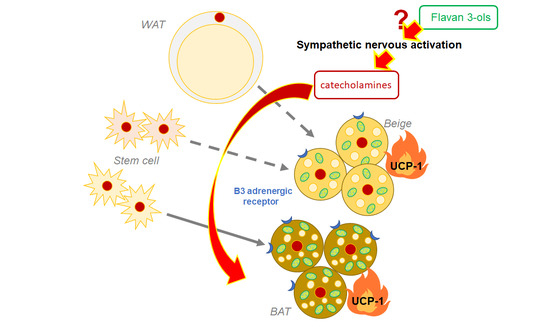
Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation
Abstract
:1. Introduction
2. Materials and Methods
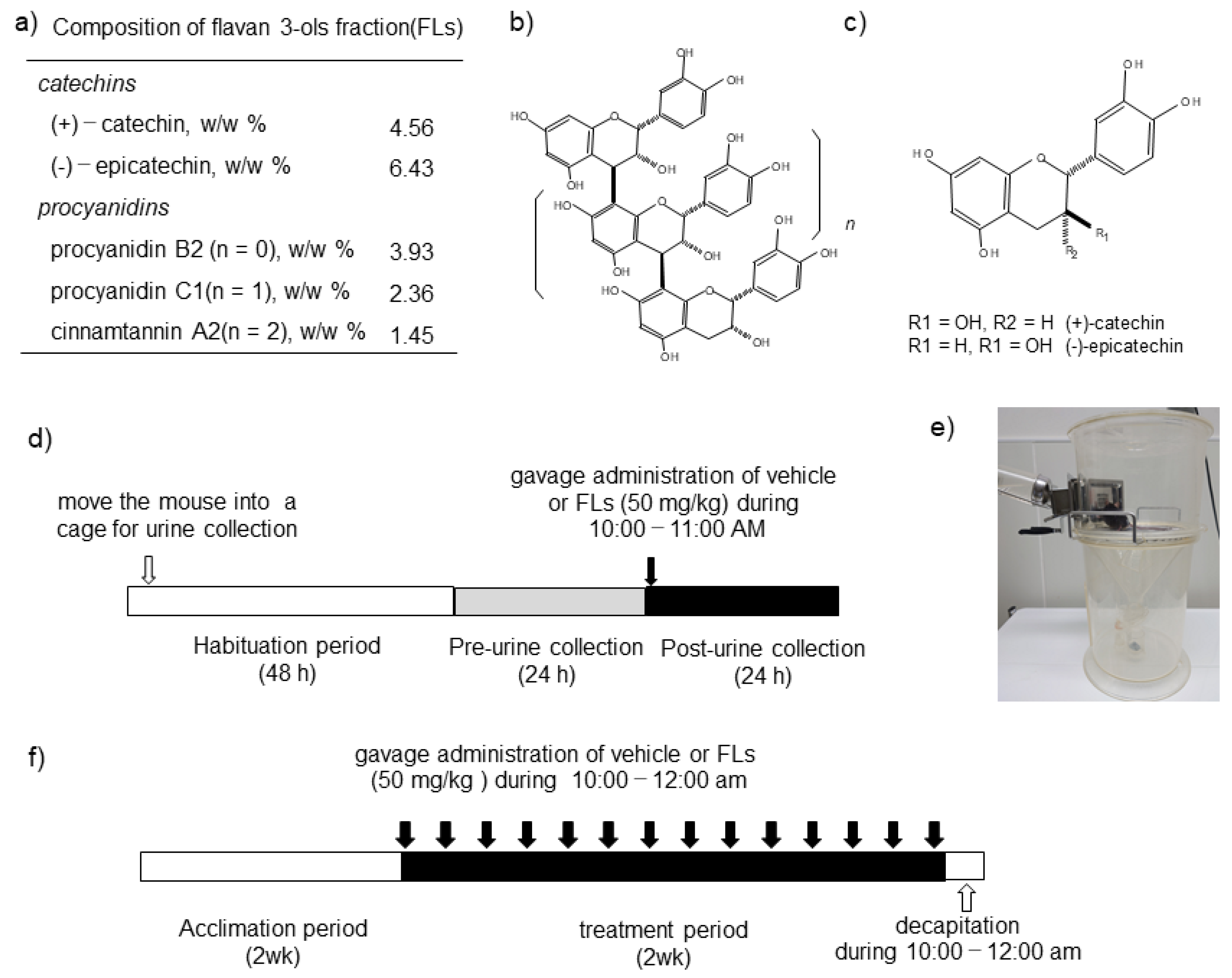
2.1. Materials
2.2. Animals and Diets
2.3. Impact of a Single Dose of FLs on the Excretion of CAs in Urine
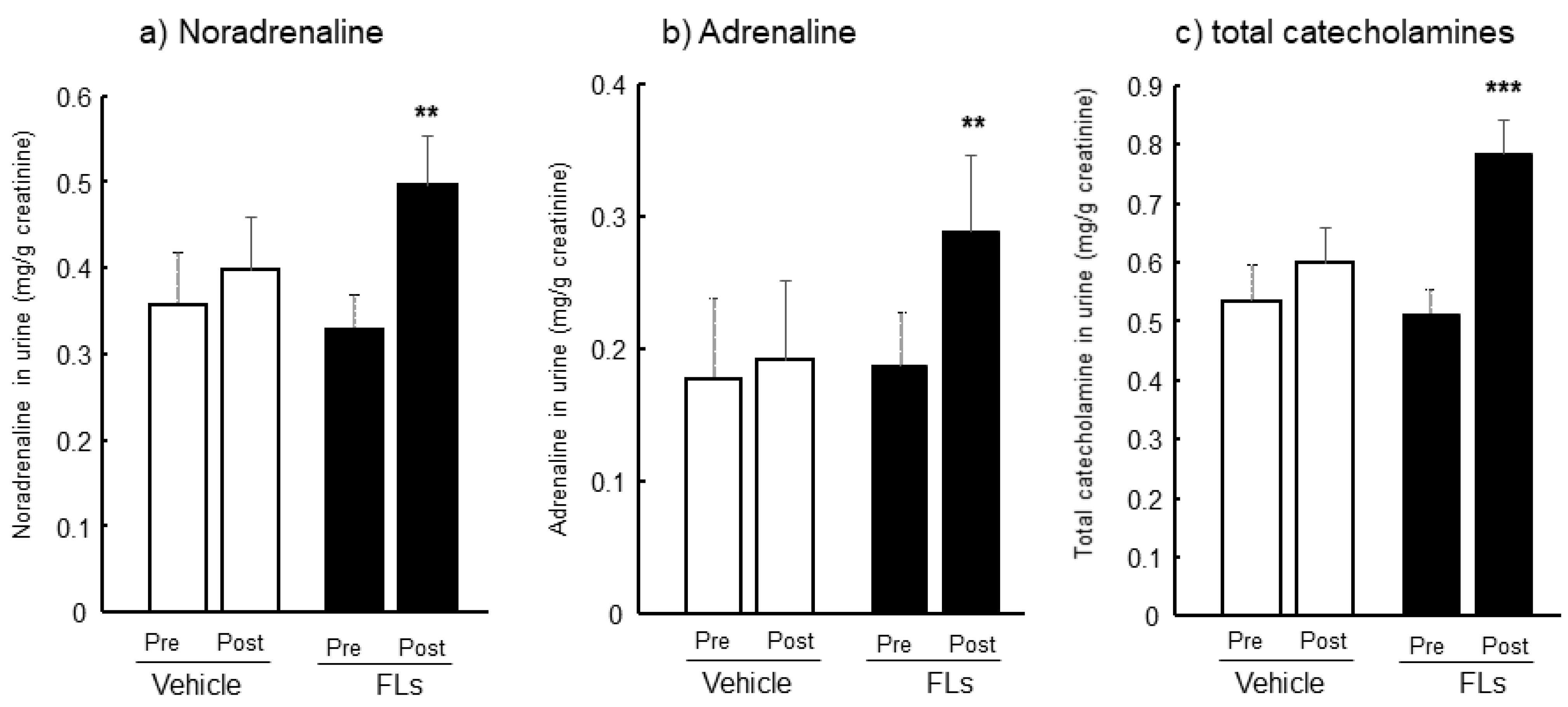
2.4. Analyses of CAs in Urine
2.5. Impact of the Repeated Oral Administration of FLs on Mouse Adipose Tissues
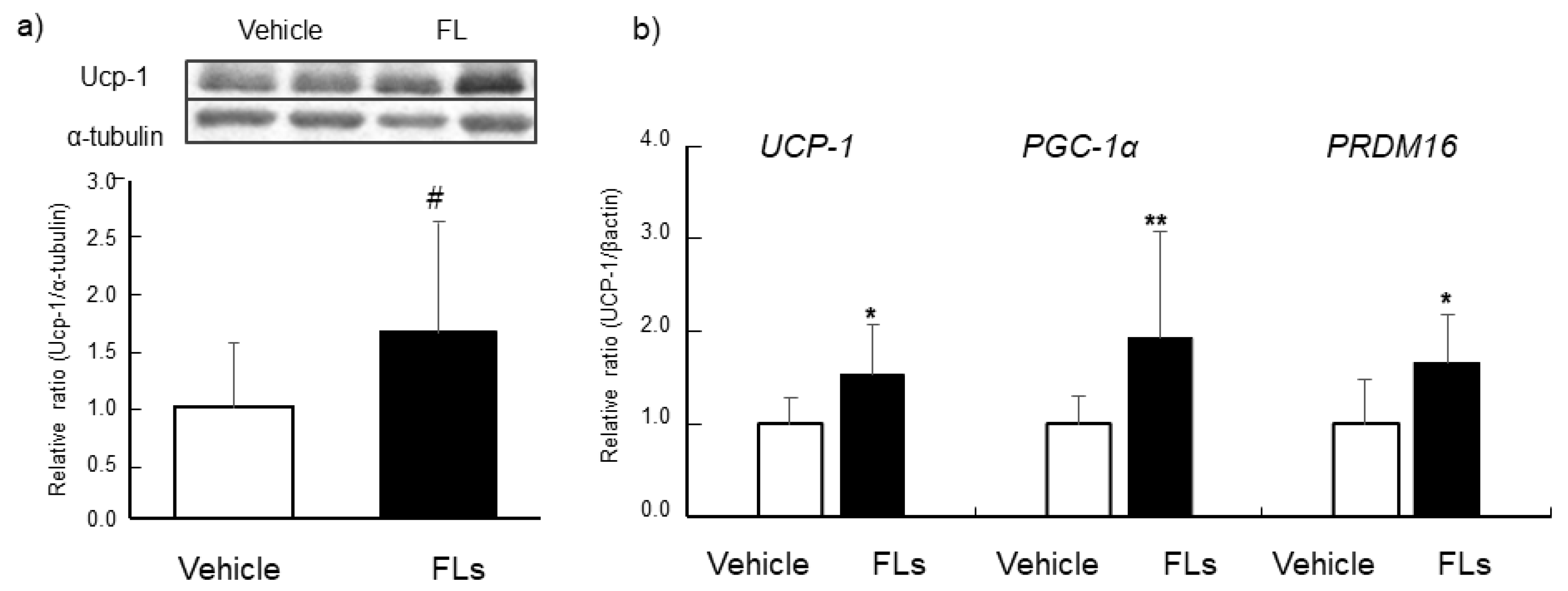
2.6. Quantitative RT-PCR Analysis
2.7. Western Blotting
2.8. Histological Analysis of iWAT
2.9. Data Analysis and Statistical Methods
3. Results
3.1. Twenty-Four Hours Urinary CAs Both Pre-and Post-Oral Administration of FL in Mice
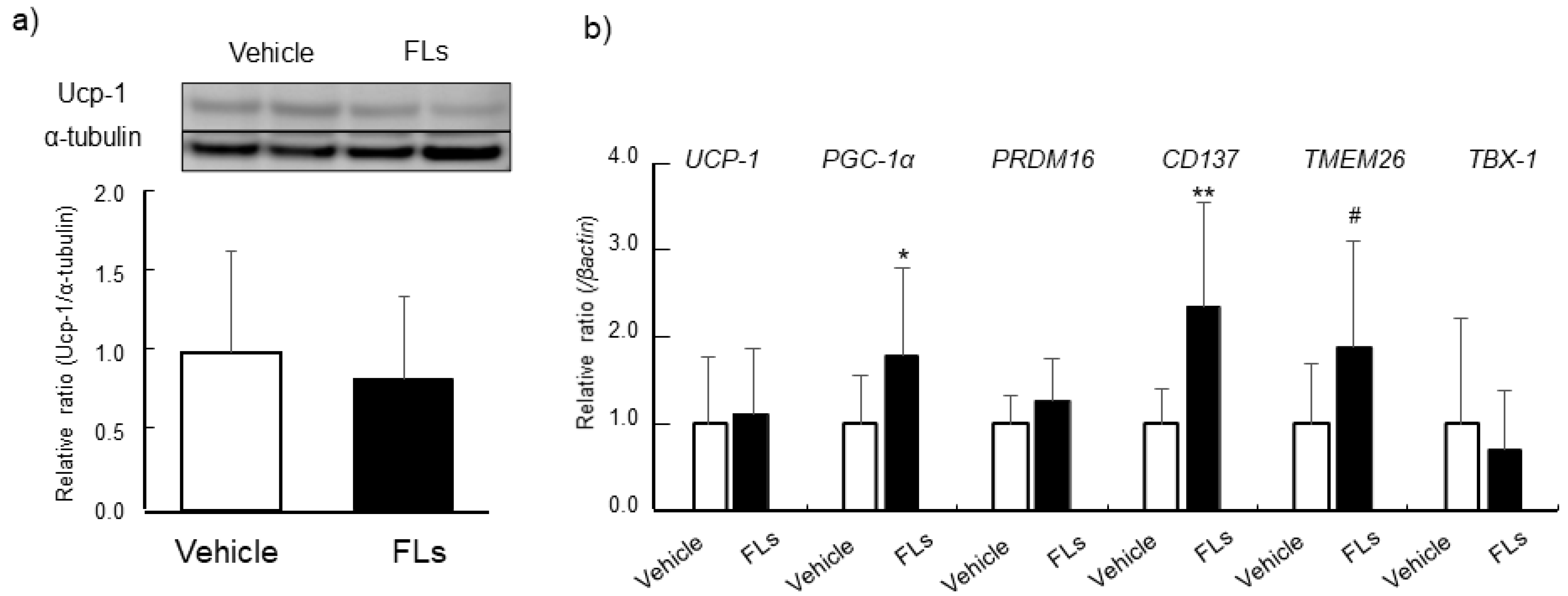
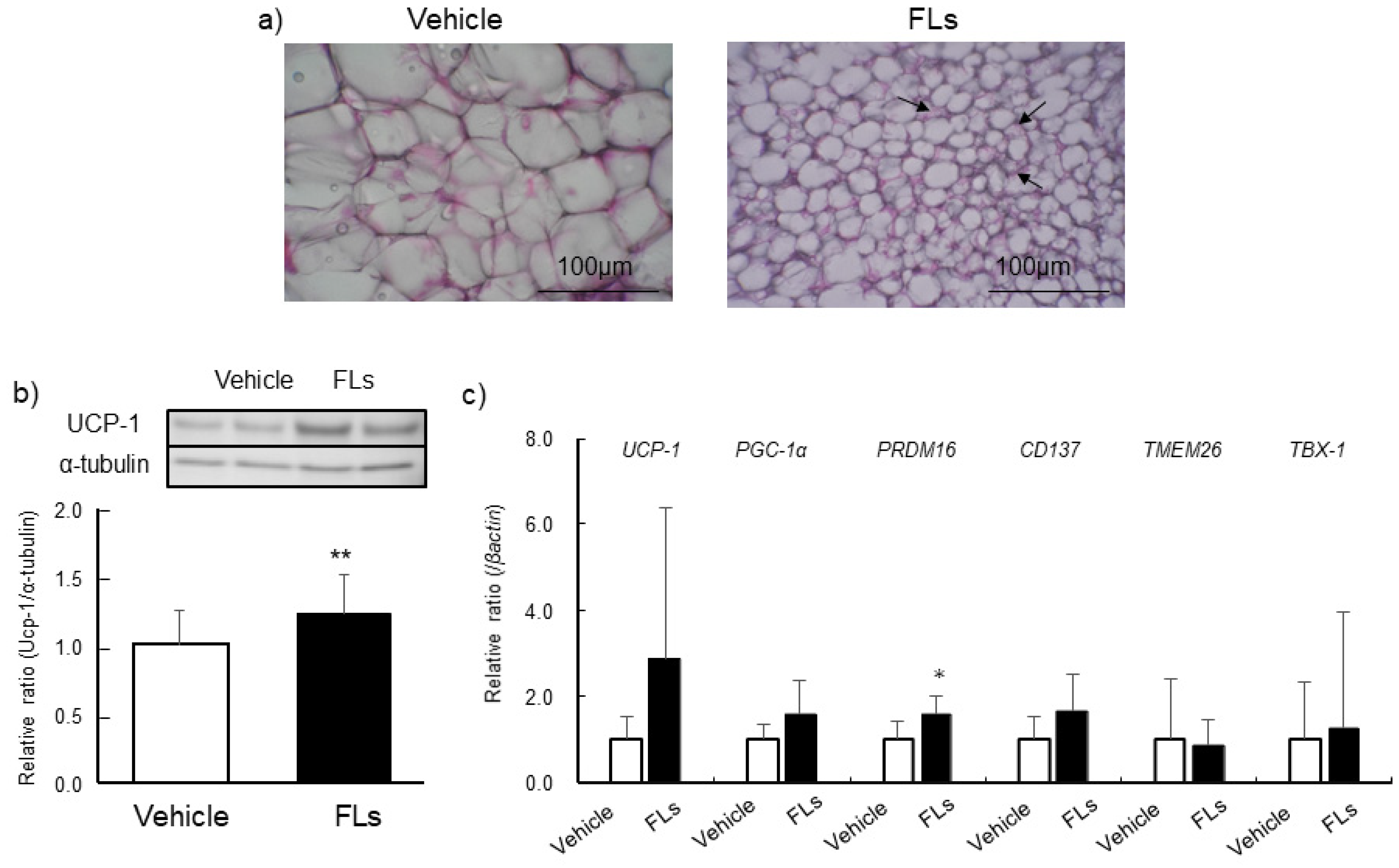
3.2. Impact of Repeated Oral Doses of FLs Adipose Tissues in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatano, T.; Miyatake, H.; Natsume, M.; Osakabe, N.; Takizawa, T.; Ito, H.; Yoshida, T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry 2002, 59, 749–758. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Yanagida, A.; Kanda, T. Gel permeation chromatography of anthocyanin pigments from Rose cider and red wine. J. Agric. Food Chem. 1999, 47, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Obara, M.; Takahashi, T.; Masumoto, S.; Hirota, H.; Miura, T. The Differences in the Flavan-3-ol and Procyanidin Contents of the Japanese ‘Fuji’ and ‘Orin’ Apples Using a Rapid Quantitative High-Performance Liquid Chromatography Method: Estimation of the Japanese Intake of Flavan-3-ols and Procyanidins from Apple as Case Study. Foods 2021, 10, 274. [Google Scholar]
