The Prognostic Role of Glutathione and Its Related Antioxidant Enzymes in the Recurrence of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
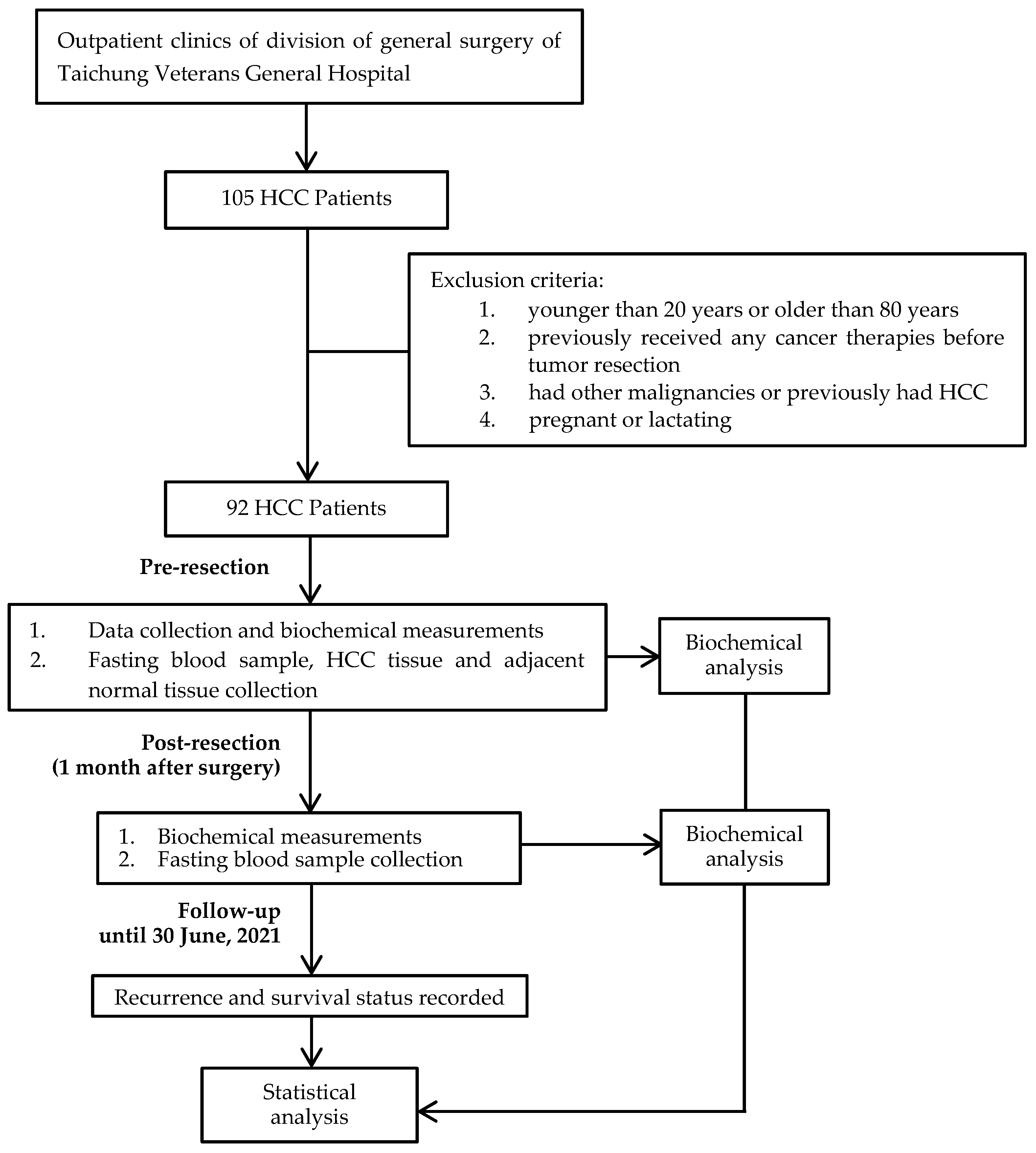
2.1. Study Design and Sample Size Calculation
2.2. Patient Eligibility and Tumor Pathology
2.3. Data Collection and Biochemical Measurements
2.4. Follow-Up Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| GSH | glutathione |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GST | glutathione S-transferase |
| GSSG | glutathione disulfide |
| HCC | hepatocellular carcinoma |
| hs-CRP | high-sensitivity C-reactive protein |
| MDA | malondialdehyde |
| TEAC | trolox equivalent antioxidant capacity |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Thandra, K.C.; Saginala, K.; Rawla, P.; Barsouk, A. Chemical Risk Factors of Primary Liver Cancer: An Update. Hepat. Med. 2020, 12, 179–188. [Google Scholar] [CrossRef]
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Mossenta, M.; Busato, D.; Dal Bo, M.; Toffoli, G. Glucose Metabolism and Oxidative Stress in Hepatocellular Carcinoma: Role and Possible Implications in Novel Therapeutic Strategies. Cancers 2020, 12, 1668. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Tsai, S.M.; Lin, S.K.; Lee, K.T.; Hsiao, J.K.; Huang, J.C.; Wu, S.H.; Ma, H.; Wu, S.H.; Tsai, L.Y. Evaluation of redox statuses in patients with hepatitis B virus-associated hepatocellular carcinoma. Ann. Clin. Biochem. 2009, 46, 394–400. [Google Scholar] [CrossRef]
- Nishimura, M.; Takaki, A.; Tamaki, N.; Maruyama, T.; Onishi, H.; Kobayashi, S.; Nouso, K.; Yasunaka, T.; Koike, K.; Hagihara, H.; et al. Serum oxidative-anti-oxidative stress balance is dysregulated in patients with hepatitis C virus-related hepatocellular carcinoma. Hepatol. Res. 2013, 43, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Yahya, R.S.; Ghanem, O.H.; Foyouh, A.A.; Atwa, M.; Enany, S.A. Role of interleukin-8 and oxidative stress in patients with hepatocellular carcinoma. Clin. Lab. 2013, 59, 969–976. [Google Scholar] [CrossRef]
- Lee, K.T.; Tsai, S.M.; Wang, S.N.; Lin, S.K.; Wu, S.H.; Chuang, S.C.; Wu, S.H.; Ma, H.; Tsai, L.Y. Glutathione status in the blood and tissues of patients with virus-originated hepatocellular carcinoma. Clin. Biochem. 2007, 40, 1157–1162. [Google Scholar] [CrossRef]
- Lin, C.C.; Yin, M.C. B vitamins deficiency and decreased anti-oxidative state in patients with liver cancer. Eur. J. Nutr. 2007, 46, 293–299. [Google Scholar] [CrossRef]
