Mokko Lactone Attenuates Doxorubicin-Induced Hepatotoxicity in Rats: Emphasis on Sirt-1/FOXO1/NF-κB Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
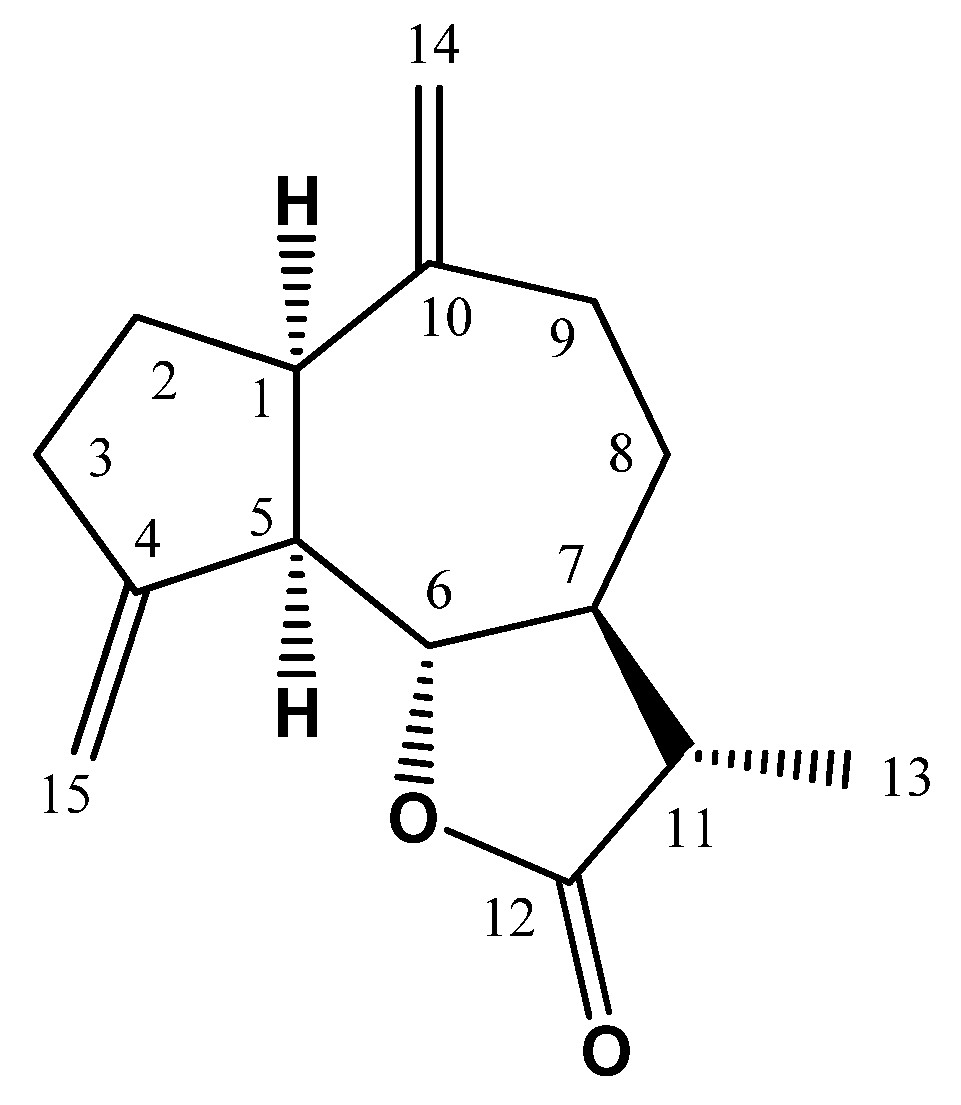
2.4. Spectral Data of ML
2.5. Chemicals
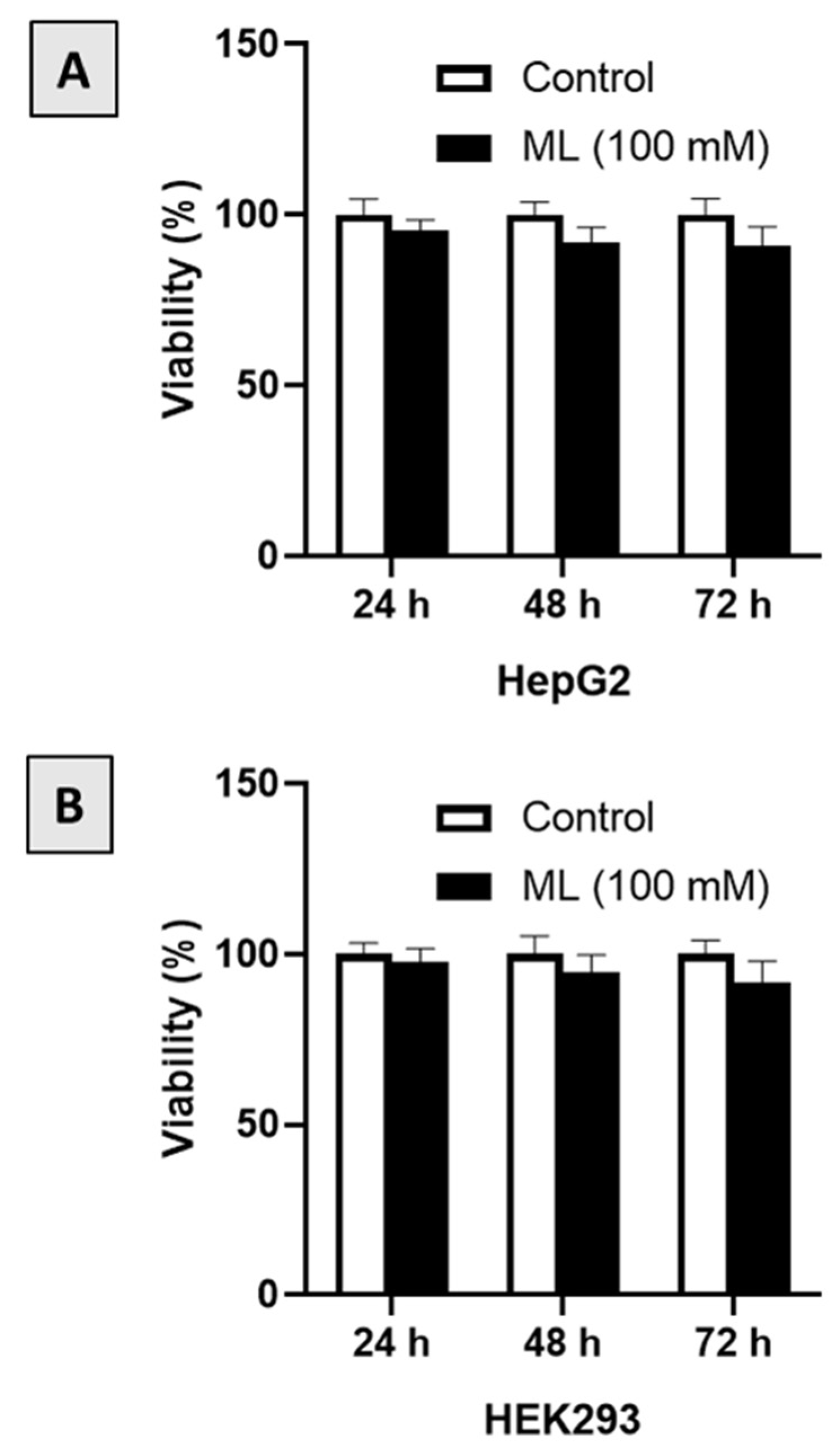
2.6. Cell Viability Assay
2.7. Animals
2.8. Experimental Design
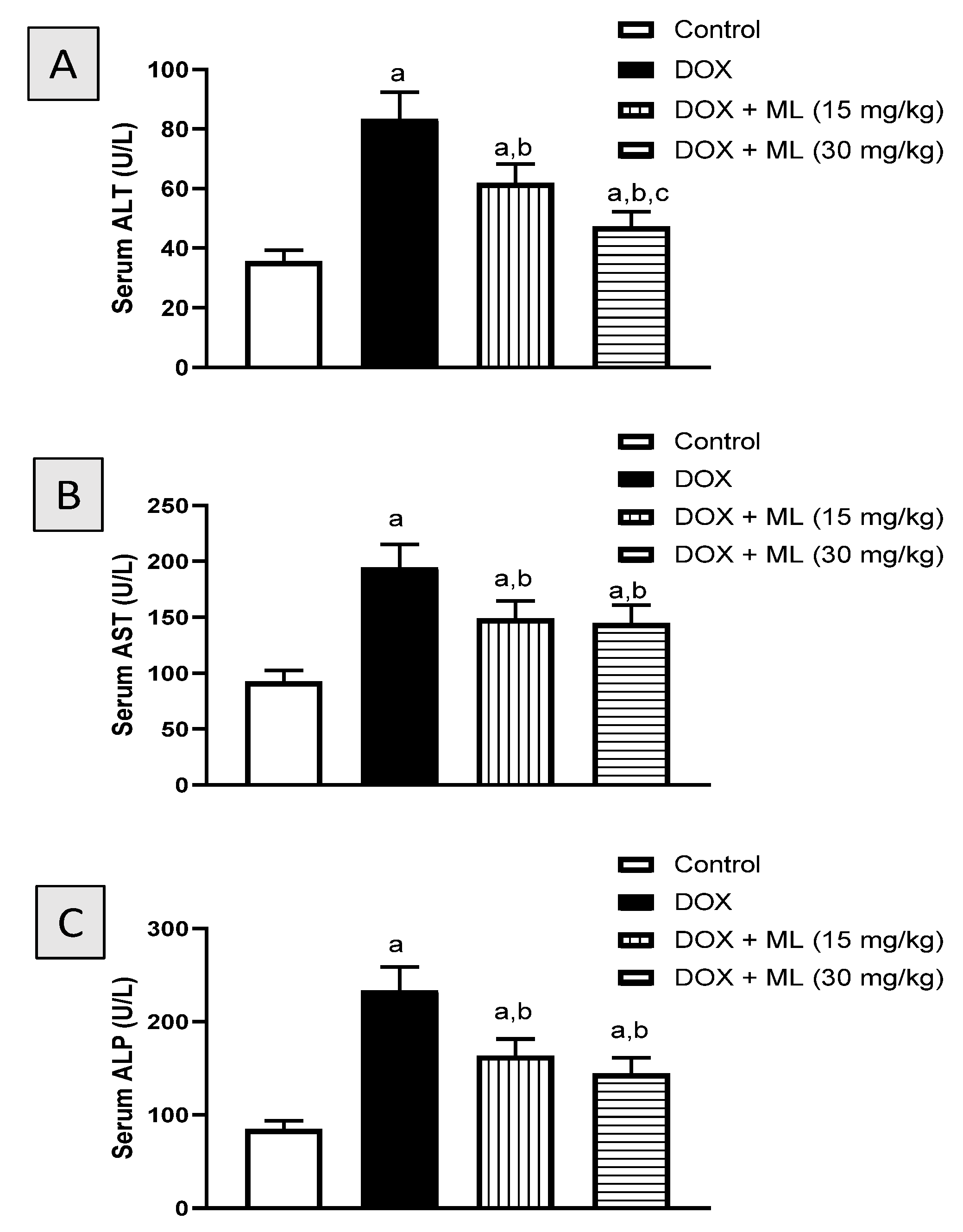
2.9. Assessment of Hepatic Function Serum Markers
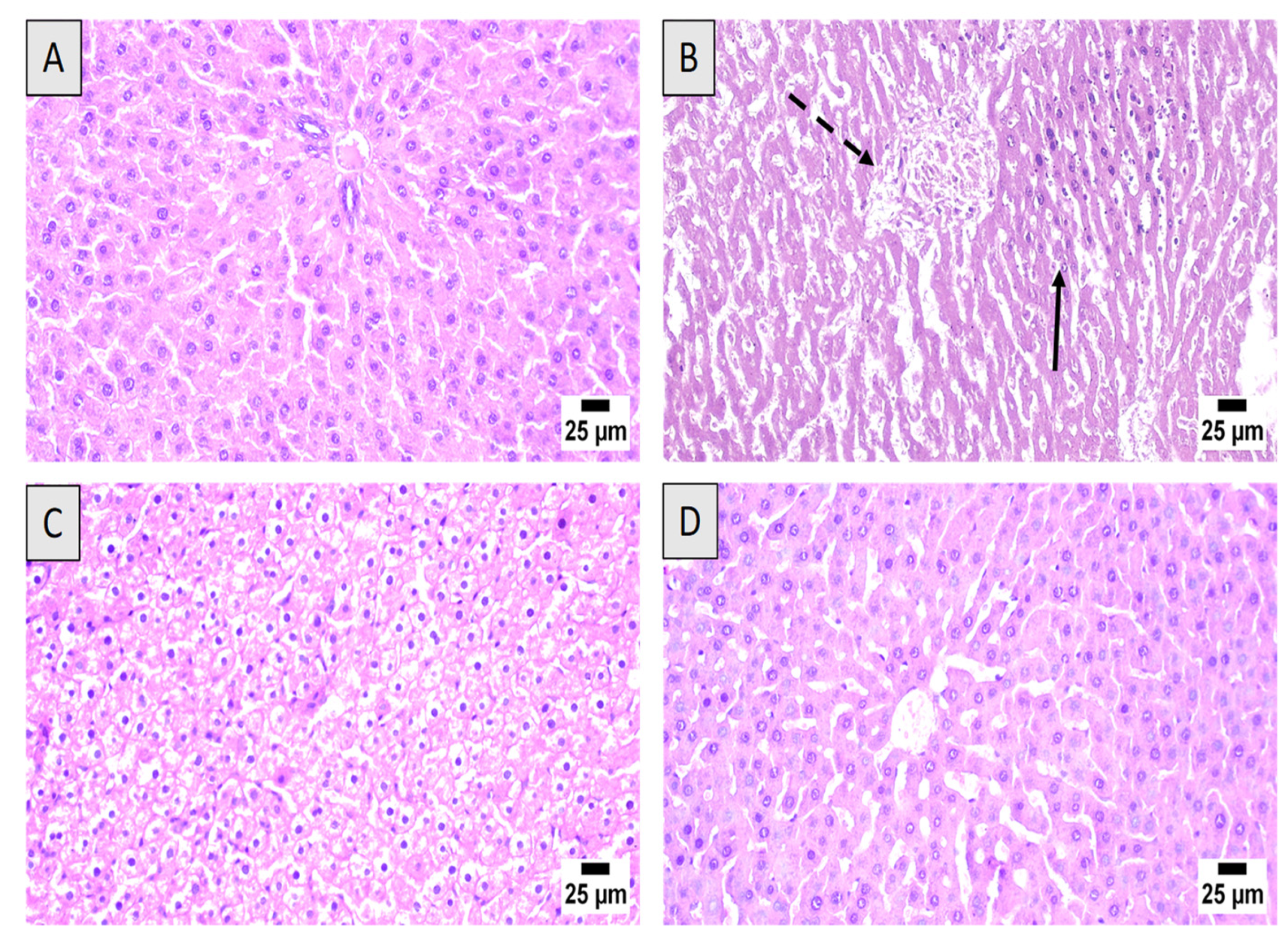
2.10. Histopathological Examination
2.11. Assessment of Oxidative Status
2.12. Immunohistochemical Staining
2.13. Quantitative Real-Time Polymerase Chain Reaction (PCR) Assay
2.14. Western Blotting Assay
2.15. Statistical Analysis
3. Results
3.1. Assessment of Cytotoxicity
3.2. Assessment of Liver Function
3.3. Histopathological Examination
3.4. Assessment of Oxidative Status
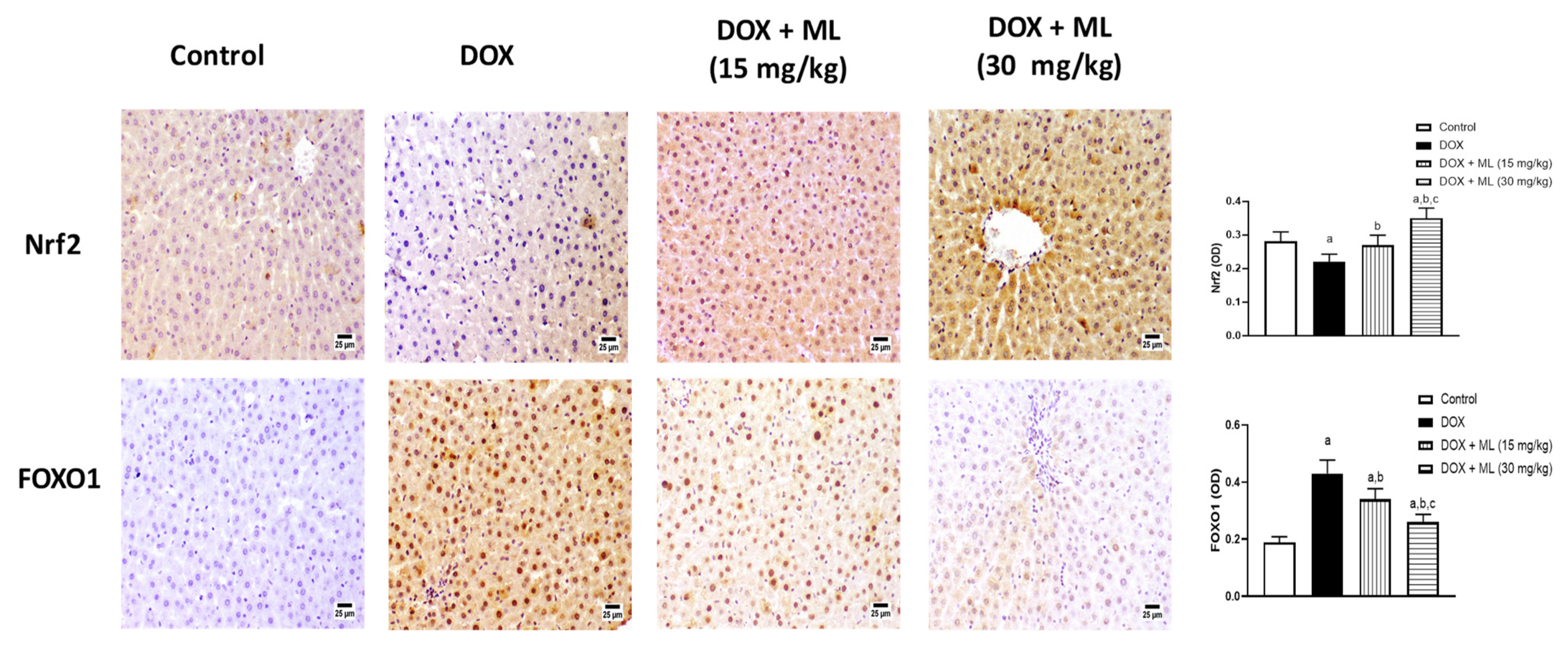
3.5. Assessment of Nrf2 and FOXO1 Expression
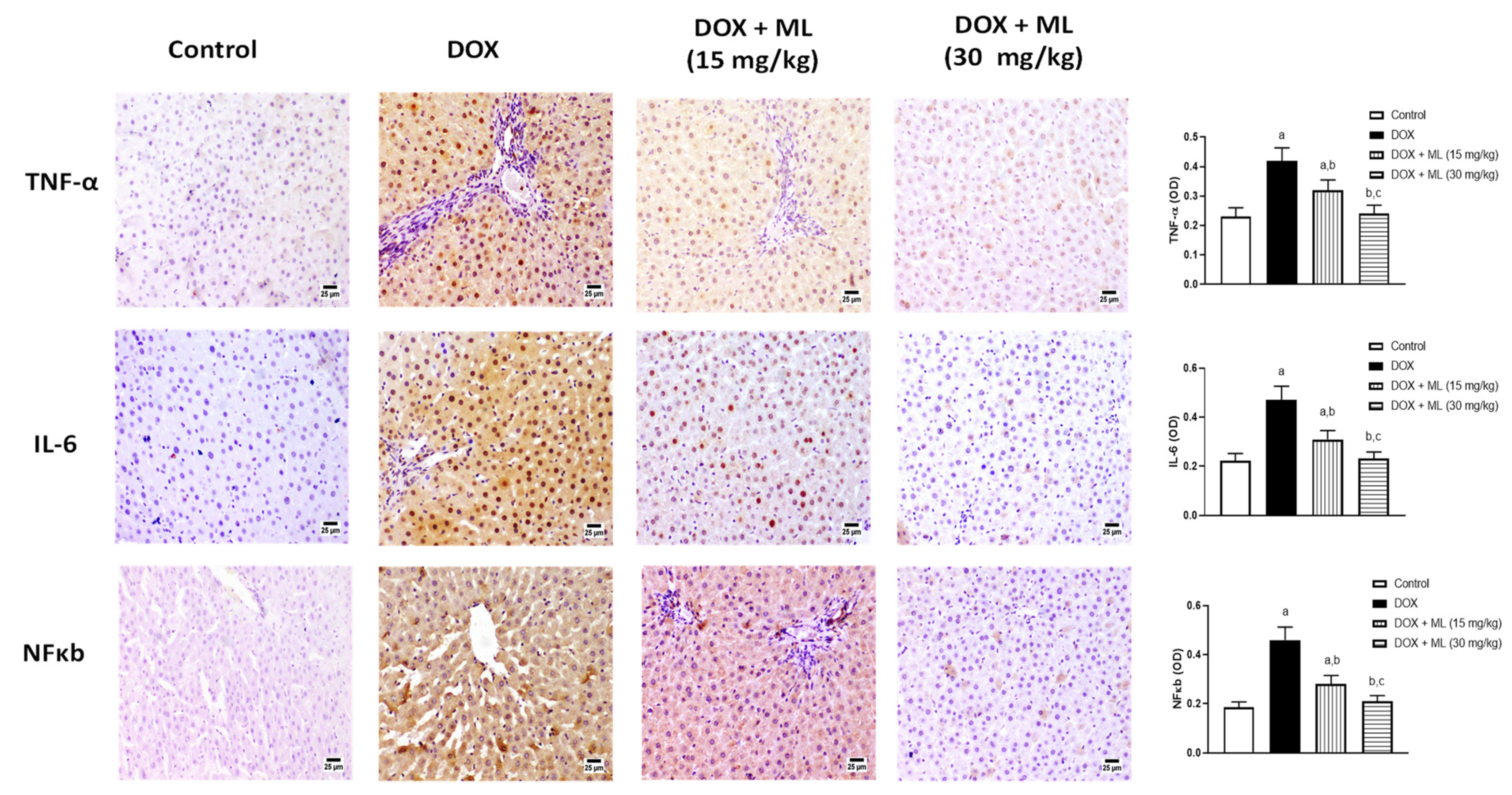
3.6. Assessment of Expression of Liver Inflammation Markers
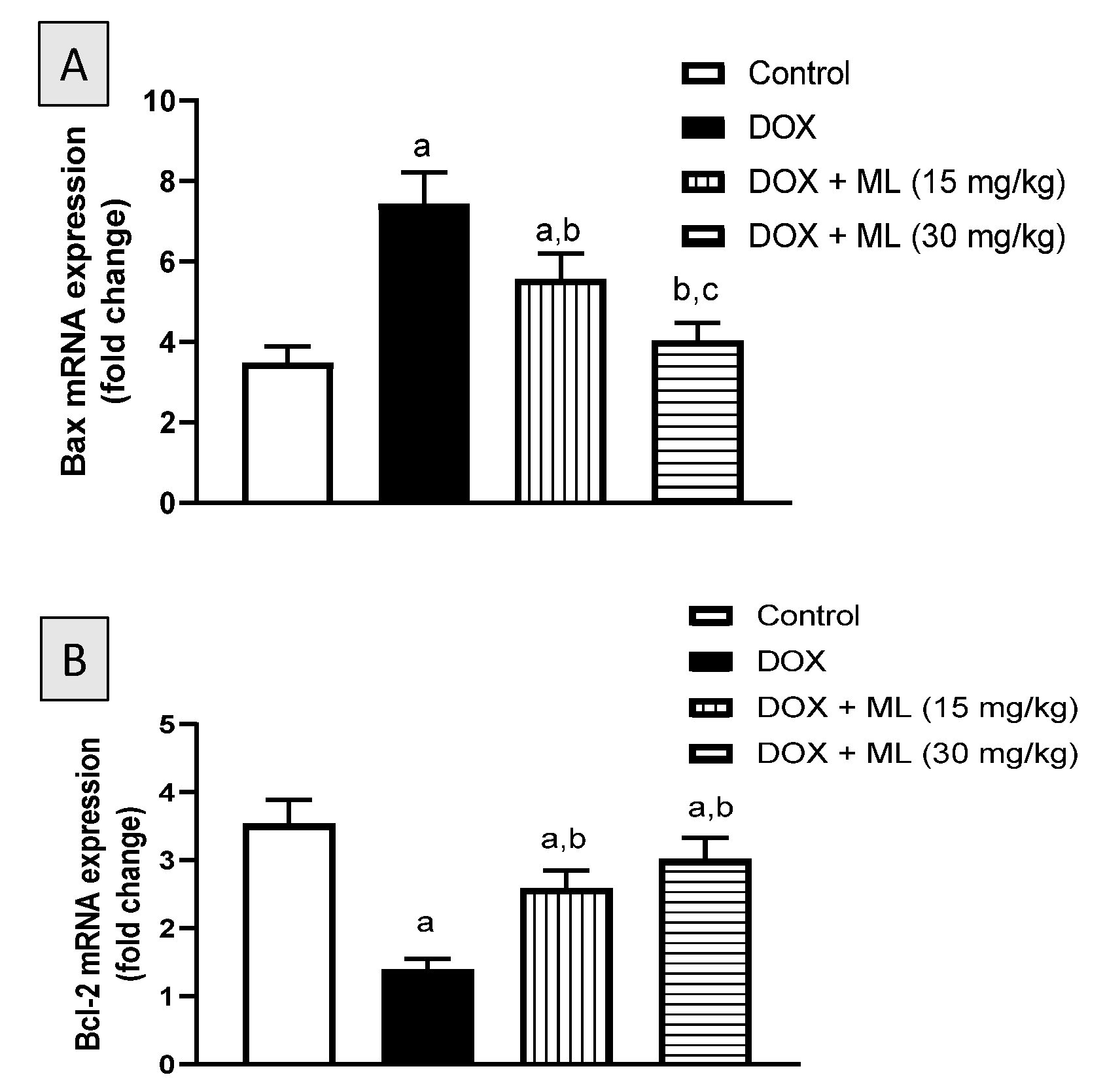
3.7. Assessment of mRNA Expression of Bax and Bcl-2