- Muñoz-González, C.; Criado, C.; Pérez-Jiménez, M.; Pozo-Bayón, M. Evaluation of the Effect of a Grape Seed Tannin Extract on Wine Ester Release and Perception Using In Vitro and In Vivo Instrumental and Sensory Approaches. Foods 2021, 10, 93. [Google Scholar] [CrossRef]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Rajesh Kumar, S.; Thomas, S.; Kalarikkal, N. Grape seed extract-soluplus dispersion and its antioxidant activity. Drug. Dev. Ind. Pharm. 2020, 46, 1219–1229. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Das, D.K. Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef]
- Merkytė, V.; Longo, E.; Jourdes, M.; Jouin, A.; Teissedre, P.L.; Boselli, E. High-Performance Liquid Chromatography-Hydrogen/Deuterium Exchange-High-Resolution Mass Spectrometry Partial Identification of a Series of Tetra- and Pentameric Cyclic Procyanidins and Prodelphinidins in Wine Extracts. J. Agric. Food Chem. 2020, 68, 3312–3321. [Google Scholar] [CrossRef]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose-response relationship between cocoa flavanols and human endothelial function: A systematic review and meta-analysis of randomized trials. Food Funct. 2019, 10, 6322–6330. [Google Scholar] [CrossRef] [Green Version]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef]
- McCullough, M.L.; Chevaux, K.; Jackson, L.; Preston, M.; Martinez, G.; Schmitz, H.H.; Coletti, C.; Campos, H.; Hollenberg, N.K. Hypertension, the Kuna, and the epidemiology of flavanols. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 2), S103–S109; discussion 119–121. [Google Scholar] [CrossRef] [PubMed]
