Abstract
Several recent studies have demonstrated that the direct precursor of vitamin D3, the calcifediol [25(OH)D3], through the binding to the nuclear vitamin D receptor (VDR), is able to regulate the expression of many genes involved in several cellular processes. Considering that itself may function as a VDR ligand, although with a lower affinity, respect than the active form of vitamin D, we have assumed that 25(OH)D3 by binding the VDR could have a vitamin’s D3 activity such as activating non-genomic pathways, and in particular we selected mesenchymal stem cells derived from human adipose tissue (hADMSCs) for the in vitro assessment of the intracellular Ca2+ mobilization in response to 25(OH)D3. Our result reveals the ability of 25(OH)D3 to activate rapid, non-genomic pathways, such as an increase of intracellular Ca2+ levels, similar to what observed with the biologically active form of vitamin D3. hADMSCs loaded with Fluo-4 AM exhibited a rapid and sustained increase in intracellular Ca2+ concentration as a result of exposure to 10−5 M of 25(OH)D3. In this work, we show for the first time the in vitro ability of 25(OH)D3 to induce a rapid increase of intracellular Ca2+ levels in hADMSCs. These findings represent an important step to better understand the non-genomic effects of vitamin D3 and its role in endocrine system.
1. Introduction
Calcitriol (1α,25-(OH)2D3), the biologically active form of vitamin D3, is a hormone that participates in many biological processes, including the regulation of the serum calcium and phosphate levels, in addition to exerting direct effects on bone and mineral metabolism [1]. In bone, its actions are mediated via the interaction with the nuclear vitamin D receptor (VDR), to promote the expression of genes related to bone remodelling, such as alkaline phosphatase (ALP), type I collagen, and non-collagenous proteins [2,3,4].
VDR acts like transcription factor forming heterodimers with retinoid X receptor (RXR) and either positively or negatively regulating the expression of target genes by binding to their promoter regions through vitamin D3 response elements (VDREs) [2].
Moreover, in addition to genomic actions, all the steroid molecules have been proven to transmit via specific membrane receptors rapid non-genomic effects [5]. In 1942, Hans Selye observed that progesterone was able to induce anaesthetic effects within minutes of its administration differently than its major hormonal activity that occurred only hours after its application, thus ascertaining for the first time the non-genomic actions of steroids molecules [6]. In 1964, Spach and Streeten demonstrated that the variation of Na+ ions induced by aldosterone administration in dog erythrocytes happened within few minutes, offering new evidence on non-genomic actions of this steroid hormone [7]. The results of later studies remained obscure until the recent recognition of rapid non-genomic effects for various steroid hormones, including 1α,25-(OH)2D3 [8]. These actions take place on a range of seconds to minutes, are not controlled by molecules that inhibit the genomic effects (i.e., cycloheximide or actinomycin D), and occur in response even to steroids bound to large proteins and therefore no capable of entering in the cells [9].
Among the rapid non-genomic actions of 1α,25-(OH)2D3, it has been reported the stimulation of specific intracellular signal transduction pathways (i.e., mitogen-activated protein kinase (MAPK) cascades, cAMP-protein kinase A), the increase of cytoplasmic calcium concentrations (Ca2+), and the activation of the chloride and calcium channels [10].
It has been postulated that non-genomic mechanisms require the interaction between 1α,25-(OH)2D3 and intracellular or membrane-bound proteins, thereby providing efficient and rapid response to the stimulus [11].
Membrane-bound VDR (mVDR) complexed with caveolin 1 (CAV1) and proto-oncogene, non-receptor tyrosine kinase Src in caveolae is involved in the regulation of different signal transduction pathways, such as transcriptional activity of Wnt [12,13,14,15], Notch [16,17,18], and sonic hedgehog (Shh) (Figure 1) [19,20,21,22,23,24]. Moreover, its interaction with CAV1 results in the activation of several intracellular signalling molecules (i.e., phospholipase A2 (PLA2), protein kinase C (PKC), phosphatidylinositol 3-kinases (PI3Ks), calcium/calmodulin-dependent protein kinase II gamma (CaMKII), and MAP kinases) (Figure 1) [10].
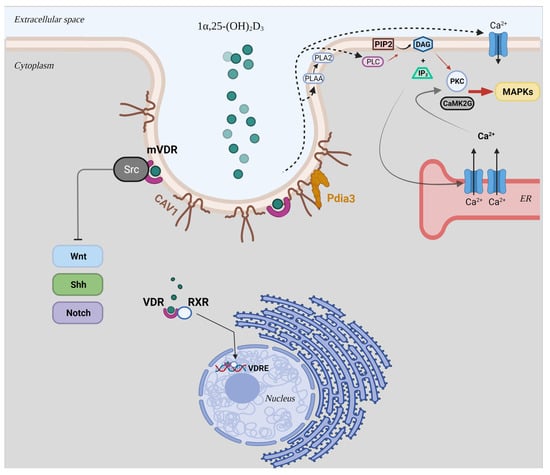
Figure 1.
