Predicting Hospital Length of Stay at Admission Using Global and Country-Specific Competing Risk Analysis of Structural, Patient, and Nutrition-Related Data from nutritionDay 2007–2015
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Variables
2.4. Statistical Methods
2.4.1. Descriptive Analysis
2.4.2. Multivariable Analysis Statistical Methods
2.4.3. Country-Specific Analyses
2.4.4. Cross-Sectional Study Bias
2.4.5. Department Clustering
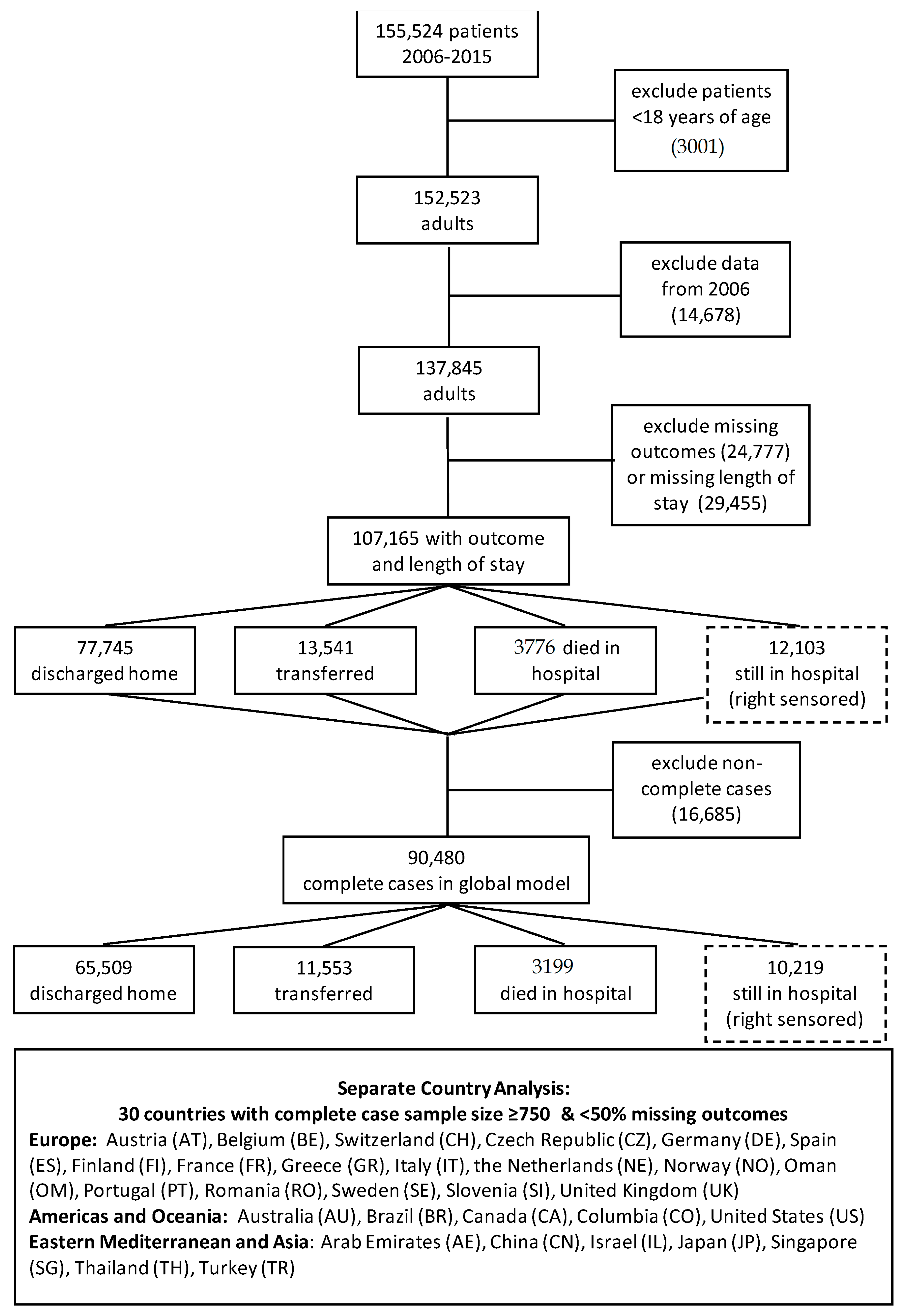
2.5. Missingness
3. Results
3.1. Outcome Timing