- Latham, L.S.; Hensen, Z.K.; Minor, D.S. Chocolate--guilty pleasure or healthy supplement? J. Clin. Hypertens. 2014, 16, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Corbi, G.; Zarrelli, A.; Arisi, M.; Calzavara-Pinton, P.; Grassi, D.; De Vivo, I.; Scapagnini, G. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J. Nutr. Biochem. 2018, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Rissanen, T.H.; Virtanen, J.K.; Kaikkonen, J.; Nyyssonen, K.; Salonen, J.T. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic. Biol. Med. 2004, 37, 1351–1359. [Google Scholar] [CrossRef]
- Almoosawi, S.; Tsang, C.; Ostertag, L.M.; Fyfe, L.; Al-Dujaili, E.A. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Funct. 2012, 3, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Rynarzewski, J.; Dicks, L.; Zimmermann, B.F.; Stoffel-Wagner, B.; Ludwig, N.; Helfrich, H.P.; Ellinger, S. Impact of a Usual Serving Size of Flavanol-Rich Cocoa Powder Ingested with a Diabetic-Suitable Meal on Postprandial Cardiometabolic Parameters in Type 2 Diabetics—A Randomized, Placebo-Controlled, Double-Blind Crossover Study. Nutrients 2019, 11, 417. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, N.; Terao, J. Possible mechanisms of postprandial physiological alterations following flavan 3-ol ingestion. Nutr. Rev. 2018, 76, 174–186. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats. Free Radic. Biol. Med. 2002, 33, 142–148. [Google Scholar] [CrossRef]