Schematic representation of the genomic and non-genomic mechanisms of the biological active form of vitamin D3, 1α,25-(OH)2D3. Abbreviations: mVDR: membrane-bound VDR; RXR: retinoid X receptor; VDRE: vitamin D3 response elements; CAV1: caveolin 1; Shh: Sonic hedgehog; Pdia3: protein disulphide isomerase family A member 3; PLA2: phospholipase A2; PLAA: PLA2 activating protein; PLC: phospholipase C; PIP2: phosphatidylinositol bisphosphate; DAG: diacylglycerol; IP3: inositol trisphosphate; PKC: protein kinase C; CaMK2G: calcium/calmodulin-dependent protein kinase II gamma; MAPK: mitogen-activated protein kinase.
In addition, these actions could be mediated via the joint interaction of CAV1 and protein disulphide isomerase family A member 3 (Pdia3) (Figure 1) [11,25,26]. This latter is an endoplasmic reticulum (ER) chaperone for calnexin (CANX) and calreticulin (CALR) and a 1α,25-(OH)2D3-membrane associated, rapid response steroid (MAARS) binding protein that interacts with vitamin D3 compounds, making it essential for the non-genomic responses of 1α,25-(OH)2D3. Although no binding site for 1α,25-(OH)2D3 has been identified in the structure of Pdia3 using crystallographic studies, in vivo studies on Pdia3-/- and VDR-/- mice strongly suggest the involvement of this ER chaperone in skeletal development and intestinal Ca2+ absorption [27,28,29,30,31]. On the basis of this evidence, despite the fact that Pdia3 could not directly interact with 1α,25-(OH)2D3, this protein could serve as a chaperone for vitamin D3 binding protein (DBP) or VDR, suggesting its importance in rapid responses to the biologically active metabolite of vitamin D3.
The most noticeable non-genomic rapid effect of 1α,25-(OH)2D3 is the increase of intracellular Ca2+ ion concentrations at subnanomolar concentrations by modulating its release from intracellular stores and its uptake in intestinal epithelia, referred as “transcaltachia” [8,32]. In addition, a rapid increase in tissue Ca2+ concentrations has been also found in primary cultured myocytes obtained from chicken embryonic heart [33] and in the osteoblast-like cells ROS 24/1 lacking the nuclear VDR [34].
Interestingly, evidence has demonstrated that 1α,25-(OH)2D3 exerts rapid, non-genomic actions in vivo. In particular, functional studies showed that the stimulation of phosphate uptake into intestinal epithelial cells isolated from chicken results from the rapid actions of the secosteroid hormone [35]. In a further study, Boyan et al. observed that the regulation of PKC activity from 1α,25-(OH)2D3 is mediated by rapid membrane associated mechanisms in cultured costochondral cartilage cells derived from VDR knockout mice [27].
Calcifediol (25(OH)D3), the major circulating form of vitamin D3 and the direct precursor of 1α,25-(OH)2D3, is produced mainly in the liver by hydroxylation of vitamin D3 through the enzyme 25-hydroxylase [36,37,38,39,40]. Recently, several studies demonstrated that 25(OH)D3 is an agonistic VDR ligand with gene regulatory and anti-proliferative properties, although with a lower affinity compared with the active form of vitamin D3 [41].
In this study we have hypothesized for the first time that the 25(OH)D3 could activate non-genomic pathways. As it had been previously shown, human mesenchymal stem cells (hMSCs) are an excellent model for studying the hormonal effects of 1α,25-(OH)2D3 [42], we selected MSCs derived from human adipose tissue (hADMSCs) for the in vitro analysis of the intracellular Ca2+ mobilization in response to 25(OH)D3.
2. Materials and Methods
2.1. Isolation of hADMSCs Cells and Cultures
hADMSC lines were prepared from small fragments of subcutaneous adipose tissue biopsies developed by Dr. Brandi during her visiting years at the National Institutes of Health (NIH, Bethesda, MD, USA). The adipose tissue biopsies were immediately placed in culture medium supplemented with 100 IU/mL penicillin and 100 µg/mL streptomycin and transported to the laboratory for their processing. Briefly, each sample was minced mechanically into small fragments (1 mm) and was subjected to the enzymatic treatment in Ham’s F12 Coon’s modification medium supplemented with 20% foetal bovine serum (FBS), 100 IU/mL penicillin, 100 µg/mL streptomycin, and 3 mg/mL collagenase type II for 3 h at 37 °C. After centrifugation, pellet fragments were mechanically dispersed by pipetting and sedimented by centrifugation at 400× g for 5 min. After removing the supernatant by aspiration, the cell pellet was suspended and cultured into a 100 mm Petri dish at 37 °C in humified atmosphere with 5% CO2 in growth medium (GM) [Ham’s F12 Coon’s modification medium supplemented with 10% FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin]. The medium was replaced with fresh GM every 3 days and once confluence was reached, the cells were detached by trypsinization and were used either for cell expansion, or cryopreserved upon reaching 5 × 103 cells/cm2, or plated on tissue culture dishes or wells for other purposes.
2.2. Multiple Differentiation Potential Assessment of hADMSCs
The characterization analysis to evaluate the stem cell potential of hADMSCs cell lines were performed by studying the ability of the cells to differentiate toward both the adipogenic and osteogenic lineage, as described below.
