The Association between Carotenoids and Head and Neck Cancer Risk
Abstract
:1. Introduction
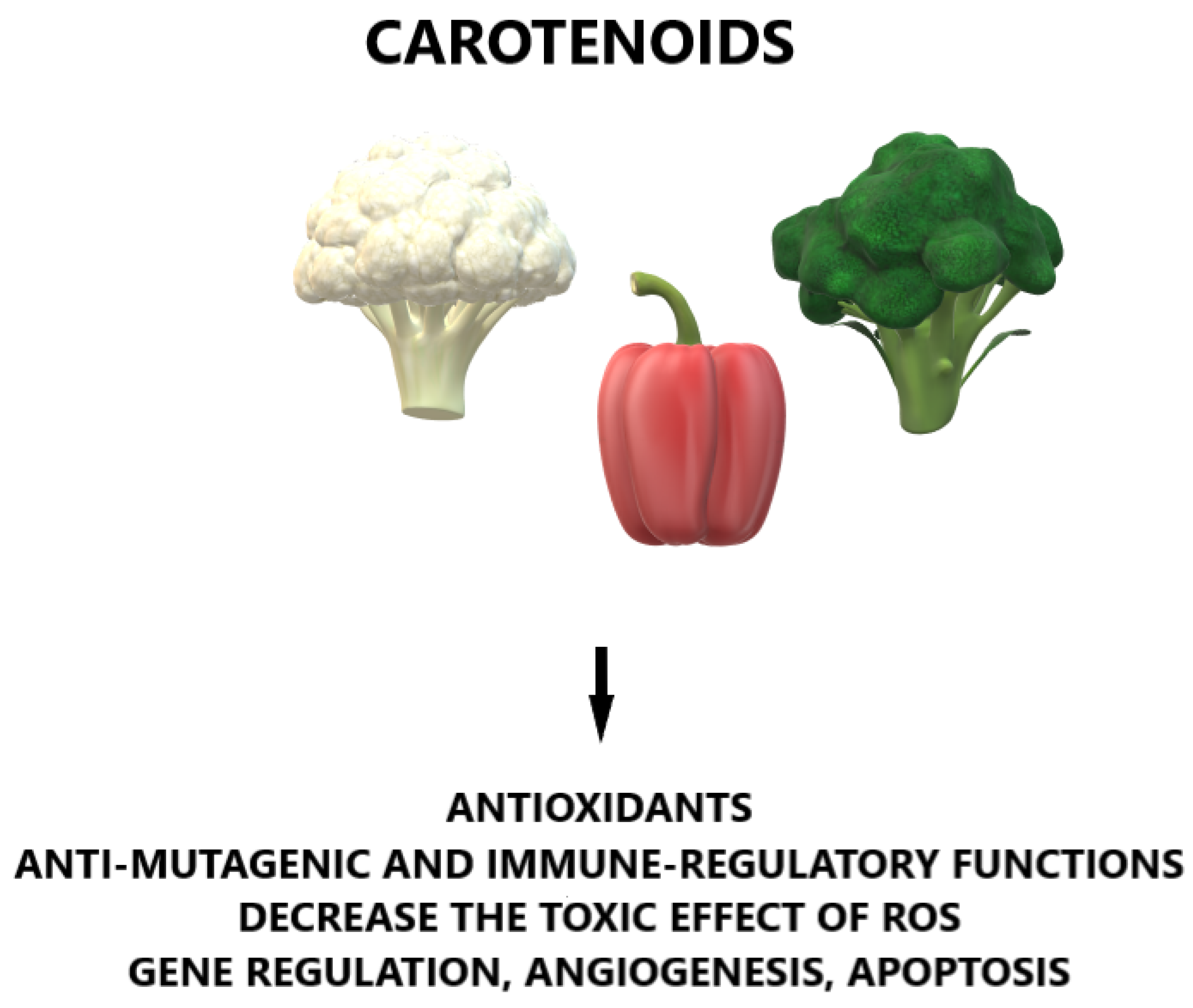
2. Characteristics of Carotenoids
2.1. General Characteristics
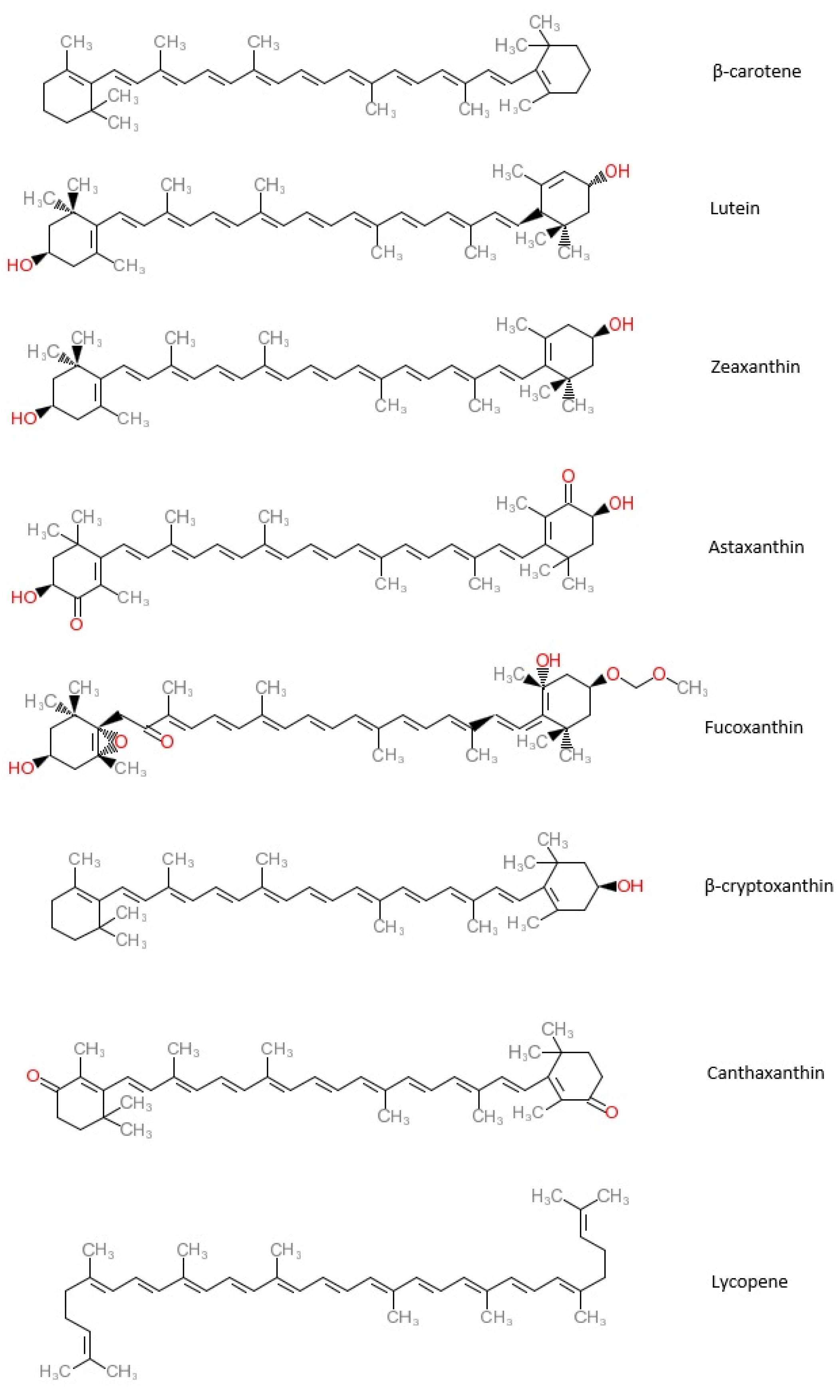
2.2. Common Types of Carotenoids
2.2.1. β-Carotene
2.2.2. Lutein (LUT)
2.2.3. Zeaxanthin (ZX)
2.2.4. Astaxanthin (ATX)
2.2.5. Fucoxanthin (FX)
2.2.6. β-Cryptoxanthin (BCX)
2.2.7. Canthaxanthin (CX)
2.2.8. Lycopene
3. Methods of the Literature Search
4. Literature Review
4.1. Studies Presenting the Relationship between Carotenoids and HNC Risk
4.1.1. Systematic Reviews and Meta-Analyses
4.1.2. Studies Investigating the Relationship between the Dietary Consumption of Carotenoids and HNC Risk
Prospective Cohort Studies
Case-Control Studies
4.1.3. Studies Investigating Relationships between the Consumption and Serum Concentrations of Specific Carotenoids and HNC Risk
Prospective Cohort Studies
Case-Control Cohort Studies
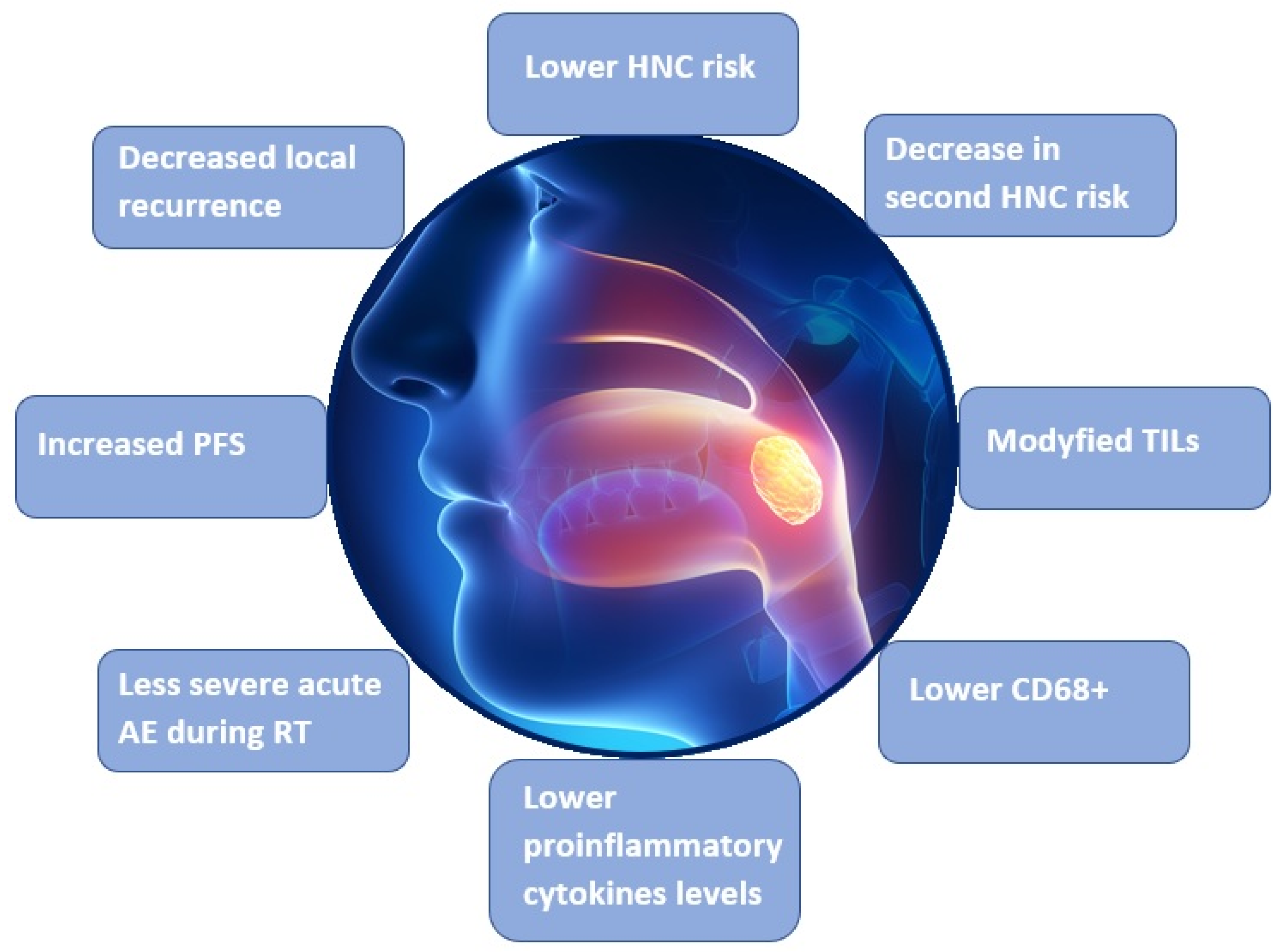
4.2. Interventional Studies on the Association between Carotenoids and HNC
4.3. Studies on the Relationship between Carotenoids and Survival in HNC Patients