- Shimomura, Y.; Takaki, A.; Wada, N.; Yasunaka, T.; Ikeda, F.; Maruyama, T.; Tamaki, N.; Uchida, D.; Onishi, H.; Kuwaki, K.; et al. The Serum Oxidative/Anti-oxidative Stress Balance Becomes Dysregulated in Patients with Non-alcoholic Steatohepatitis Associated with Hepatocellular Carcinoma. Intern. Med. 2017, 56, 243–251. [Google Scholar] [CrossRef]
- Sanabria, J.R.; Kombu, R.S.; Zhang, G.F.; Sandlers, Y.; Ai, J.; Ibarra, R.A.; Abbas, R.; Goyal, K.; Brunengraber, H. Glutathione species and metabolomic prints in subjects with liver disease as biological markers for the detection of hepatocellular carcinoma. HPB 2016, 18, 979–990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samant, H.; Amiri, H.S.; Zibari, G.B. Addressing the worldwide hepatocellular carcinoma: Epidemiology, prevention and management. J. Gastrointest. Oncol. 2021, 12, S361–S373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Liu, H.T.; Chen, S.Y.; Lin, P.T.; Lai, C.Y.; Huang, Y.C. Changes of Oxidative Stress, Glutathione, and Its Dependent Antioxidant Enzyme Activities in Patients with Hepatocellular Carcinoma before and after Tumor Resection. PLoS ONE 2017, 12, e0170016. [Google Scholar] [CrossRef]
- Liao, K.F.; Lai, S.W.; Lin, C.Y.; Huang, C.H.; Lin, Y.Y. Risk factors of recurrence after curative resection of hepatocellular carcinoma in Taiwan. Am. J. Med. Sci. 2011, 341, 301–304. [Google Scholar]
- Shehta, A.; Han, H.S.; Ahn, S.; Yoon, Y.S.; Cho, J.Y.; Choi, Y.R. Post-resection recurrence of hepatocellular carcinoma in cirrhotic patients: Is thrombocytopenia a risk factor for recurrence? Surg. Oncol. 2016, 25, 364–369. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Ahn, C.S.; Kim, K.H.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Park, G.C.; Hong, S.M. Postresection prognosis of combined hepatocellular carcinoma-cholangiocarcinoma according to the 2010 World Health Organization classification: Single-center experience of 168 patients. Ann. Surg. Treat. Res. 2021, 100, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yuan, S.X.; Yang, F.; Tao, Q.F.; Yang, Y.; Xu, Q.G.; Wang, Z.G.; Yu, J.; Lin, K.Y.; Wang, Z.Y.; et al. Paraoxonase 3 inhibits cell proliferation and serves as a prognostic predictor in hepatocellular carcinoma. Oncotarget 2016, 7, 70045–70057. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, Y.M.; Wu, J.; Yang, F.C.; Lv, Z.; Xu, X.F.; Zheng, S.S. Expression of FOXO6 is Associated With Oxidative Stress Level and Predicts the Prognosis in Hepatocellular Cancer: A Comparative Study. Medicine 2016, 95, e3708. [Google Scholar] [CrossRef]