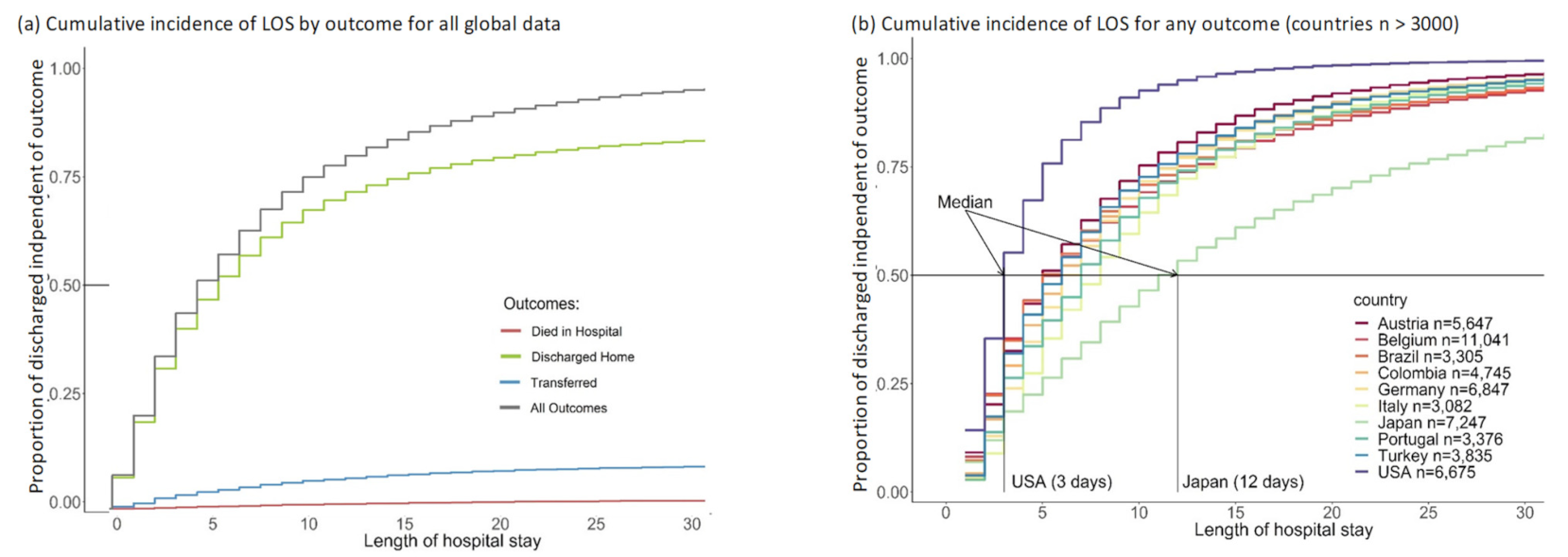
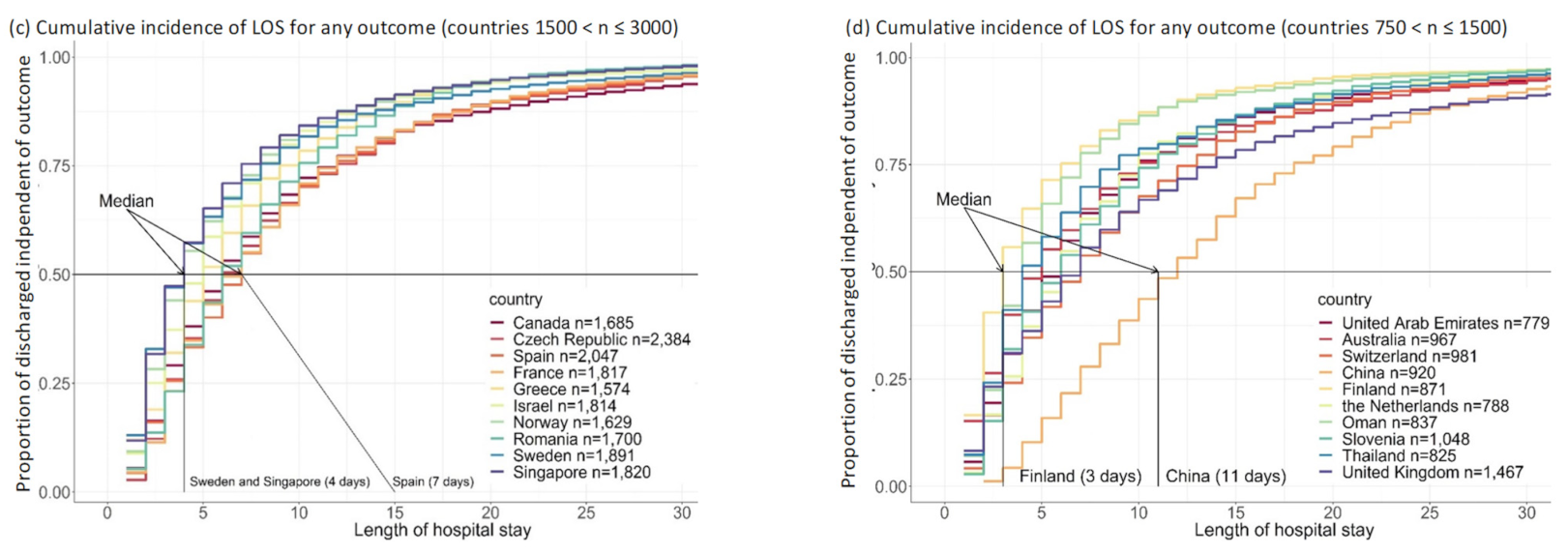
3.2. Length of Stay (LOS)
3.3. Multivariable Analysis
3.3.1. Predictors of LOS
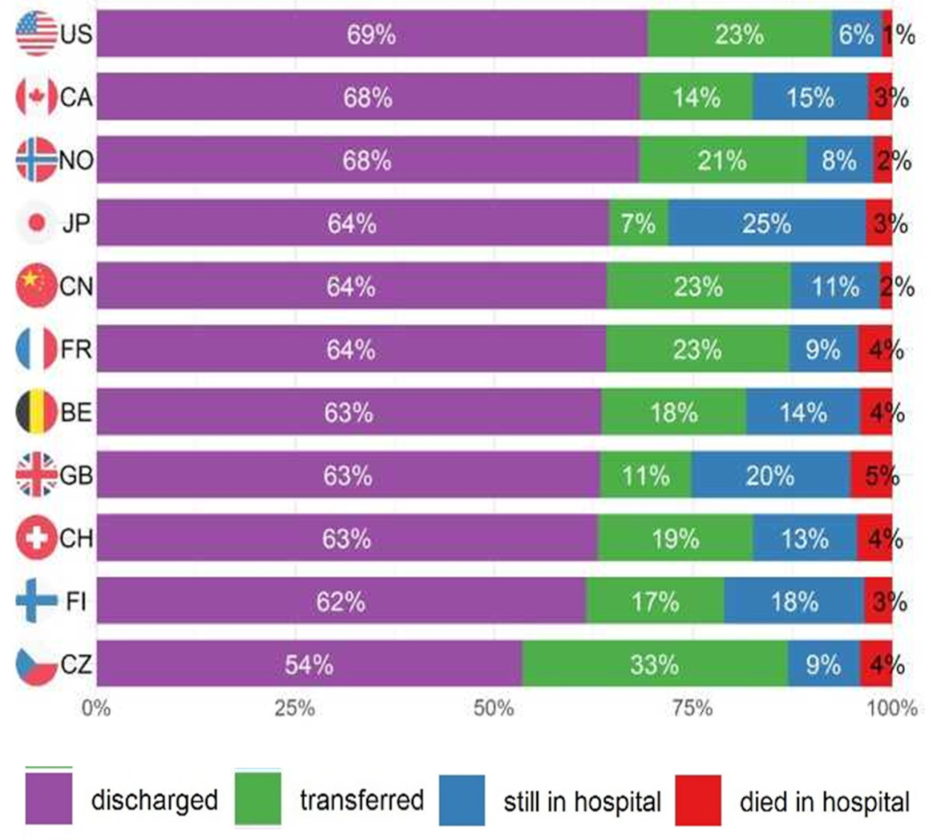
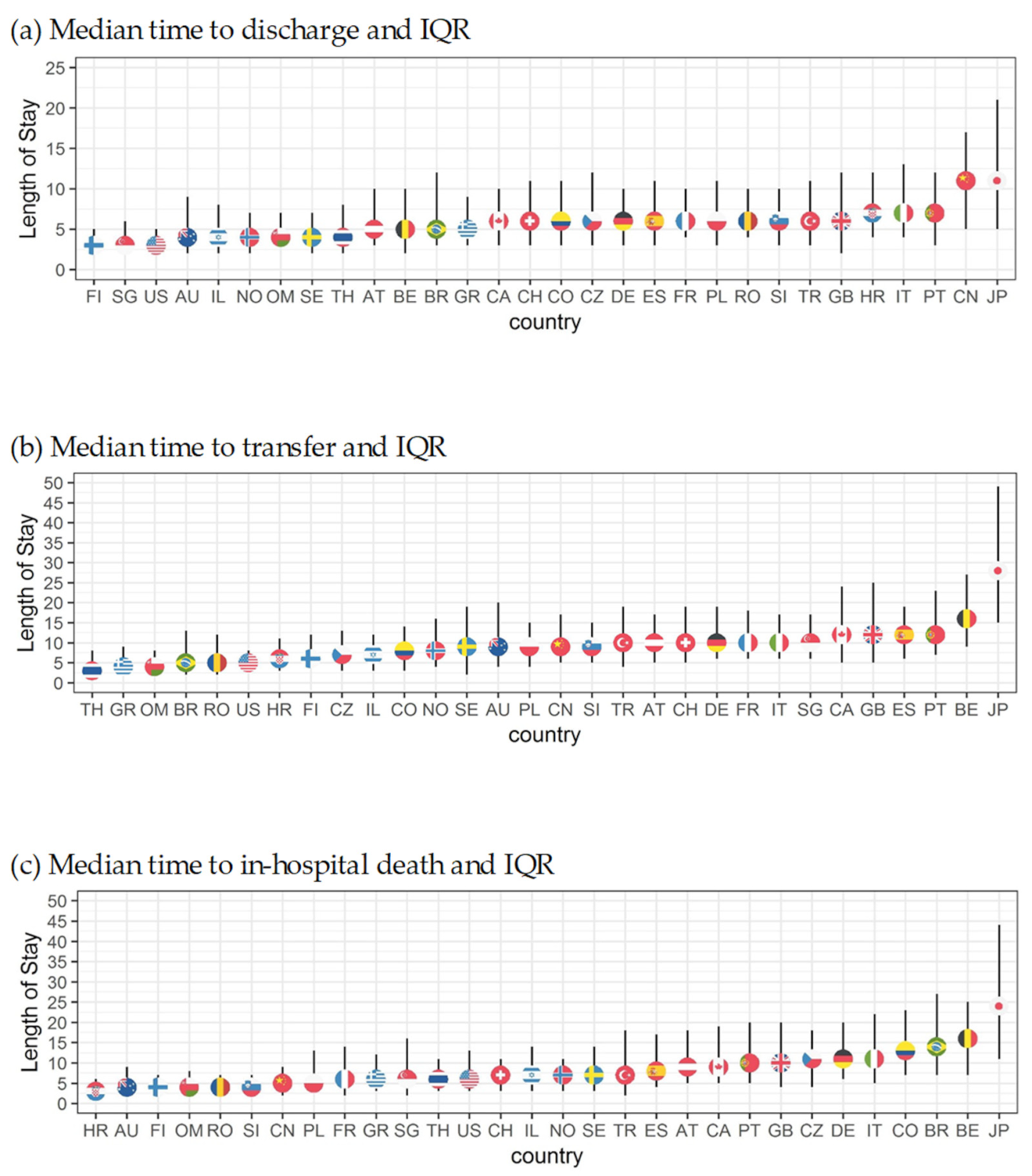
3.3.2. Country-Specific Analyses
4. Discussion
4.1. Discussion
4.2. Country Comparisons
4.3. Limitations, Mitigations, and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Variables
Appendix A.2. Results
References
- Hiesmayr, M.; Schindler, K.; Pernicka, E.; Schuh, C.; Schoeniger-Hekele, A.; Bauer, P.; Laviano, A.; Lovell, A.; Mouhieddine, M.; Schuetz, T.; et al. Decreased food intake is a risk factor for mortality in hospitalised patients: The nutritionday survey 2006. Clin. Nutr. 2009, 28, 484–491. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Hiesmayr, M.; Frantal, S.; Schindler, K.; Themessl-Huber, M.; Mouhieddine, M.; Schuh, C.; Pernicka, E.; Schneider, S.; Singer, P.; Ljunqvist, O.; et al. The patient- and nutrition-derived outcome risk assessment score (pandora): Development of a simple predictive risk score for 30-day in-hospital mortality based on demographics, clinical observation, and nutrition. PLoS ONE 2015, 10, e0127316. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Health at a Glance 2019: OECD Indicators; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Lorenzoni, L.; Marino, A. Understanding Variations in Hospital Length of Stay and Cost: Results of a Pilot Project; OECD Health Working Papers, No. 94; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Guide to Reducing Long Hospital Stays; NHS Improvement: London, UK, 2018.
