neurosci. 2014, 34, 15139–15149. [Google Scholar] [CrossRef] [PubMed]
- Toval, A.; Baños, R.; De la Cruz, E.; Morales-Delgado, N.; Pallares, J.G.; Ayad, A.; Tseng, Y.K.; Ferran, L.J. Habituation training improves locomotor performance in a forced running wheel system in rats. Front. Behav. Neurosci. 2017, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, M.W.; Chang, C.P.; Chan, S.C.; Chang, W.Y.; Yang, C.L.; Lin, M.T. A forced running wheel system with a microcontroller that provides high-intensity exercise training in an animal ischemic stroke model. Braz. J. Med. Biol. Res. 2014, 47, 858–868. [Google Scholar] [CrossRef]
- Shields, G.S.; McCullough, A.M.; Ritchey, M.; Ranganath, C.; Yonelinas, A.P. Stress and the medial temporal lobe at rest: Functional connectivity is associated with both memory and cortisol. Psychoneuroendocrinology 2019, 106, 138–146. [Google Scholar] [CrossRef]
- Treviño, S.; Aguilar-Alonso, P.; Flores Hernandez, J.A.; Brambila, E.; Guevara, J.; Flores, G.; Lopez-Lopez, G.; Muñoz-Arenas, G.; Morales-Medina, J.C.; Toxqui, V.; et al. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse 2015, 69, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Berchicci, M.; Menotti, F.; Macaluso, A.; Di Russo, F. The neurophysiology of central and peripheral fatigue during sub-maximal lower limb isometric contractions. Front. Hum. Neurosci. 2013, 7, 135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bugg, J.M.; Head, D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 2011, 32, 506–514. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, Q.; Wang, L.; Jiang, L.; Hu, M.; Jiang, Y.; Tang, J.; Liang, X.; Qi, Y.; Dou, X.; et al. Running exercise protects oligodendrocytes in the medial prefrontal cortex in chronic unpredictable stress rat model. Trans. Psychiatr. 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Chella, K.K.; Mehrabian, M.; Lusis, A. Sex differences in metabolism and cardiometabolic disorders. Curr. Opin. Lipidol. 2018, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Tronieri, J.S.; Wurst, C.M.; Pearl, R.L.; Allison, K.C. Sex Differences in Obesity and Mental Health. Curr. Psychiatr. Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Nowacka-Chmielewska, M.; Liśkiewicz, D.; Liśkiewicz, A.; Marczak, L.; Wojakowska, A.; Barski, J.J.; Małecki, A. Cerebrocortical proteome profile of female rats subjected to the western diet and chronic social stress. Nutr. Neurosci. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER:a library of protein families and subfamilies indexed by function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Kejariwal, A.; Guo, N.; Mi, H.; Campbell, M.J.; Muruganujan, A.; Lazareva-Ulitsky, B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006, 34, W645–W650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Hintze, K.J.; Benninghoff, A.D.; Cho, C.E.; Ward, R.E. Modeling the Western Diet for Preclinical Investigations. Adv. Nutr. 2018, 9, 263–271. [Google Scholar] [CrossRef]
- Leigh, S.-J.; Kendig, M.D.; Morris, M.J. Palatable Western-style Cafeteria Diet as a Reliable Method for Modeling Diet-induced Obesity in Rodents. J. Vis. Exp. 2019, 153, e60262. [Google Scholar] [CrossRef]
- Leasure, J.L.; Jones, M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 2008, 156, 456–465. [Google Scholar] [CrossRef]
- Uysal, N.; Kiray, M.; Sisman, A.R.; Camsari, U.; Gencoglu, C.; Baykara, B.; Cetinkaya, C.; Aksu, I. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2015, 90, 55–68. [Google Scholar] [CrossRef]
- Foright, R.M.; Johnson, G.C.; Kahn, D.; Catherine, A.; Charleston, C.A.; Presby, D.M.; Bouchet, C.A.; Wellberg, E.A.; Sherk, V.D.; Jackman, M.R.; et al. Compensatory eating behaviors in male and female rats in response to exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R171–R183. [Google Scholar] [CrossRef] [PubMed]
- Nouws, J.; Nijtmans, L.; Houten, S.M.; den Brand, M.; Huynen, M.; Venselaar, H.; Hoefs, S.; Gloerich, J.; Kronick, J.; Hutchin, T.; et al. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell Metab. 2010, 12, 283–294. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Santos-Alves, E.; Balça, M.M.; Rizo-Roca, D.; Moreira, P.I.; Oliveira, P.J.; Magalhães, J.; Ascensão, A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience 2015, 301, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Huertas, J.R.; Al Fazazi, S.; Hidalgo-Gutierrez, A.; López, L.C.; Casuso, R.A. Antioxidant effect of exercise: Exploring the role of the mitochondrial complex I superassembly. Redox Biol. 2017, 13, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Taylor, A.W.; Ohno, H.; Goto, S. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc. Immunol. Rev. 2001, 7, 90–107. [Google Scholar] [PubMed]