- Fang, Y.; He, J.; Janssen, H.L.A.; Wu, J.; Dong, L.; Shen, X.Z. Peroxiredoxin 1, restraining cell migration and invasion, is involved in hepatocellular carcinoma recurrence. J. Dig. Dis. 2018, 19, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Yu, J.; Huang, Y.H.; Lin, Y.H.; Yeh, C.T. The lipid peroxidation derived DNA adduct gamma-OHPdG levels in paraneoplastic liver tissues predict postoperative outcomes of hepatoma. J. Cancer 2021, 12, 4064–4074. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Lin, P.T.; Liu, H.T.; Peng, Y.S.; Huang, S.C.; Huang, Y.C. Vitamin B-6 Supplementation Could Mediate Antioxidant Capacity by Reducing Plasma Homocysteine Concentration in Patients with Hepatocellular Carcinoma after Tumor Resection. BioMed Res. Int. 2016, 2016, 7658981. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ng, K.T.; Shao, Y.; Li, C.X.; Geng, W.; Ling, C.C.; Ma, Y.Y.; Liu, X.B.; Liu, H.; Liu, J.; et al. The Clinical Significance and Potential Therapeutic Role of GPx3 in Tumor Recurrence after Liver Transplantation. Theranostics 2016, 6, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Frankel, T.L.; Sonnenday, C.; Cho, C.S.; Nathan, H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): A Surveillance, Epidemiology, End Results (SEER) analysis. J. Surg. Oncol. 2018, 117, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic. Biol. Med. 2001, 31, 331–335. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J.; García-Cánovas, F.; Acosta, M. Inhibition by L-ascorbic acid and other antioxidants of the 2.2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase: A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [PubMed]
- Sentellas, S.; Morales-Ibanez, O.; Zanuy, M.; Alberti, J.J. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. In Vitro 2014, 28, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Chen, C.; An, J.; Shang, Y.; Li, H.; Xia, H.; Yu, J.; Wang, C.; Liu, Y.; et al. Regulation of TBBPA-induced oxidative stress on mitochondrial apoptosis in L02cells through the Nrf2 signaling pathway. Chemosphere 2019, 226, 463–471. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.H.; Hassan, M.H.; Al Robaian, M.M.; Maghrabi, I.A. Cadmium-induced hepatocellular injury: Modulatory effects of gamma-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J. Trace Elem. Med. Biol. 2019, 52, 74–82. [Google Scholar] [CrossRef]