3.8. Evaluation of Sirt-1 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, D.; Aronow, W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp. Pract. 2019, 47, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Renu, K.; Abilash, V.G.; PB, T.P.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy–An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin regulates doxorubicin-induced liver dysfunction: Impact on oxidative stress and inflammation. Plants 2020, 9, 550. [Google Scholar] [CrossRef]
- Camaggi, C.M.; Comparsi, R.; Strocchi, E.; Testoni, F.; Angelelli, B.; Pannuti, F. Epirubicin and doxorubicin comparative metabolism and pharmacokinetics. Cancer Chemother. Pharmacol. 1988, 21, 221–228. [Google Scholar] [CrossRef]
- Shivakumar, P.; Rani, M.U.; Reddy, A.G.; Anjaneyulu, Y. A study on the toxic effects of doxorubicin on the histology of certain organs. Toxicol. Int. 2012, 19, 241. [Google Scholar] [PubMed] [Green Version]
- Damodar, G.; Smitha, T.; Gopinath, S.; Vijayakumar, S.; Rao, Y.A. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann. Med. Health Sci. Res. 2014, 4, 74–79. [Google Scholar] [CrossRef]
- Mansouri, E.; Jangaran, A.; Ashtari, A. Protective effect of pravastatin on doxorubicin-induced hepatotoxicity. Bratisl. Lek. Listy 2017, 118, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Nagai, K.; Oda, A.; Konishi, H. Theanine prevents doxorubicin-induced acute hepatotoxicity by reducing intrinsic apoptotic response. Food Chem. Toxicol. 