- Thompson, G.; O’Horo, J.C.; Pickering, B.W.; Herasevich, V. Impact of the electronic medical record on mortality, length of stay, and cost in the hospital and icu: A systematic review and metaanalysis. Crit. Care Med. 2015, 43, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Busse, R. Do diagnosis-related groups explain variations in hospital costs and length of stay?—Analyses from the eurodrg project for 10 episodes of care across 10 European countries. Health Econ. 2012, 21, 1–5. [Google Scholar] [CrossRef]
- Wiley, M.M.; Tomas, R.; Casas, M. A cross-national, casemix analysis of hospital length of stay for selected pathologies. Eur. J. Public Health 1999, 9, 86–92. [Google Scholar] [CrossRef]
- Rotter, T.; Kugler, J.; Koch, R.; Gothe, H.; Twork, S.; van Oostrum, J.M.; Steyerberg, E.W. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs and patient outcomes. BMC Health Serv. Res. 2008, 8, 265. [Google Scholar] [CrossRef]
- Gaughan, J.; Kobel, C.; Linhart, C.; Mason, A.; Street, A.; Ward, P. Why do patients having coronary artery bypass grafts have different costs or length of stay? An analysis across 10 european countries. Health Econ. 2012, 21, 77–88. [Google Scholar] [CrossRef]
- Vrijens, F.; Hulstaert, F.; Devriese, S.; Van de Sande, S. Hospital-acquired infections in belgian acute-care hospitals: An estimation of their global impact on mortality, length of stay and healthcare costs. Epidemiol. Infect. 2011, 140, 126–136. [Google Scholar] [CrossRef]
- Taylor, S.; Sen, S.; Greenhalgh, D.G.; Lawless, M.; Curri, T.; Palmieri, T.L. A competing risk analysis for hospital length of stay in patients with burns. JAMA Surg. 2015, 150, 450–456. [Google Scholar] [CrossRef]
- Jean, S.; Harvey, E.; Belzile, E.; Brown, J.; Morin, S. Temporal trends in length of stay after hip, femur and pelvic fracture in Québec, Canada. J. Bone Miner. Res. 2017, 32, S250–S251. [Google Scholar] [CrossRef]
- Bai, A.D.; Showler, A.; Burry, L.; Steinberg, M.; Ricciuto, D.R.; Fernandes, T.; Chiu, A.; Raybardhan, S.; Science, M.; Fernando, E.; et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in staphylococcus aureus bacteremia: Results from a large multicenter cohort study. Clin. Infect. Dis. 2015, 60, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Huebner, M.; Larson, D.W.; Cima, R.R.; Habermann, E. Impact of key factors of enhanced recovery pathway and preexisting comorbidities on complications and length of stay following colorectal surgery. Gastroenterology 2013, 144, S1061. [Google Scholar] [CrossRef]
- Huebner, M.; Hübner, M.; Cima, R.R.; Larson, D.W. Timing of complications and length of stay after rectal cancer surgery. J. Am. Coll. Surg. 2014, 218, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Barili, F.; Cheema, F.H.; Barzaghi, N.; Grossi, C. The analysis of intensive care unit length of stay in a competing risk setting. Eur. J. Cardio. Thorac. Surg. 2012, 41, 232. [Google Scholar] [CrossRef] [PubMed]
- Barili, F.; Barzaghi, N.; Cheema, F.H.; Capo, A.; Jiang, J.; Ardemagni, E.; Argenziano, M.; Grossi, C. An original model to predict intensive care unit length-of stay after cardiac surgery in a competing risk framework. Int. J. Cardiol. 2013, 168, 219–225. [Google Scholar] [CrossRef] [PubMed]
- NutritionDay Worldwide: Benchmark and Monitor your Nutrition Care. Available online: https://www.nutritionday.org/ (accessed on 16 November 2021).