- Williamson, G.; Dionisi, F.; Renouf, M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Ishiyama, K.; Watanabe, A.; Kawano, S.; Miyase, T.; Sano, M. Determination of theaflavins including methylated theaflavins in black tea leaves by solid-phase extraction and HPLC analysis. J. Agric. Food Chem. 2007, 55, 7252–7257. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Lamuela-Raventos, R.M.; Santos-Buelga, C.; Sacanella, E.; Castell, M.; Permanyer, J.; Andres-Lacueva, C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal. Bioanal. Chem. 2009, 394, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.P.; Montoliu, I.; Nagy, K.; Moco, S.; Collino, S.; Guy, P.; Redeuil, K.; Scherer, M.; Rezzi, S.; Kochhar, S. Specific dietary preferences are linked to differing gut microbial metabolic activity in response to dark chocolate intake. J. Proteome Res. 2012, 11, 6252–6263. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar] [CrossRef]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Betts, N.M.; Leyva, M.J.; Fu, D.; Aston, C.E.; Lyons, T.J. Acute Cocoa Supplementation Increases Postprandial HDL Cholesterol and Insulin in Obese Adults with Type 2 Diabetes after Consumption of a High-Fat Breakfast. J. Nutr. 2015, 145, 2325–2332. [Google Scholar] [CrossRef]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of acute red wine polyphenol consumption on postprandial lipaemia in postmenopausal women. Atherosclerosis 2004, 177, 401–408. [Google Scholar] [CrossRef]