2015, 78, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chu, L.; Liang, H.; Chen, J.; Liang, J.; Huang, Z.; Zhang, B.; Chen, X. Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-κb signal. Front. Pharmacol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- De la Torre, B.G.; Albericio, F. The pharmaceutical industry in 2018. An analysis of FDA drug approvals from the perspective of molecules. Molecules 2019, 24, 809. [Google Scholar] [CrossRef] [Green Version]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.D.; Wilson, I.D. Insect hormones and insect chemical ecology. ChemInform 2001, 32. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Weitzel, C.; Christensen, S.B. Guaianolide sesquiterpenoids: Pharmacology and biosynthesis. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3069–3098. [Google Scholar]
- Kim, D.Y.; Choi, B.Y. Costunolide—A bioactive sesquiterpene lactone with diverse therapeutic potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef] [Green Version]
- El-Far, A.H.; Shaheen, H.M.; Alsenosy, A.W.; El-Sayed, Y.S.; Al Jaouni, S.K.; Mousa, S.A. Costus speciosus: Traditional Uses, Phytochemistry, and Therapeutic Potentials. Pharmacogn. Rev. 2018, 12, 23. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; El-Shaer, A.-D.A.; Salah, N.; Asfou, H.Z.; Elshali, K.Z.; Shaaban, M.I.A.; Al-Attas, A.A.M.; Mohamed, G.A.A. Antimicrobial, antiquorum sensing and antiproliferative activities of sesquiterpenes from Costus speciosus rhizomes. Pak. J. Pharm. Sci. 2019, 32, 109–115. [Google Scholar] [PubMed]
- AlSaadi, B.H.; AlHarbi, S.H.; Ibrahim, S.R.M.; El-Kholy, A.A.; El-Agamy, D.S.; Mohamed, G.A. Hepatoprotective activity of Costus speciosus (Koen. Ex. Retz.) Against paracetamol induced liver injury in mice. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 35–41. [Google Scholar]
- Al-Attas, A.A.M.; El-Shaer, N.S.; Mohamed, G.A.; Ibrahim, S.R.M.; Esmat, A. Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes. J. Ethnopharmacol. 2015, 176, 365–374. [Google Scholar] [CrossRef]
- Injac, R.; Strukelj, B. Recent advances in protection against doxorubicin-induced toxicity. Technol. Cancer Res. Treat. 2008, 7, 497–516. [Google Scholar] [CrossRef]
- Quiles, J.L.; Huertas, J.R.; Battino, M.; Mataix, J.; Ramírez-Tortosa, M.C. Antioxidant nutrients and adriamycin toxicity. Toxicology 2002, 180, 79–95. [Google Scholar] [CrossRef]
- Eliza, J.; Daisy, P.; Ignacimuthu, S.; Duraipandiyan, V. Normo-glycemic and hypolipidemic effect of costunolide isolated from Costus speciosus (Koen ex. Retz.) Sm. in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2009, 179, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, I.; Özmen, Ö.; Tutun, H.; Yalçın, A.; Türk, A. Artemisinin attenuates doxorubicin induced cardiotoxicity and hepatotoxicity in rats. Biotech. Histochem. 2020, 95, 121–128. [Google Scholar] [CrossRef]
- Mahesh, A.; Jeyachandran, R.; Cindrella, L.; Thangadurai, D.; Veerapur, V.; Muralidhara Rao, D. Hepatocurative potential of sesquiterpene lactones of Taraxacum officinale on carbon tetrachloride induced liver toxicity in mice. Acta Biol. Hung. 2010, 61, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective effect of boswellic acids against doxorubicin-induced hepatotoxicity: Impact on Nrf2/HO-1 defense pathway. Oxidative Med. Cell. Longev. 2018, 2018, 8296451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Yan, M.; Chen, L.; Fang, P.; Li, Z.; Wan, Z.; Zhang, B. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2018, 16, 3333–3344. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, W.; Niu, T.; Wang, H.; Li, B.; Shao, L.; Cui, T. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxidative Med. Cell. Longev. 2014, 2014, 748524. [Google Scholar] [CrossRef] [Green Version]
- Niture, S.K.; Jaiswal, A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2 by costunolide provides neuroprotective effect in PC12 cells. Food Funct. 2019, 10, 4143–4152. [Google Scholar] [CrossRef]