- Radak, Z.; Kumagai, S.; Taylor, A.W.; Naito, H.; Goto, S. Effects of exercise on brain function: Role of free radicals. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2007, 32, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Darshi, M.; Ma, Y.; Perkins, G.A.; Shen, Z.; Haushalter, K.J.; Saito, R.; Chen, A.; Lee, Y.S.; Patel, H.H.; et al. Quantitative proteomic and functional analysis of liver mitochondria from high fat diet (HFD) diabetic mice. Mol. Cell Proteom. 2013, 12, 3744–3758. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Melton, E.M.; Li, H.; Sohn, P.; Jung, D.; Tsai, C.-Y.; Ma, T.; Sano, H.; Ha, H.; Friedline, R.H.; et al. Myeloid-specific Acat1 ablation attenuates inflammatory responses in macrophages, improves insulin sensitivity, and suppresses diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Song, Y.; Zeng, Y.; Chen, L.; Yan, F.; Chen, A.; Wu, B.; Wang, Y. Improvement of hyperlipidemia by aerobic exercise in mice through a regulatory effect of miR-21a-5p on its target genes. Sci. Rep. 2021, 11, 11966. [Google Scholar] [CrossRef]
- Iadecola, C. Sugar and Alzheimer’s disease: A bittersweet truth. Nat. Neurosci. 2015, 18, 477–478. [Google Scholar] [CrossRef][Green Version]
- Dunn, L.; Allen, G.F.; Mamais, A.; Ling, H.; Li, A.; Duberley, K.E.; Hargreaves, L.P.; Pope, S.; Holton, J.L.; Lees, A.; et al. Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol. Aging 2014, 35, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; López-Rodas, G.; Casanova, B.; Marti, M.B. Perturbed glucose metabolism: Insights into multiple sclerosis pathogenesis. Front. Neurol. 2014, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, V.; Adriaanse, M.C.; van’t Riet, E.; Snoek, F.J.; Dekker, J.M.; Nijpels, G. Depression, anxiety and glucose metabolism in the general dutch population: The new Hoorn study. PLoS ONE 2010, 5, e9971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lachance, B.B.; Mattson, M.P.; Jia, X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog. Neurobiol. 2021, 204, 102089. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, Y.; Li, Y.; Gao, L.; Zhao, J. Alpha-lipoic acid attenuates insulin resistance and improves glucose metabolism in high fat diet-fed mice. Acta Pharmacol. Sin. 2014, 35, 1285–1292. [Google Scholar] [CrossRef]
- Pierre, K.; Parent, A.; Jayet, P.Y.; Halestrap, A.P.; Scherrer, U.; Pellerin, L. Enhanced expression of three monocarboxylate transporter isoforms in the brain of obese mice. J. Physiol. 2007, 583, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants (Basel) 2020, 9, 140. [Google Scholar] [CrossRef]
- Bunprajun, T.; Henriksen, T.I.; Scheele, C.; Pedersen, B.K.; Green, C.J. Lifelong Physical Activity Prevents Aging-Associated Insulin Resistance in Human Skeletal Muscle Myotubes via Increased Glucose Transporter Expression. PLoS ONE 2013, 8, e66628. [Google Scholar] [CrossRef]
- Takimoto, M.; Hamada, T. Acute exercise increases brain region-specific expression of MCT1, MCT2, MCT4, GLUT1, and COX IV proteins. J. Appl. Physiol. 2014, 116, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka-Chmielewska, M.M.; Liśkiewicz, D.; Grabowska, K.; Liśkiewicz, A.; Marczak, Ł.; Wojakowska, A.; Pondel, N.; Grabowski, M.; Barski, J.J.; Małecki, A. Effects of Simultaneous Exposure to a Western Diet and Wheel-Running Training on Brain Energy Metabolism in Female Rats. Nutrients 2021, 13, 4242. https://doi.org/10.3390/nu13124242
Nowacka-Chmielewska MM, Liśkiewicz D, Grabowska K, Liśkiewicz A, Marczak Ł, Wojakowska A, Pondel N, Grabowski M, Barski JJ, Małecki A. Effects of Simultaneous Exposure to a Western Diet and Wheel-Running Training on Brain Energy Metabolism in Female Rats. Nutrients. 2021; 13(12):4242. https://doi.org/10.3390/nu13124242
Chicago/Turabian StyleNowacka-Chmielewska, Marta Maria, Daniela Liśkiewicz, Konstancja Grabowska, Arkadiusz Liśkiewicz, Łukasz Marczak, Anna Wojakowska, Natalia Pondel, Mateusz Grabowski, Jarosław Jerzy Barski, and Andrzej Małecki. 2021. "Effects of Simultaneous Exposure to a Western Diet and Wheel-Running Training on Brain Energy Metabolism in Female Rats" Nutrients 13, no. 12: 4242. https://doi.org/10.3390/nu13124242
APA StyleNowacka-Chmielewska, M. M., Liśkiewicz, D., Grabowska, K., Liśkiewicz, A., Marczak, Ł., Wojakowska, A., Pondel, N., Grabowski, M., Barski, J. J., & Małecki, A. (2021). Effects of Simultaneous Exposure to a Western Diet and Wheel-Running Training on Brain Energy Metabolism in Female Rats. Nutrients, 13(12), 4242. https://doi.org/10.3390/nu13124242