- Schindler, K.; Pichard, C.; Sulz, I.; Volkert, D.; Streicher, M.; Singer, P.; Ljungqvist, O.; Van Gossum, A.; Bauer, P.; Hiesmayr, M. Nutritionday: 10 years of growth. Clin. Nutr. 2017, 36, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R_R Package Version 3.2-13. 2021. Available online: https://CRAN.R-project.org/package=survival> (accessed on 15 November 2021).
- Terry, M.; Therneau, P.; Grambsch, M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Frantal, S.; Pernicka, E.; Hiesmayr, M.; Schindler, K.; Bauer, P. Length bias correction in one-day cross-sectional assessments—The nutritionday study. Clin. Nutr. 2016, 35, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Aalen, O.O.; Johansen, S. An empirical transition matrix for non-homogeneous markov chains based on censored observations. Scand. J. Stat. 1978, 5, 141–150. [Google Scholar]
- Dignam, J.J.; Zhang, Q.; Kocherginsky, M.N. The use and interpretation of competing risks regression models. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2301–2308. [Google Scholar] [CrossRef]
- Wears, R.L. Advanced statistics: Statistical methods for analyzing cluster and cluster-randomized data. Acad. Emerg. Med. 2002, 9, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Scealy, J.L.; Welsh, A.H. Model selection in linear mixed models. Stat. Sci. 2013, 28, 135–167. [Google Scholar] [CrossRef]
- Bauer, P.; Pötscher, B.M.; Hackl, P. Model selection by multiple test procedures. Statistics 1988, 19, 39–44. [Google Scholar] [CrossRef]
- Binder, D.A. Fitting cox’s proportional hazards models from survey data. Biometrika 1992, 79, 139–147. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Zheng, Y. Survival model predictive accuracy and roc curves. Biometrics 2005, 61, 92–105. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Saha-Chaudhur, P. Risksetroc: Riskset Roc Curve Estimation from Censored Survival Data; 2012. [Google Scholar]
- Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Lu, M.; Sajobi, T.; Lucyk, K.; Lorenzetti, D.L.; Quan, H. Systematic review of risk adjustment models of hospital length of stay (los). Med. Care 2015, 53, 355–365. [Google Scholar] [CrossRef]
- Lainscak, M.; Schols, A.; Farkas, J.; Sulz, I.; Themessl-Huber, M.; Laviano, A.; Kosak, S.; Hiesmayr, M.; Schindler, K. Weight loss, food intake and mortality in hospitalized patients with chronic obstructive pulmonary disease (copd): The nutritionday survey analysis. Eur. Respir. J. 2016, 48, OA1507. [Google Scholar] [CrossRef]
- Cereda, E.; Klersy, C.; Hiesmayr, M.; Schindler, K.; Singer, P.; Laviano, A.; Caccialanza, R. Body mass index, age and in-hospital mortality: The nutritionday multinational survey. Clin. Nutr. 2017, 36, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; Anker, S.; Farkas, J.; Themessl-Huber, M.; Laviano, A.; Hiesmayr, M.; Schindler, K. Nutritional indices, weight change, and mortality in heart failure: The nutrition day survey analysis. Eur. J. Heart Fail. 2016, 18, 92. [Google Scholar] [CrossRef]