- Cheong, C.-U.; Yeh, C.-S.; Hsieh, Y.-W.; Lee, Y.-R.; Lin, M.-Y.; Chen, C.-Y.; Lee, C.-H. Protective effects of Costunolide against hydrogen peroxide-induced injury in PC12 cells. Molecules 2016, 21, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.; Yi, M.; Wang, R.; Huang, Y.; Chen, M. Protective effects of costunolide against D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Front. Pharmacol. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, D.T.; Milovanova, T.N. Mucosal immunity and the FOXO1 transcription factors. Front. Immunol. 2019, 10, 2530. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Lee, K.H.; Park, S.-K.; Kim, H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004, 313, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.H.; Lee, J.-H.; Park, Y.J.; Hong, Y.-S.; Kim, H.S.; Kim, K.-W.; Lee, J.J. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-κB by targeting IκB phosphorylation. Planta Med. 2001, 67, 103–107. [Google Scholar] [CrossRef]
- Rayan, N.A.; Baby, N.; Pitchai, D.; Indraswari, F.; Ling, E.-A.; Lu, J.; Dheen, T. Costunolide inhibits proinflammatory cytokines and iNOS in activated murine BV2 microglia. Front. Biosci. 2011, 3, 1079–1091. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Gopalakrishnan, A.V. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, J.E.; Lee, A.S.; Kang, K.P.; Lee, S.; Park, S.K.; Lee, S.Y.; Han, M.K.; Kim, D.H.; Kim, W. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem. Biophys. Res. Commun. 2012, 419, 206–210. [Google Scholar] [CrossRef]
- Omran, M.M.; Mansour, H.H.; Hasan, H.F. Metformin and/or low dose radiation reduces cardiotoxicity and apoptosis induced by cyclophosphamide through SIRT-1/SOD and BAX/Bcl-2 pathways in rats. Mol. Biol. Rep. 2020, 47, 5115–5126. [Google Scholar]
- Takayama, K.; Ishida, K.; Matsushita, T.; Fujita, N.; Hayashi, S.; Sasaki, K.; Tei, K.; Kubo, S.; Matsumoto, T.; Fujioka, H. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009, 60, 2731–2740. [Google Scholar] [CrossRef] [PubMed]

| Control | DOX | DOX + ML (15 mg/kg) | DOX + ML (30 mg/kg) | |
|---|---|---|---|---|
| MDA (nmol/mg protein) | 0.37 ± 0.04 | 2.72 a ± 0.30 | 2.04 a,b ± 0.23 | 1.34 a,b,c ± 0.16 |
| GSH (nmol/mg protein) | 21.64 ± 0.24 | 8.63 a ± 0.96 | 12.70 a,b ± 1.36 | 14.87 a,b ± 1.52 |
| SOD (U/mg protein) | 3.36 ± 0.43 | 1.23 a ± 0.14 | 1.88 a,b ± 0.20 | 2.75 a,b,c ± 0.30 |