- Lorente, L.; Rodriguez, S.T.; Sanz, P.; Abreu-Gonzalez, P.; Diaz, D.; Moreno, A.M.; Borja, E.; Martin, M.M.; Jimenez, A.; Barrera, M.A. Association between Pre-Transplant Serum Malondialdehyde Levels and Survival One Year after Liver Transplantation for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 500. [Google Scholar] [CrossRef]
- Lorente, L.; Rodriguez, S.T.; Sanz, P.; Perez-Cejas, A.; Abreu-Gonzalez, P.; Padilla, J.; Diaz, D.; Gonzalez, A.; Martin, M.M.; Jimenez, A.; et al. Serum total antioxidant capacity prior to liver transplantation for hepatocellular carcinoma is associated with 1-year liver transplantation survival. J. Int. Med. Res. 2018, 46, 2641–2649. [Google Scholar] [CrossRef]
- Qi, X.; Ng, K.T.; Lian, Q.Z.; Liu, X.B.; Li, C.X.; Geng, W.; Ling, C.C.; Ma, Y.Y.; Yeung, W.H.; Tu, W.W.; et al. Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget 2014, 5, 11103–11120. [Google Scholar] [CrossRef]
- Liu, T.; Kan, X.F.; Ma, C.; Chen, L.L.; Cheng, T.T.; Zou, Z.W.; Li, Y.; Cao, F.J.; Zhang, W.J.; Yao, J.; et al. GPX2 overexpression indicates poor prognosis in patients with hepatocellular carcinoma. Tumour Biol. 2017, 39, 1010428317700410. [Google Scholar] [CrossRef]
- Alves, A.F.; Moura, A.C.; Andreolla, H.F.; Veiga, A.; Fiegenbaum, M.; Giovenardi, M.; Almeida, S. Gene expression evaluation of antioxidant enzymes in patients with hepatocellular carcinoma: RT-qPCR and bioinformatic analyses. Genet. Mol. Biol. 2021, 44, e20190373. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Ma, Q.; Chen, W.; Atyah, M.; Yin, Y.; Fu, P.; Liu, S.; Hu, B.; Ren, N.; et al. High GCLC level in tumor tissues is associated with poor prognosis of hepatocellular carcinoma after curative resection. J. Cancer 2019, 10, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Ma-On, C.; Sanpavat, A.; Whongsiri, P.; Suwannasin, S.; Hirankarn, N.; Tangkijvanich, P.; Boonla, C. Oxidative stress indicated by elevated expression of Nrf2 and 8-OHdG promotes hepatocellular carcinoma progression. Med. Oncol. 2017, 34, 57. [Google Scholar] [CrossRef]
- Wang, Z.X.; Peng, W.; Zhang, X.Y.; Wen, T.F.; Li, C. Prognostic significance of postoperative change of PALBI grade for patients with hepatocellular carcinoma after hepatectomy. Medicine 2021, 100, e24476. [Google Scholar] [CrossRef]