2.2.1. Adipogenic Differentiation
hADMSCs cell lines were plated on 24-well plates at a cell density of 1 × 104 cells/cm2 in GM until reaching 80–90% confluence. Afterward, the medium was changed to adipogenic medium (AM), composed as follow: Ham’s F12 Coon’s modification medium supplemented with 10%FBS, 100 IU/mL penicillin, 100 µg/mL streptomycin, 1 µM dexamethasone, 1 µM bovine insulin, 0.5 mM IsoButylMethylXanthine (IBMX), and 100 µM indomethacin. The AM was refreshed twice a week. The expression of the adipogenic phenotype was assessed on cells cultured in AM or GM (negative control) for 21 days, stained directly with freshly prepared Oil Red O working solution in order to prevent the burst of lipid droplets, and immediately observed in brightfield microscopy (Axiovert 200, Zeiss, Oberkochen, Germany).
2.2.2. Osteogenic Differentiation
hADMSCs cell lines were seeded on 24-well plates at a cell density of 1 × 104 cells/cm2 in GM until reaching the 70–80% confluence. Afterward, the medium was switched to osteogenic medium (OM), composed as follow: Ham’s F12 Coon’s modification medium supplemented with 10% FBS (South America origin), 100 IU/mL penicillin, 100 µg/mL streptomycin, 10 nM dexamethasone, 0.2 mM sodium L-ascorbyl-2-phosphate, 10 mM β-glycerol phosphate, and 1 µg/mL calcein. The OM was refreshed twice a week. The osteogenic phenotype was evaluated up to 35 days of osteogenic induction both by monitoring alkaline phosphatase (ALP) activity and Ca2+ deposition by cythochemical staining. Thus, the cells were washed twice with Dulbecco’s phosphate buffered saline (DPBS), fixed in 4% paraformaldehyde (PFA)/DPBS, and washed three times with ultrapure distilled H2O. For ALP cytochemical staining, the fixed cells were washed twice with DPBS, and stained with a specific dye mixture composed of 5 mg naphtol-AS-MX phosphate sodium salt in 1mL dimethyl sulfoxide (DMSO), 40 mg Fast Red Violet LB salt in 50 mL Tris-HCl Buffer pH 9 for 30 min at 37 °C, by monitoring the course of staining every 10 min through the microscope. ALP-positive cells were stained in red, and nuclei, counterstained in green with methyl green were observed in LSCM (Zeiss, Oberkochen, Germany) under brightfield observation. For calcium mineralized deposits, hydroxyapatite (HA) deposits were stained with 1% silver nitrate solution and placed under ultraviolet light for 4 h. After that, the unreacted silver solution was removed with 5% sodium thiosulfate for 5 min and rinsed several times with distilled water. ALP+ cells and HA deposits were observed in bright field microscopy (Axiovert 200, ZEISS).
2.3. Evaluation of the Intracellular [Ca2+] Levels Variation on hADMSCs by LSCM
The variation of intracellular Ca2+ levels on hADMSCs exposed to 10−5 M of 25(OH)D3 was evaluated by LSCM. Briefly, cells were plated on 24-well plates at a cell density of 1 × 104 cells/cm2 in GM, until they reaching 70% confluence on the day of imaging and were loaded with 4 × 10−4 M Fluo-4 acetoxymethil ester form (Fluo-4 AM) fluorescent dye (λex/λem: 494/506 nm) diluted 1/200 in Ca2+ free Hank’s buffered salt solution (HBSS) for 60 min at RT. Fluo-4 AM is a calcium indicator that once inside the cell, is cleaved by cellular esterases, resulting in a fluorescent form which exhibits increased fluorescence intensity at the emission wavelength of 506 nm, upon excitation at 494 nm, reflecting the cytoplasmic [Ca2+]. After this period, cells were washed two times with HBSS, and further incubated with 300 µL of HBSS for 60 min at RT, so that esterase activity were inhibited and allowing Fluo-4 to bind intracellular Ca2+. The mobilisation of intracellular Ca2+ was measured in untreated cells (negative control), cells exposed to 10−5 M of 25(OH)D3, and 10−5 M calcium ionophore-treated cells (positive control) by LSCM. Briefly, we set the LSCM to scan images every 8 s for around 8 min to establish baseline fluorescence for Fluo-4 (negative control), by exciting cells at 488 nm. Subsequently, cells were exposed to 10−5 M 25(OH)D3 added directly to a single well after 35 s of acquisition with a pipette. Once imaging was completed, the maximum intensities for Fluo-4 signals in single cells were determined by using image analysis software through the selection of multiple regions of interest (ROI). The fluorescence was then normalised by pixel-pixel adjustment to the fluorescence measured in a single image acquired before addition of the 25(OH)D3 (baseline fluorescence). Maximum fluorescence intensity derived from cells exposed to 25(OH)D3 was compared to negative control. Cells treated with 10−5 M calcium ionophore A23187 (Merck KGaA, Darmstadt, Germany) were used as positive control for influx of intracellular Ca2+ experiment.