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Leoncini, E.; Edefonti, V.; Hashibe, M.; Parpinel, M.; Cadoni, G.; Ferraroni, M.; Serraino, D.; Matsuo, K.; Olshan, A.F.; Zevallos, J.P.; et al. Carotenoid intake and head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Eur. J. Epidemiol. 2016, 31, 369–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC. List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans. Volumes 1–109. Available online: http://monographs.iarc.fr/ENG/Classification/index.php (accessed on 8 December 2021).
- Brewczyński, A.; Jabłońska, B.; Mazurek, A.; Mrochem-Kwarciak, J.; Mrowiec, S.; Śnietura, M.; Kentnowski, M.; Kołosza, Z.; Składowski, K.; Rutkowski, T. Comparison of Selected Immune and Hematological Parameters and Their Impact on Survival in Patients with HPV-Related and HPV-Unrelated Oropharyngeal Cancer. Cancers 2021, 13, 3256. [Google Scholar] [CrossRef]
- World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund International: Washington, DC, USA, 2007. [Google Scholar]
- Chew, B.P.; Park, J.S. Carotenoid Action on the Immune Response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Wakai, K.; Matsuo, K.; Hirose, K.; Ito, H.; Kuriki, K.; Sato, S.; Ueda, R.; Hasegawa, Y.; Tajima, K. Effect of dietary antioxidants and risk of oral, pharyngeal and laryngeal squamous cell carcinoma according to smoking and drinking habits. Cancer Sci. 2006, 97, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.P.; Krishna, T.P.; Pasricha, S.; Quereshi, M.A.; Krishnaswamy, K. Diet and oral cancer—A case control study. Asia Pac. J. Clin. Nutr. 1995, 4. [Google Scholar]
- Zheng, W.; Blot, W.J.; Shu, X.O.; Diamond, E.L.; Gao, Y.T.; Ji, B.T.; Fraumeni, J.F. Risk factors for oral and pharyngeal cancer in Shanghai, with emphasis on diet. Cancer Epidemiol. Biomark. Prev. 1992, 1, 441–448. [Google Scholar]
- Zheng, T.; Boyle, P.; Willett, W.C.; Hu, H.; Dan, J.; Evstifeeva, T.V.; Niu, S.; MacMahon, B. A case-control study of oral cancer in Beijing, People’s Republic of China. Associations with nutrient intakes, foods and food groups. Oral Oncol. 1993, 29, 45–55. [Google Scholar] [CrossRef]
- Marshall, J.R.; Graham, S.; Haughey, B.P.; Shedd, D.; O’Shea, R.; Brasure, J.; Wilkinson, G.S.; West, D. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Oral Oncol. 1992, 28, 9–15. [Google Scholar] [CrossRef]
- Negri, E.; Franceschi, S.; Bosetti, C.; Levi, F.; Conti, E.; Parpinel, M.; La Vecchia, C. Selected micronutrients and oral and pharyngeal cancer. Int. J. Cancer 2000, 86, 122–127. [Google Scholar] [CrossRef]