- Sear, J.W. 26-Antihypertensive Drugs and Vasodilators. In Pharmacology and Physiology for Anesthesia, 2nd ed.; Hemmings, H.C., Egan, T.D., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 535–555. [Google Scholar]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 2010, 34 (Suppl. 1), S36–S42. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Nakagawa, Y.; Mikome, K.; Yamamoto, H.; Osakabe, N. Enhancement of energy expenditure following a single oral dose of flavan-3-ols associated with an increase in catecholamine secretion. PLoS ONE 2014, 9, e112180. [Google Scholar] [CrossRef]
- Kamio, N.; Suzuki, T.; Watanabe, Y.; Suhara, Y.; Osakabe, N. A single oral dose of flavan-3-ols enhances energy expenditure by sympathetic nerve stimulation in mice. Free Radic. Biol. Med. 2015, 91, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [Green Version]
- Tentolouris, N.; Liatis, S.; Katsilambros, N. Sympathetic system activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 129–152. [Google Scholar] [CrossRef]
- Niebylski, A.; Boccolini, A.; Bensi, N.; Binotti, S.; Hansen, C.; Yaciuk, R.; Gauna, H. Neuroendocrine changes and natriuresis in response to social stress in rats. Stress Health 2012, 28, 179–185. [Google Scholar] [CrossRef]
- Kaija, H.; Pakanen, L.; Uusitalo, J.; Nikkilä, S.; Kortelainen, M.L.; Porvari, K.S. Changes in cardiac thrombomodulin and heat shock transcription factor 1 expression and peripheral thrombomodulin and catecholamines during hypothermia in rats. Stress 2014, 17, 504–511. [Google Scholar] [CrossRef]
- Martinho, R.; Correia, G.; Seixas, R.; Oliveira, A.; Silva, S.; Serrão, P.; Fernandes-Lopes, C.; Costa, C.; Moreira-Rodrigues, M. Treatment With Nepicastat Decreases Contextual Traumatic Memories Persistence in Post-traumatic Stress Disorder. Front. Mol. Neurosci. 2021, 14, 745219. [Google Scholar] [CrossRef]