2.4. Statistical Analysis
The statistical analysis was carried out by using GraphPad Prism 9 (GraphPad Software, San Diego, California, CA, USA). The normality distribution of the data was analyzed by the Kolmogorov-Smirnov and Shapiro-Wilk tests. Statistical analysis was performed by ANOVA followed by Bonferroni’s test with a predetermined experimentwise αT = 0.05.
3. Results
3.1. Isolation of hADMSCs
From the adipose tissue biopsies (Figure 2A) have been established four cell lines, named respectively preadipocyte cell lines (PA) 60, PA 67, PA 70, and PA 73. The above cell lines displayed a spindle-shaped fibroblast-like morphology with long cytoplasmic extensions (Figure 2B). All cell lines adhered to the plastic of culture dishes without any additional surface modifications and maintained their morphology throughout their expansion.
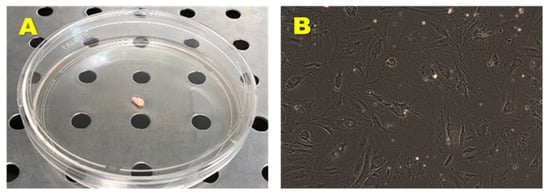
Figure 2.
Biopsy sample obtained by surgical resection from healthy donor (A) and primary hADMSCs cell line (B). Observation with a phase contrast microscopy (AxioVision, ZEISS). Original Magnification: 10×.
3.2. Multipotentiality of hADMSCs
To evaluate the potential for their multipotentiality, cells were induced to differentiate toward the osteogenic and adipogenic phenotypes, by using appropriate medium defined in the ‘Materials and Methods’ section.
3.2.1. Osteogenic Differentiation
Osteogenic phenotype of the established hADMSCs lines was evaluated in OM up to 35 days, by monitoring the ALP expression and the production of calcium mineralized deposits. Results obtained by cytochemical staining showed that the above-mentioned cell lines cultured in OM displayed an increase of ALP-positive cells in a time-dependent manner, reaching a maximum ALP activity at 14 days of induction (Figure 3A). In contrast, control cells cultured in GM did not exhibit any ALP-positive cells in the same time span (data not shown). Furthermore, a time-dependent increase in terms of number and size of mineralized nodules was observed in the PA 60, PA 67, PA 70 lines grown in OM (Figure 3B). In contrast, control cells cultured in GM did not show any calcium mineralized deposits in the same time span (data not shown).
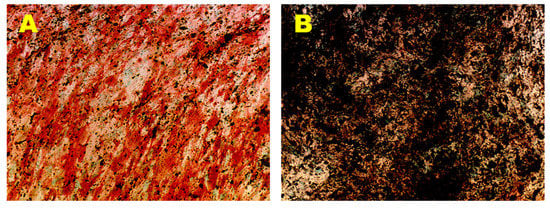
Figure 3.
Osteogenic Differentiation Assay—ALP and HA. Osteogenic differentiation at 14 days (A) and 35 days (B) of induction by cytochemical staining for ALP with Fast Red Violet B and for HA with Von Kossa staining. The ALP+ cells are in red and the grainy deposits are in black. Nuclei are counterstained in green. Observation in brightfield (AxioVision, ZEISS). Original magnification: 20×.
3.2.2. Adipogenic Differentiation
Adipogenic phenotype of the established hADMSCs lines was evaluated in AM for 21 days, monitoring the formation of intracellular vesicles containing drops of lipids by Oil Red O staining. The presence of multiple intracellular lipid droplets was observed in brightfield (AxioVision, ZEISS) (Figure 4A). In contrast, control cells cultured in GM did not show any lipid vesicles in the same time span (Figure 4B).
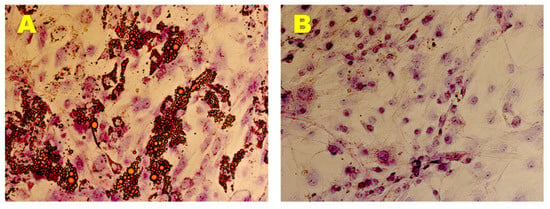
Figure 4.
Adipogenic Differentiation Assay. Adipogenic differentiation at 35 days (A) and after 0 days (B) of induction by cytochemical staining with Oil Red O. In red the lipidic vesicles and in violet the nuclei counterstained by Toluidine Blue. Observation in brightfield. Original magnification: 20×.