- Lurje, G.; Bednarsch, J.; Czigany, Z.; Amygdalos, I.; Meister, F.; Schoning, W.; Ulmer, T.F.; Foerster, M.; Dejong, C.; Neumann, U.P. Prognostic factors of disease-free and overall survival in patients with hepatocellular carcinoma undergoing partial hepatectomy in curative intent. Langenbecks Arch. Surg. 2018, 403, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Xing, H.; Han, J.; Li, Z.L.; Lau, W.Y.; Zhou, Y.H.; Gu, W.M.; Wang, H.; Chen, T.H.; Zeng, Y.Y.; et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019, 154, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.W.; Ning, W.; Chen, X.; Smith, J.J.; Washington, M.K.; Hill, K.E.; Coburn, L.A.; Peek, R.M.; Chaturvedi, R.; Wilson, K.T.; et al. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013, 73, 1245–1255. [Google Scholar] [CrossRef]

| Characteristics | Recurrence (n = 48) | Non-Recurrence (n = 44) | ||
|---|---|---|---|---|
| Pre-Resection | Post-Resection | Pre-Resection | Post-Resection | |
| Age (years) | 60.9 ± 9.7 | 58.6 ± 10.3 | ||
| Gender (male/female) | 39/9 | 36/8 | ||
| BMI (kg/m2) | 24.0 ± 3.0 | 23.6 ± 2.8 2,* | 24.9 ± 2.8 | 24.5 ± 2.4 3,* |
| Blood pressure | ||||
| SBP (mmHg) | 131.3 ± 16.2 | 126.7 ± 18.6 2,* | 126.8 ± 13.4 | 129.8 ± 16.8 3 |
| DBP (mmHg) | 76.4 ± 11.2 | 73.6 ± 13.3 2 | 77.5 ± 11.6 | 79.2 ± 11.4 3 |
| Smoking habit (n, %) | ||||
| Yes | 13 (27.1%) | 14 (31.8%) | ||
| No | 35 (72.9%) | 30 (68.2%) | ||
| Drinking habit (n, %) | ||||
| Yes | 6 (12.5%) | 8 (18.2%) | ||
| No | 42 (87.5%) | 36 (81.8%) | ||
| Cirrhosis (n, %) | ||||
| Yes | 17 (35.4%) | 7 (15.9%) | ||
| No | 31 (64.6%) | 37 (84.1%) | ||
| Hepatitis (n, %) | ||||
| No hepatitis | 3 (6.3%) | 3 (6.8%) | ||
| Hepatitis B | 28 (58.3%) | 30 (68.2%) | ||
| Hepatitis C | 16 (33.3%) | 10 (22.7%) | ||
| Co-hepatitis B and C | 1 (2.1%) | 1 (2.3%) | ||
| Use of nutritional supplement | ||||
| Yes | 10 (20.8%) | 5 (11.4%) | ||
| No | 38 (79.2%) | 39 (88.6%) | ||
| Cancer stage (n, %) | ||||
| Stage I | 27 (56.3%) | 21 (47.7%) | ||
| Stage II | 21 (43.8%) | 23 (52.3%) | ||
| Histological grading (n, %) | ||||
| Well-differentiated | 1 (2.1%) | 0 (0%) | ||
| Moderately differentiated | 21 (43.8%) | 25 (56.8%) | ||
| Poorly differentiated | 26 (54.2%) | 19 (43.2%) | ||
| Tumor number (n, %) | ||||
| Solitary | 39 (81.3%) | 43 (97.7%) | ||
| Multifocal | 9 (18.8%) | 1 (2.3%) | ||
| Tumor size (n, %) | ||||
| ≤5 cm | 34 (70.8%) | 27 (61.4%) | ||
| >5 cm | 14 (29.2%) | 17 (38.6%) | ||
| Lymph-Vascular Invasion (n, %) | ||||
| Absent | 33 (68.8%) | 22 (50%) | ||