- Fujii, Y.; Suzuki, K.; Hasegawa, Y.; Nanba, F.; Toda, T.; Adachi, T.; Taira, S.; Osakabe, N. Single oral administration of flavan-3-ols induces stress responses monitored with stress hormone elevations in the plasma and paraventricular nucleus. Neurosci. Lett. 2018, 682, 106–111. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Takizawa, T.; Nakamura, T.; Terao, J. Cocoa powder enhances the level of antioxidative activity in rat plasma. Br. J. Nutr. 2000, 84, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.; Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Nakamura, T.; Terao, J. Bioavailability of (-)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Paulo, E.; Wang, B. Towards a Better Understanding of Beige Adipocyte Plasticity. Cells 2019, 8, 1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metab. Clin. Exp. 2018, 81, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Barquissau, V.; Leger, B.; Beuzelin, D.; Martins, F.; Amri, E.Z.; Pisani, D.F.; Saris, W.H.M.; Astrup, A.; Maoret, J.J.; Iacovoni, J.; et al. Caloric Restriction and Diet-Induced Weight Loss Do Not Induce Browning of Human Subcutaneous White Adipose Tissue in Women and Men with Obesity. Cell Rep. 2018, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Jing, J.; Cui, X.; Shi, H.; Xue, B. Sympathetic nerve innervation is required for beigeing in white fat. Physiol. Rep. 2019, 7, e14031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, J.J. Green tea reduces body fat accretion caused by high-fat diet in rats through beta-adrenoceptor activation of thermogenesis in brown adipose tissue. J. Nutr. Biochem. 2003, 14, 671–676. [Google Scholar] [CrossRef]