| Total n = 90,480 | Time to Discharge n = 65,509 | Time to Transfer n = 11,553 | Time to Death n = 3199 | |
|---|---|---|---|---|
| Weight Δ in the last 3 months (prior to hospitalization) | ||||
| Lost weight | 7 (3–13) | 6 (3–12) | 10 (4–19) | 14 (7–26) |
| Idem (stayed the same) | 4 (3–9) | 4 (2–8) | 7 (3–14) | 11 (5–21) |
| Gained weight | 4 (3–8) | 4 (2–8) | 7 (3–13) | 9 (3–22) |
| Unsure | 7 (3–13) | 6 (3–12) | 10 (5–18) | 12 (5–21) |
| Missing | 6 (4–12) | 4 (2–9) | 10 (5–20) | 10 (6–20) |
| Nutrition risk screening at admission | ||||
| Not Screened | 7 (4–12) | 5 (3–10) | 10 (5–17) | 12 (7–22) |
| Screened | 5 (3–10) | 5 (3–9) | 8 (4–16) | 12 (6–24) |
| Missing | 5 (4–11) | 5 (4–11) | 3 (2–6) | 2 (2–2) |
| Dedicated nutrition care person (department) | ||||
| Yes | 5 (3–10) | 5 (3–9) | 8 (4–16) | 13 (6–24) |
| No | 7 (4–13) | 6 (3–11) | 9 (5–18) | 11 (5–22) |
| Nutrition team available (hospital) | ||||
| Yes | 6 (4–12) | 5 (3–10) | 10 (5–18) | 13 (7–24) |
| No | 5 (3–9) | 5 (2–9) | 7 (3–14) | 11 (5–20) |
| Dietician available | ||||
| Yes | 5 (3–10) | 5 (3–9) | 8 (3–15) | 12 (6–23) |
| No | 7 (4–12) | 5 (3–10) | 10 (5–18) | 13 (6–24) |
| Missing | 6 (3–11) | 5 (3–10) | 10 (5–19) | 12 (6–22) |
| Discharged | Transferred | Died in Hospital | ||||
|---|---|---|---|---|---|---|
| Variable | Increase LOS | Decrease LOS | Increase LOS | Decrease LOS | Increase LOS | Decrease LOS |
| Patient characteristics | ||||||
| Age (reference 61–70) | - | - | - | - | - | - |
| 18–30 | - | 1.18 (1.12–1.24) | - | - | 0.31 (0.14–0.71) | - |
| 31–40 | - | 1.15 (1.09–1.21) | - | - | 0.49 (0.29–0.85) | - |
| 41–50 | - | 1.10 (1.05–1.15) | - | - | - | - |
| 51–60 | - | 1.06 (1.02–1.10) | - | - | - | - |
| 71–80 | 0.92 (0.89–0.96) | - | - | 1.25 (1.10–1.43) | - | 1.40 (1.10–1.77) |
| 81–120 | 0.78 (0.74–0.82) | - | - | 1.77 (1.54–2.04) | - | 2.25 (1.78–2.84) |
| Male | - | - | - | - | - | 1.19 (1.03–1.39) |
| Affected Organs | ||||||
| Brain/nerves | 0.86 (0.82–0.91) | - | 1.35 (1.19–1.55) | - | - | - |
| Skeleton/bone/muscle | 0.86 (0.81–0.90) | - | 1.22 (1.07–1.39) | - | 0.62 (0.47–0.82) | - |
| Blood/bone marrow | 0.86 (0.80–0.93) | - | - | - | - | - |
| Skin | 0.87 (0.81–0.93) | - | - | - | - | - |
| Cancer | 0.91 (0.86–0.96) | - | - | - | - | 2.34 (1.88–2.90) |
| Infection | 0.86 (0.81–0.91) | - | - | - | - | 1.34 (1.04–1.73) |
| Eye/ear | - | 1.12(1.02–1.22) | - | - | - | - |
| Lung | 0.88 (0.85–0.92) | - | - | - | - | 1.83 (1.50–2.24) |
| Liver | 0.86 (0.81–0.91) | - | - | - | - | 1.90 (1.45–2.49) |
| Comorbidities | - | - | - | - | - | - |
| Diabetes I/II | 0.94 (0.91–0.97) | - | - | - | - | |
| Stroke | 0.90 (0.84–0.97) | - | - | - | - | - |
| Others | 0.96 (0.93–0.99) | - | - | 1.13 (1.01–1.27) | - | - |
| Setting characteristics | ||||||
| Regions (ref. Eur A) | ||||||