| CAT (U/mg protein) | 4.79 ± 0.50 | 1.87 a ± 0.20 | 3.42 a,b ± 0.36 | 3.64 a,b ± 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirwi, A.; Shaik, R.A.; Alamoudi, A.J.; Eid, B.G.; Kammoun, A.K.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdallah, H.M.; Abdel-Naim, A.B. Mokko Lactone Attenuates Doxorubicin-Induced Hepatotoxicity in Rats: Emphasis on Sirt-1/FOXO1/NF-κB Axis. Nutrients 2021, 13, 4142. https://doi.org/10.3390/nu13114142
Sirwi A, Shaik RA, Alamoudi AJ, Eid BG, Kammoun AK, Ibrahim SRM, Mohamed GA, Abdallah HM, Abdel-Naim AB. Mokko Lactone Attenuates Doxorubicin-Induced Hepatotoxicity in Rats: Emphasis on Sirt-1/FOXO1/NF-κB Axis. Nutrients. 2021; 13(11):4142. https://doi.org/10.3390/nu13114142
Chicago/Turabian StyleSirwi, Alaa, Rasheed A. Shaik, Abdulmohsin J. Alamoudi, Basma G. Eid, Ahmed K. Kammoun, Sabrin R. M. Ibrahim, Gamal A. Mohamed, Hossam M. Abdallah, and Ashraf B. Abdel-Naim. 2021. "Mokko Lactone Attenuates Doxorubicin-Induced Hepatotoxicity in Rats: Emphasis on Sirt-1/FOXO1/NF-κB Axis" Nutrients 13, no. 11: 4142. https://doi.org/10.3390/nu13114142
APA StyleSirwi, A., Shaik, R. A., Alamoudi, A. J., Eid, B. G., Kammoun, A. K., Ibrahim, S. R. M., Mohamed, G. A., Abdallah, H. M., & Abdel-Naim, A. B. (2021). Mokko Lactone Attenuates Doxorubicin-Induced Hepatotoxicity in Rats: Emphasis on Sirt-1/FOXO1/NF-κB Axis. Nutrients, 13(11), 4142. https://doi.org/10.3390/nu13114142