| Present | 15 (31.3%) | 22 (50%) | ||
| Parameters | Recurrence (n = 48) | Non-Recurrence (n = 44) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Resection | Post-Resection | p Value 2 | Δ(Post—Pre-Resection) | Pre-Resection | Post-Resection | p Value 2 | Δ(Post—Pre-Resection) | |
| ALT (U/L) | 64.4 ± 8.2 (43.5) | 54.2 ± 7.2 (39.5) | 0.12 | −10.3 ± 8.0 (−3.0) | 76.4 ± 15.6 (37.0) | 44.2 ± 4.7 (35.5) | 0.10 | −33.9 ± 16.5 (−2.0) |
| AST (U/L) | 63.0 ± 8.7 (36.5) | 42.8 ± 5.6 (31.5) | 0.01 | −20.2 ± 9.0 (−5.5) | 77.5 ± 15.4 (37.0) | 37.3 ± 3.5 (30.0) | 0.003 | −42.9 ± 16.4 (−5.5) |
| α-fetoprotein (ng/mL) | 1240.1 ± 941.8 (19.1) | 34.1 ± 10.0 (9.8) | <0.001 | −1205.3 ± 937.4 (−3.1) | 2562.8 ± 1504.8 (31.3) | 119.8 ± 67.0 # (5.7) | <0.001 | −2652.5 ± 1594.0 (−21.8) |
| Total bilirubin (mg/dL) | 0.6 ± 0.04 (0.6) | 1.1 ± 0.3 3 (0.8) | 0.01 | 0.5 ± 0.3 (0.2) | 0.7 ± 0.1 (0.6) | 0.7 ± 0.1 4 (0.6) | 0.58 | 0.02 ±0.03 (0.0) |
| Albumin (g/dL) | 4.1 ± 0.1 (4.1) | 4.1 ± 0.1 3 (4.1) | 0.69 | −0.01 ± 0.8 (0.1) | 4.1 ± 0.1 (4.1) | 4.3 ± 0.04 # (4.3) | 0.004 | 0.2 ± 0.1 † (0.1) |
| hs-CRP (mg/dL) | 0.9 ± 0.3 (0.2) | 0.7 ± 0.1 (0.4) | 0.07 | −0.2 ± 0.3 (0.1) | 0.4 ± 0.1 (0.1) | 0.5 ± 0.2 (0.3) | 0.01 | 0.1 ± 0.2 (0.1) |
| BUN (mg/dL) | 14.3 ± 0.8 (13.0) | 16.1 ± 0.9 (15.0) | 0.01 | 1.7 ± 0.7 (2.0) | 14.6 ± 0.9 (13.5) | 16.1 ± 0.9 (15.0) | 0.49 | 0.6 ± 0.8 (0.5) |
| Parameters | Recurrence (n = 48) | Non-Recurrence (n = 44) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Resection | Post-Resection | p Value 2 | Δ(Post—Pre-Resection) | Pre-Resection | Post-Resection | p Value 2 | Δ(Post—Pre-Resection) | |
| Oxidative stress marker | ||||||||
| MDA (μmol/L) | 0.9 ± 0.04 (0.9) | 0.8 ± 0.5 (0.7) | 0.01 | −0.1 ± 0.1 (−0.2) | 1.0 ± 0.04 (1.0) | 1.0 ± 0.2 (0.8) | 0.01 | 0.0 ± 0.1 (−0.1) |
| GSSG/GSH ratio | 34.6 ± 20.1 (12.3) | 24.8 ± 7.6 (8.8) | 0.20 | −12.5 ± 17.4 (−1.9) | 17.6 ± 4.1 (9.4) | 23.5 ± 13.0 (7.7) | 0.10 | 6.7 ± 12.5 (−1.2) |
| Antioxidant capacities | ||||||||
| GSH (μmol/L) | 54.0 ± 5.2 (47.6) | 67.9 ± 6.8 (62.9) | 0.01 | 14.9 ± 5.5 (12.2) | 64.8 ± 5.9 (60.9) | 83.8 ± 8.3 (77.9) | 0.003 | 17.6 ± 6.1 (13.2) |
| GSSG (μmol/L) | 555.1 ± 15.4 (575.7) | 610.1 ± 16.5 (606.0) | 0.01 | 52.5 ± 17.6 (50.6) | 552.7 ± 15.6 (554.1) | 611.1 ± 18.4 (593.9) | <0.001 | 58.1 ± 12.7 (37.7) |
| GPx (nmol min−1 mL−1) | 130.9 ± 13.4 (132.4) | 152.3 ± 11.2 (160.5) | 0.03 | 22.4 ± 14.5 (15.3) | 144.5 ± 7.4 (149.0) | 157.4 ± 9.4 (175.7) | 0.17 | 16.5 ± 10.2 (15.3) |