| American Region A | - | 1.10 (1.02–1.17) | - | 2.59 (2.06–3.26) | - | - |
| American Region B | 0.92 (0.85–0.99) | - | - | - | - | 1.82 (1.34–2.47) |
| Europe Region B/C | 0.86 (0.79–0.93) | - | 0.50 (0.30–0.83) | - | - | - |
| Japan | 0.80 (0.68–0.93) | - | 0.31 (0.23–0.43) | - | 0.46 (0.31–0.70) | - |
| Specialty (ref. Internal medicine) | - | - | - | - | - | - |
| Cardiothoracic surgery | - | - | - | - | 0.38 (0.15–0.95) | - |
| Ear Nose Throat (ENT) | - | - | - | - | 0.22 (0.07–0.69) | - |
| General surgery | - | 1.07 (1.00–1.14) | - | - | 0.54 (0.39–0.76) | - |
| Geriatrics | 0.67 (0.60–0.76) | - | - | - | 0.67 (0.49–0.91) | - |
| Gynaecology | - | - | - | - | 0.34 (0.13–0.94) | - |
| Long-term care | 0.72 (0.53–0.99) | - | 0.51 (0.29–0.91) | - | - | - |
| Orthopaedic surgery | - | - | - | - | 0.47 (0.23–0.97) | - |
| Psychiatry | 0.63 (0.45–0.87) | - | 0.43 (0.21–0.88) | - | 0.11 (0.02–0.70) | - |
| Nutrition-related characteristics | ||||||
| Dietician available | - | - | 0.77 (0.63–0.94) | - | - | - |
| Nutrition team available | 0.95 (0.91–1.00) | - | - | - | - | - |
| Weight Δ in the last 3 months (reference idem) | ||||||
| Lost weight | 0.86 (0.83–0.88) | - | - | - | - | 1.75 (1.40–2.18) |
| Unsure | 0.87 (0.80–0.93) | - | - | 1.47 (1.21–1.79) | - | 4.50 (3.35–6.05) |
| Missing | 0.83 (0.78–0.89) | - | - | 1.32 (1.13–1.54) | - | 2.88 (2.07–4.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, N.; Hiesmayr, M.; Sulz, I.; Bauer, P.; Heinze, G.; Mouhieddine, M.; Schuh, C.; Tarantino, S.; Simon, J. Predicting Hospital Length of Stay at Admission Using Global and Country-Specific Competing Risk Analysis of Structural, Patient, and Nutrition-Related Data from nutritionDay 2007–2015. Nutrients 2021, 13, 4111. https://doi.org/10.3390/nu13114111
Kiss N, Hiesmayr M, Sulz I, Bauer P, Heinze G, Mouhieddine M, Schuh C, Tarantino S, Simon J. Predicting Hospital Length of Stay at Admission Using Global and Country-Specific Competing Risk Analysis of Structural, Patient, and Nutrition-Related Data from nutritionDay 2007–2015. Nutrients. 2021; 13(11):4111. https://doi.org/10.3390/nu13114111
Chicago/Turabian StyleKiss, Noemi, Michael Hiesmayr, Isabella Sulz, Peter Bauer, Georg Heinze, Mohamed Mouhieddine, Christian Schuh, Silvia Tarantino, and Judit Simon. 2021. "Predicting Hospital Length of Stay at Admission Using Global and Country-Specific Competing Risk Analysis of Structural, Patient, and Nutrition-Related Data from nutritionDay 2007–2015" Nutrients 13, no. 11: 4111. https://doi.org/10.3390/nu13114111
APA StyleKiss, N., Hiesmayr, M., Sulz, I., Bauer, P., Heinze, G., Mouhieddine, M., Schuh, C., Tarantino, S., & Simon, J. (2021). Predicting Hospital Length of Stay at Admission Using Global and Country-Specific Competing Risk Analysis of Structural, Patient, and Nutrition-Related Data from nutritionDay 2007–2015. Nutrients, 13(11), 4111. https://doi.org/10.3390/nu13114111