3.3. Effect of 25(OH)D3 on the Mobilization of Intracellular Ca2+
To assess in vitro the capacity of 25(OH)D3 to trigger rapid non-genomic response, we evaluated its effects on the mobilization of intracellular Ca2+ levels in hADMSC by using LSCM (Figure 5). Overall, 4 cells, one for each tested cell line, responded positively to 10−5 M calcium ionophore A23187 as well as 17 cells treated with 10−5 M of 25(OH)D3. As shown in Figure 6, three of them reached a maximum peak comparable to that of calcium ionophore A23187 (positive control), while the other cells showed a weaker response compared to ionophore. The estimated recovery time from exposure to 10−5 M 25(OH)D3, which is the time required for the returning to the basal conditions, was about 8 min for the cells treated with 10−5 M 25(OH)D3. In contrast, in cells exposed to 10−5 M calcium ionophore, no recovery in the same time span was observed. The maximum peak of calcium spikes was recorded after around 48 s from the beginning of the experiment (13 s) for the 10−5 M calcium ionophore A23187-treated cells (5.10). Similar results were obtained in cells exposed to 10−5 M 25(OH)D3 where the maximum calcium response was reached between 64 s to 240 s (29 to 235 s from the addition of 25(OH)D3), varying between cells (Figure 6). The maximum fluorescence intensity derived from untreated cells was 0.84 ± 0.55 (mean ± ds), from cells exposed to 10−5 M of 25(OH)D3 was 3.83 ± 0.62 (mean ± ds), and from 10−5 M calcium ionophore-treated cells was 4.81 ± 0.21 (mean ± ds) (Figure 6B). The rate of increase of the maximum fluorescence intensity of the cells treated with 25(OH)D3 and calcium ionophore compared with cells untreated was 356% and 473%, respectively. Altogether, our experiments have revealed that the increase of intracellular Ca2+ levels in response to 10−5 M 25(OH)D3 was comparable to what observed with the calcium ionophore A23187 for every cell line tested.
Figure 5.
Calcium imaging on hADMSCs before (A) and following exposure to 10−5 M 25(OH)D3 (B).
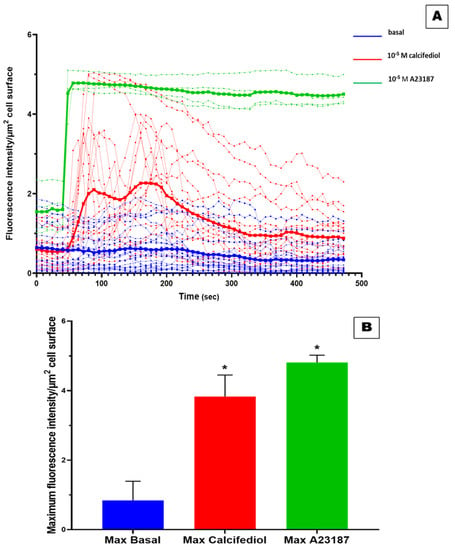
Figure 6.
Effect of 25(OH)D3 on the mobilization of intracellular Ca2+. Time courses experiments has revealed the changes in intracellular Ca2+ levels in response to 25(OH)D3: blue for untreated cells, red for cells exposed to 10−5 M 25(OH)D3, and green for 10−5 M calcium ionophore-treated cells (A). The bold curves represent the average intensity values for Fluo-4 signals for all the cells in response to the treatment for each time (A). Maximum fluorescence intensity derived from cells exposed to 25(OH)D3 was compared to negative control (B). * = p-value < 0.0005.
4. Discussion
Vitamin D has attracted attention because its deficiency underlies the pathogenesis of well-known bone pathological conditions, such as rickets in children and osteomalacia in adults [43,44,45].
Following the identification of the molecular structure of vitamin D, a great interest has been focused on the mode of action of vitamin D at cellular level. 1α,25-(OH)2D3, the biologically active form of vitamin D, has been showed classically to mediate its effects through the interaction with a VDR, a nuclear receptor found to be expressed in virtually all cell types [1,3].
The discovery of non-genomic steroid actions opened to the characterization of non-genomic effects for all steroid hormones, including the sterol 1α,25-(OH)2D3 [6,7]. It is recognized that 1α,25-(OH)2D3 exerts non-genomic actions such as the activation of both intracellular signalling molecules (i.e., phospholipase A2 (PLA2), p21ras, phospholipase C, and phosphatidylinositol-3 kinase (PI3K)) and second messengers generation (i.e., cyclic AMP, phosphatidylinositol 3,4,5 trisphosphate, Ca2+) together with the activation of protein kinases [46,47,48,49,50]. The non-genomic mechanisms of 1α,25-(OH)2D3 encompass also the opening of Cl− and Ca2+ channels [51].
The major difference between genomic and non-genomic actions seems to be attributable to the time to onset of action [5], with the non-genomic ones taking place within minutes, differently than genomic responses, which require the accumulation of newly formed proteins, and thus occurring in the range of hours or even days [8].
In addition to the rapid time course, non-genomic mechanisms are unresponsive to inhibitors of protein synthesis or transcription, such as cycloheximide and actinomycin D [5]. The identification of non-genomic effects could be made through the use of steroid bounded to large macromolecules, such as bovine serum albumin, preventing steroid molecules from entering the cell, even though the endocytosis-mediated uptake of active molecules could disprove these conclusions [8].
25(OH)D3, for a long time considered only a metabolic precursor of 1α,25-(OH)2D3, has been demonstrated to be an agonist VDR ligand with gene regulatory function and anti-proliferative properties despite having a reduced affinity compared with that of 1α,25-(OH)2D3 [41].