| GR (nmol min−1 mL−1) | 54.8 ± 2.4 (54.7) | 69.2 ± 2.7 (67.4) | <0.001 | 14.1 ± 2.7 (9.6) | 64.7 ± 3.2 * (59.6) | 70.4 ± 2.9 (70.8) | 0.047 | 5.7 ± 3.7 (7.8) |
| GST (nmol min−1 mL−1) | 38.7 ± 4.1 (35.8) | 30.0 ± 3.0 (25.8) | 0.03 | −8.9 ± 4.3 (−6.0) | 24.7 ± 3.1 * (15.2) | 22.1 ± 2.8 (18.2) | 0.31 | −2.6 ± 2.7 (−3.0) |
| TEAC (μmol/L) | 4346.4 ± 83.3 (4334.7) | 4592.6 ± 65.7 (4602.6) | 0.01 | 269.2 ± 101.1 (318.0) | 4394. 9 ± 76.0 (4401.3) | 4610.1 ± 69.5 (4642.1) | 0.02 | 215.2 ± 92.0 (219.1) |
| Characteristics | Recurrence (n = 48) | Non-Recurrence (n = 44) | ||
|---|---|---|---|---|
| Adjacent Normal Tissue | HCC Tissue | Adjacent Normal Tissue | HCC Tissue | |
| Oxidative stress marker | ||||
| MDA (μmol/g protein) | 0.7 ± 0.1 (0.7) | 0.5 ± 0.1 (0.3) | 0.6 ± 0.1 (0.4) | 0.7 ± 0.1 (0.5) |
| GSSG/GSH ratio | 14.5 ± 1.8 2 (14.1) | 16.4 ± 3.4 2 (13.8) | 21.9 ± 2.8 3,# (19.8) | 23.9 ± 3.6 3 (18.2) |
| Antioxidant capacities | ||||
| GSH (μmol/g protein) | 20.4 ± 2.4 (21.4) | 30.6 ± 3.9 * (26.5) | 14.5 ± 3.2 # (3.3) | 20.0 ± 3.9 *,# (6.6) |
| GSSG (μmol/g protein) | 33.5 ± 1.5 2 (32.5) | 47.0 ± 4.4 2,* (43.1) | 38.7 ± 2.1 3 (35.7) | 54.0 ± 4.7 3,* (48.4) |
| TEAC (μmol/g protein) | 214.1 ± 9.7 (209.6) | 268.3 ± 16.5 * (262.7) | 202.6 ± 11.6 (189.3) | 247.8 ± 17.0 * (218.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-F.; Cheng, S.-B.; Lai, C.-Y.; Liu, H.-T.; Huang, S.-C.; Huang, Y.-C. The Prognostic Role of Glutathione and Its Related Antioxidant Enzymes in the Recurrence of Hepatocellular Carcinoma. Nutrients 2021, 13, 4071. https://doi.org/10.3390/nu13114071
Hsiao Y-F, Cheng S-B, Lai C-Y, Liu H-T, Huang S-C, Huang Y-C. The Prognostic Role of Glutathione and Its Related Antioxidant Enzymes in the Recurrence of Hepatocellular Carcinoma. Nutrients. 2021; 13(11):4071. https://doi.org/10.3390/nu13114071
Chicago/Turabian StyleHsiao, Yung-Fang, Shao-Bin Cheng, Chia-Yu Lai, Hsiao-Tien Liu, Shih-Chien Huang, and Yi-Chia Huang. 2021. "The Prognostic Role of Glutathione and Its Related Antioxidant Enzymes in the Recurrence of Hepatocellular Carcinoma" Nutrients 13, no. 11: 4071. https://doi.org/10.3390/nu13114071
APA StyleHsiao, Y.-F., Cheng, S.-B., Lai, C.-Y., Liu, H.-T., Huang, S.-C., & Huang, Y.-C. (2021). The Prognostic Role of Glutathione and Its Related Antioxidant Enzymes in the Recurrence of Hepatocellular Carcinoma. Nutrients, 13(11), 4071. https://doi.org/10.3390/nu13114071