- Day, G.L.; Blot, W.J.; Austin, D.F.; Bernstein, L.; Greenberg, R.S.; Preston-Martin, S.; Schoenberg, J.B.; Winn, D.M.; McLaughlin, J.K.; Fraumeni, J.F. Racial Differences in Risk of Oral and Pharyngeal Cancer: Alcohol, Tobacco, and Other Determinants. J. Natl. Cancer Inst. 1993, 85, 465–473. [Google Scholar] [CrossRef]
- Gridley, G.; McLaughlin, J.K.; Block, G.; Blot, W.J.; Winn, D.M.; Greenberg, R.S.; Schoenberg, J.B.; Preston-Martin, S.; Austin, N.F.; Fraumeni, J.F. Diet and oral and pharyngeal cancer among blacks. Nutr. Cancer 1990, 14, 219–225. [Google Scholar] [CrossRef]
- McLaughlin, J.K.; Gridley, G.; Block, G.; Winn, D.M.; Preston-Martin, S.; Schoenberg, J.B.; Greenberg, R.S.; Stemhagen, A.; Austin, D.F.; Ershow, A.G.; et al. Dietary Factors in Oral and Pharyngeal Cancer. J. Natl. Cancer Inst. 1988, 80, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Rossing, M.A.; Vaughan, T.L.; McKnight, B. Diet and pharyngeal cancer. Int. J. Cancer 1989, 44, 593–597. [Google Scholar] [CrossRef]
- De Stefani, E.; Oreggia, F.; Boffetta, P.; Deneo-Pellegrini, H.; Ronco, A.; Mendilaharsu, M. Tomatoes, tomato-rich foods, lycopene and cancer of the upper aerodigestive tract: A case-control in Uruguay. Oral Oncol. 2000, 36, 47–53. [Google Scholar] [CrossRef]