Our hypothesis has been that to test whether 25(OH)D3 could also activate non-genomic pathways. In particular, we analyzed in vitro the intracellular Ca2+ mobilization in response to 10−5 M 25(OH)D3 in MSCs derived from human adipose tissue, hADMSCs. Previous studies showed that hMSCs can differentiate into several different types of cell, such as osteoblasts, chondrocytes, adipocytes, and muscle cells [52]. Furthermore, they have been showed as good models for studying hormonal-mediated effects in vitro [42]. So, we have decided to test the non-genomic effects of 25(OH)D3 on previously characterized hADMSCs cell lines.
Here we show that 25(OH)D3 has the ability to activate rapid non-genomic pathways, such as an increase of intracellular Ca2+ levels, similarly to what observed with the biologically active form of vitamin D3 [53].
In hADMSCs loaded with Fluo-4 AM the 25(OH)D3 (10−5 M) induce a rapid (48 s) and sustained increase in intracellular Ca2+ concentration in line with what has been observed with the action of the secosteroid 1,25α(OH)2D3 [53].
In fact, we found that the rise of intracellular Ca2+ concentrations induced by 10−5 M 25(OH)D3 was 356% higher than that of untreated cells. This response was comparable to that of the calcium ionophore A23187 for every cell line tested (473% above the cells untreated).
This variation of intracellular concentration of calcium could result from an initial transient 25(OH)D3-induced IP3-dependent Ca2+ mobilization from intracellular stores into the cytoplasm followed by Ca2+ influx from the extracellular environment which accounts for the endorsed Ca2+ phase.
Regarding the 1α,25(OH)2D3, it has been proposed that the generation of non-genomic responses could be mediated by the binding of the secosteroid hormone to caveolae-associated VDR, resulting in the generation of second messengers and activation of different signal transduction pathways [54]. Recent studies have also shown that the interaction of PDIA3 and CAV1 could be involved in the activation of these rapid responses to the biologically active form of vitamin D3 [47,55,56]. However, the mechanisms underpinning these rapid responses to hormones at the cellular and molecular levels are not well established so far, and their elucidation could provide novel therapeutic strategies able to modulate their actions.
Another interesting non-genomic mechanism of 25(OH)D3 involves the processing and subsequent degradation of sterol regulatory element-binding proteins (SREBPs) cleavage-activating protein (SCAP) in the ER to control lipogenesis. Since studies provided evidence of an inverse correlation between serum levels of 25(OH)D3 and metabolic syndrome severity, a study of Asano et al. [57] evaluated whether SREBPs could be inhibited by secosteroids compounds. From the chemical library of substances analysed, 25(OH)D3 induces SCAP ubiquitin-mediated proteasomal degradation via a non-genomic mechanism independently from VDR. This mechanism results in the destabilization of SREBP thus reducing the expression of SREBP-responsive genes.
According to these findings, 25(OH)D3 has a non-genomic action responsible for the regulation of intracellular Ca2+ at concentrations that are orders of magnitude higher than those subnanomolar under normal physiological conditions. This response at a higher dose could be caused by the remarked reduced affinity of 25(OH)D3 approximately 500 times for VDR respect that of active form of vitamin D [58]. In this respect, subnanomolar concentrations of 25(OH)D3 have been demonstrated unable to trigger an increase in intracellular Ca2+ concentration even though a marked but delayed response was observed at higher concentrations in human spermatozoa [58].
In summary, to the best of our knowledge, this is the first report that demonstrate in vitro that 25(OH)D3 induce a rapid increase of intracellular Ca2+ levels in hADMSCs. These findings could improve the understanding of the vitamin D endocrine system, thereby paving the way for the identification of novel therapeutical targets.
Author Contributions
M.L.B., S.D., G.P., F.M. (Francesca Marini), G.M. and R.Z. conceived the study and designed the experiments. M.L.B. provided the bioptic samples. G.P., C.R., G.G. and R.Z. developed the cellular model. S.D., G.P., C.R., R.Z., G.G. performed the experiments. G.P., G.G. and R.Z. supervised the experiments. S.D., G.P., C.A., F.M. (Francesca Miglietta), I.F. and R.Z. performed the data and statistical analysis. S.D., G.P., R.Z. and M.L.B. wrote the manuscript. T.I. and M.L.B. revised the manuscript. M.L.B. supervised the entire research group. All the authors contributed to the interpretation and discussion of the results. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by a research award (to Dr. Gemma Marcucci) by OrtoMed Society through an unrestricted grant by Bruno Farmaceutici.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported by the Società Italiana di Ortopedia, Medicina e delle Malattie Rare dello Scheletro (OrtoMed) society.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Malloy, P.J.; Gross, C. Chapter 9—Vitamin D: Biology, Action, and Clinical Implications. In Osteoporosis, 2nd ed.; Marcus, R., Feldman, D., Kelsey, J., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 257–303. ISBN 978-0-12-470862-4. [Google Scholar]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- DeLuca, H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008, 66, S73–S87. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–55. [Google Scholar] [CrossRef]