- Andrade, J.M.; Frade, A.C.; Guimaraes, J.B.; Freitas, K.M.; Lopes, M.T.; Guimaraes, A.L.; de Paula, A.M.; Coimbra, C.C.; Santos, S.H. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar] [CrossRef]
- Ingawa, K.; Aruga, N.; Matsumura, Y.; Shibata, M.; Osakabe, N. Alteration of the systemic and microcirculation by a single oral dose of flavan-3-ols. PLoS ONE 2014, 9, e94853. [Google Scholar]
- Saito, A.; Inagawa, K.; Ebe, R.; Fukase, S.; Horikoshi, Y.; Shibata, M.; Osakabe, N. Onset of a hypotensive effect following ingestion of flavan-3-ols involved in the activation of adrenergic receptors. Free Radic. Biol. Med. 2016, 99, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Grouzmann, E.; Lamine, F. Determination of catecholamines in plasma and urine. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Lidell, M.E.; Betz, M.J.; Enerbäck, S. Brown adipose tissue and its therapeutic potential. J. Intern. Med. 2014, 276, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Choi, H.R.; Park, S.G.; Ko, Y.; Bae, K.H.; Lee, S.C. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int. J. Biochem. Cell Biol. 2012, 44, 327–334. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Cannon, B.; de Jong, J.M.A.; Fischer, A.W.; Nedergaard, J.; Petrovic, N. Human brown adipose tissue: Classical brown rather than brite/beige? Exp. Physiol. 2020, 105, 1191–1200. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Long, J.Z.; Svensson, K.J.; Tsai, L.; Zeng, X.; Roh, H.C.; Kong, X.; Rao, R.R.; Lou, J.; Lokurkar, I.; Baur, W.; et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014, 19, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Seale, P. Brown adipose tissue biology and therapeutic potential. Front. Endocrinol. 2013, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenwald, M.; Perdikari, A.; Rülicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013, 15, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.M.; Larsson, O.; Cannon, B.; Nedergaard, J. A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1085–E1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, Y.; Muta, O.; Teshima, T.; Hirasima, N.; Odaka, M.; Fushimi, T.; Fujii, Y.; Osakabe, N. Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients 2021, 13, 4214. https://doi.org/10.3390/nu13124214
Ishii Y, Muta O, Teshima T, Hirasima N, Odaka M, Fushimi T, Fujii Y, Osakabe N. Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients. 2021; 13(12):4214. https://doi.org/10.3390/nu13124214
Chicago/Turabian StyleIshii, Yuko, Orie Muta, Tomohiro Teshima, Nayuta Hirasima, Minayu Odaka, Taiki Fushimi, Yasuyuki Fujii, and Naomi Osakabe. 2021. "Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation" Nutrients 13, no. 12: 4214. https://doi.org/10.3390/nu13124214
APA StyleIshii, Y., Muta, O., Teshima, T., Hirasima, N., Odaka, M., Fushimi, T., Fujii, Y., & Osakabe, N. (2021). Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients, 13(12), 4214. https://doi.org/10.3390/nu13124214