- Selye, H. Correlations between the chemical structure and the pharmacological actions of the steroids. Endocrinology 1942, 30, 437–453. [Google Scholar] [CrossRef]
- Spach, C.; Streeten, D.H.P. Retardation of Sodium Exchange in Dog Erythrocytes by Physiological Concentrations of Aldosterone, in Vitro. J. Clin. Investig. 1964, 43, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.M.; Gerdes, D.; Feuring, M.; Falkenstein, E.; Christ, M.; Wehling, M. Rapid, nongenomic steroid actions: A new age? Front. Neuroendocrinol. 2000, 21, 57–94. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, D.; Christ, M.; Haseroth, K.; Notzon, A.; Falkenstein, E.; Wehling, M. Nongenomic Actions of Steroids—From the Laboratory to Clinical Implications. J. Pediatric Endocrinol. Metab. 2000, 13, 853–878. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Tapia, C.; Suares, A.; De Genaro, P.; González-Pardo, V. In vitro studies revealed a downregulation of Wnt/β-catenin cascade by active vitamin D and TX 527 analog in a Kaposi’s sarcoma cellular model. Toxicol. Vitr. 2020, 63, 104748. [Google Scholar] [CrossRef]
- Muralidhar, S.; Filia, A.; Nsengimana, J.; Poźniak, J.; O’Shea, S.J.; Diaz, J.M.; Harland, M.; Randerson-Moor, J.A.; Reichrath, J.; Laye, J.P.; et al. Vitamin D–VDR Signaling Inhibits Wnt/β-Catenin–Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019, 79, 5986–5998. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Fang, W.; Lin, J.; Li, J.; Wu, W.; Xu, J. Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/β-catenin signaling. Lab. Investig. 2018, 98, 1527–1537. [Google Scholar] [CrossRef]
- Larriba, M.J.; González-Sancho, J.M.; Bonilla, F.; Muñoz, A. Interaction of vitamin D with membrane-based signaling pathways. Front. Physiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, X.; Xu, L.; Zhang, J.; Cao, H. A molecular sub-cluster of colon cancer cells with low VDR expression is sensitive to chemotherapy, BRAF inhibitors and PI3K-mTOR inhibitors treatment. Aging 2019, 11, 8587–8603. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, E.; Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Jiang, Y.; Nguyen, T.; Oda, Y.; Tu, C. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front. Physiol. 2016, 7, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandera Merchan, B.; Morcillo, S.; Martin-Nuñez, G.; Tinahones, F.J.; Macías-González, M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J. Steroid Biochem. Mol. Biol. 2017, 167, 203–218. [Google Scholar] [CrossRef]
- Hadden, M.K. Hedgehog and Vitamin D Signaling Pathways in Development and Disease. Vitam. Horm. 2016, 100, 231–253. [Google Scholar]
- Lisse, T.S.; Saini, V.; Zhao, H.; Luderer, H.F.; Gori, F.; Demay, M.B. The Vitamin D Receptor Is Required for Activation of cWnt and Hedgehog Signaling in Keratinocytes. Mol. Endocrinol. 2014, 28, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Teichert, A.E.; Elalieh, H.; Elias, P.M.; Welsh, J.; Bikle, D.D. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Investig. Dermatol. 2011, 131, 2289–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teichert, A.; Elalieh, H.; Bikle, D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J. Cell. Physiol. 2010, 225, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanal, R.; Nemere, I. Membrane receptors for vitamin D metabolites. Crit. Rev.™ Eukaryot. Gene Expr. 2007, 17, 31–47. [Google Scholar] [CrossRef]
- Yang, W.S.; Yu, H.; Kim, J.J.; Lee, M.J.; Park, S.-K. Vitamin D-induced ectodomain shedding of TNF receptor 1 as a nongenomic action: D3 vs D2 derivatives. J. Steroid Biochem. Mol. Biol. 2016, 155, 18–25. [Google Scholar] [CrossRef]
- Boyan, B.D.; Sylvia, V.L.; McKinney, N.; Schwartz, Z. Membrane actions of vitamin D metabolites 1alpha,25(OH)2D3 and 24R,25(OH)2D3 are retained in growth plate cartilage cells from vitamin D receptor knockout mice. J. Cell. Biochem. 2003, 90, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Garbi, N.; Winger, Q. The 1,25D3-MARRS receptor/PDIA3/ERp57 and lifespan. J. Cell. Biochem. 2015, 116, 380–385. [Google Scholar] [CrossRef]
- Nemere, I.; Garbi, N.; Hammerling, G.; Hintze, K.J. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids 2012, 77, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Garbi, N.; Hämmerling, G.J.; Khanal, R.C. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J. Biol. Chem. 2010, 285, 31859–31866. [Google Scholar] [CrossRef] [Green Version]
- Wilkin, A.M.; Harnett, A.; Underschultz, M.; Cragg, C.; Meckling, K.A. Role of the ERp57 protein (1,25D3-MARRS receptor) in murine mammary gland growth and development. Steroids 2018, 135, 63–68. [Google Scholar] [CrossRef]
- Nemere, I.; Zhou, L.X.; Norman, A.W. Nontranscriptional effects of steroid hormones. Receptor 1993, 3, 277–291. [Google Scholar]
- Selles, J.; Boland, R. Evidence on the participation of the 3′,5′-cyclic AMP pathway in the non-genomic action of 1,25-dihydroxy-vitamin D3 in cardiac muscle. Mol. Cell. Endocrinol. 1991, 82, 229–235. [Google Scholar] [CrossRef]
- Baran, D.T.; Ray, R.; Sorensen, A.M.; Honeyman, T.; Holick, M.F. Binding characteristics of a membrane receptor that recognizes 1 alpha,25-dihydroxyvitamin D3 and its epimer, 1 beta,25-dihydroxyvitamin D3. J. Cell. Biochem. 1994, 56, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Farach-Carson, M.C.; Rohe, B.; Sterling, T.M.; Norman, A.W.; Boyan, B.D.; Safford, S.E. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc. Natl. Acad. Sci. USA 2004, 101, 7392–7397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Guardia, G.; Parikh, N.; Eskridge, T.; Phillips, E.; Divine, G.; Rao, D.S. Prevalence of vitamin D depletion among subjects seeking advice on osteoporosis: A five-year cross-sectional study with public health implications. Osteoporos. Int. 2008, 19, 13–19. [Google Scholar] [CrossRef]
- van Schoor, N.M.; Visser, M.; Pluijm, S.M.F.; Kuchuk, N.; Smit, J.H.; Lips, P. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 2008, 42, 260–266. [Google Scholar] [CrossRef]
- Beck, B.R.; Shoemaker, M.R. Osteoporosis: Understanding key risk factors and therapeutic options. Physician Sportsmed. 2000, 28, 69–84. [Google Scholar] [CrossRef]
- Ruohola, J.-P.; Laaksi, I.; Ylikomi, T.; Haataja, R.; Mattila, V.M.; Sahi, T.; Tuohimaa, P.; Pihlajamäki, H. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J. Bone Miner. Res. 2006, 21, 1483–1488. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Molnár, F.; Peräkylä, M.; Qiao, S.; Kalueff, A.V.; St-Arnaud, R.; Carlberg, C.; Tuohimaa, P. 25-Hydroxyvitamin D3 is an agonistic vitamin D receptor ligand. J. Steroid Biochem. Mol. Biol. 2010, 118, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D 3 induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 7, 42816. [Google Scholar] [CrossRef] [Green Version]
- Mellanby, E. An experimental investigation on rickets. 1919. Nutrition 1989, 5, 81–86, discussion 87. [Google Scholar]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on Experimental Rickets: XXI. An Experimental Demonstration of the Existence of a Vitamin Which Promotes Calcium Deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Esvelt, R.P.; Schnoes, H.K.; DeLuca, H.F. Vitamin D3 from rat skins irradiated in vitro with ultraviolet light. Arch. Biochem. Biophys. 1978, 188, 282–286. [Google Scholar] [CrossRef]
- Fleet, J.C. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin D: What are they and what do they mean? J. Nutr. 2004, 134, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: A review of the roles of phospholipase A2 activating protein and Ca(2+)/calmodulin-dependent protein kinase II. J. Steroid Biochem. Mol. Biol. 2015, 147, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, P.P.; Hii, C.S.T.; Ferrante, A.; Tan, J.; Der, C.J.; Omdahl, J.L.; Morris, H.A.; May, B.K. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J. Biol. Chem. 2002, 277, 29643–29653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutchey, B.K.; Kaplan, J.S.; Dwivedi, P.P.; Omdahl, J.L.; Ferrante, A.; May, B.K.; Hii, C.S.T. Molecular action of 1,25-dihydroxyvitamin D3 and phorbol ester on the activation of the rat cytochrome P450C24 (CYP24) promoter: Role of MAP kinase activities and identification of an important transcription factor binding site. Biochem. J. 2005, 389, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, P.P.; Gao, X.-H.; Tan, J.C.-T.; Evdokiou, A.; Ferrante, A.; Morris, H.A.; May, B.K.; Hii, C.S.T. A role for the phosphatidylinositol 3-kinase--protein kinase C zeta--Sp1 pathway in the 1,25-dihydroxyvitamin D3 induction of the 25-hydroxyvitamin D3 24-hydroxylase gene in human kidney cells. Cell. Signal. 2010, 22, 543–552. [Google Scholar] [CrossRef]
- Norman, A.W. Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, G.; de Boland, A.R.; Boland, R. Stimulation of Ca2+ release-activated Ca2+ channels as a potential mechanism involved in non-genomic 1,25(OH)2-vitamin D3-induced Ca2+ entry in skeletal muscle cells. Biochem. Biophys. Res. Commun. 1997, 239, 562–565. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)₂vitamin D₃: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Doroudi, M.; Chen, J.; Boyan, B.D.; Schwartz, Z. New insights on membrane mediated effects of 1α,25-dihydroxy vitamin D3 signaling in the musculoskeletal system. Steroids 2014, 81, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Phospholipase A2 activating protein is required for 1α,25-dihydroxyvitamin D3 dependent rapid activation of protein kinase C via Pdia3. J. Steroid Biochem. Mol. Biol. 2012, 132, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.-I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.B.; Dissing, S. Non-genomic effects of vitamin D in human spermatozoa. Steroids 2012, 77, 903–909. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).