- Bidoli, E.; Bosetti, C.; La Vecchia, C.; Levi, F.; Parpinel, M.; Talamini, R.; Negri, E.; Maso, L.D.; Franceschi, S. Micronutrients and laryngeal cancer risk in Italy and Switzerland: A case–control study. Cancer Causes Control. 2003, 14, 477–484. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract—II. Nutrients. Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef]
- Schantz, S.P.; Zhang, Z.-F.; Spitz, M.S.; Sun, M.; Hsu, T.C. Genetic susceptibility to head and neck cancer: Interaction between nutrition and mutagen sensitivity. Laryngoscope 1997, 107, 765–781. [Google Scholar] [CrossRef]
- Petridou, E.; Zavras, A.I.; Lefatzis, D.; Dessypris, N.; Laskaris, G.; Dokianakis, G.; Segas, J.; Douglas, C.W.; Diehl, S.R.; Trichopoulos, D. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer 2002, 94, 2981–2988. [Google Scholar] [CrossRef]
- Kune, G.A.; Kune, S.; Field, B.; Watson, L.F.; Cleland, H.; Merenstein, D.; Vitetta, L. Oral and pharyngeal cancer, diet, smoking, alcohol, and serum vitamin a and β-carotene levels: A case-control study in men. Nutr. Cancer 1993, 20, 61–70. [Google Scholar] [CrossRef]
- Gallus, S.; Bosetti, C.; Franceschi, S.; Levi, F.; Negri, E.; La Vecchia, C. Laryngeal cancer in women: Tobacco, alcohol, nutritional, and hormonal factors. Cancer Epidemiol. Biomark. Prev. 2003, 12, 514–517. [Google Scholar]
- Riboli, E.; Terracini, B.; Merletti, F.; Crosignani, P.; Ascunce, N.; Zubiri, L.; Blanchet, F.; Raymond, L.; Repetto, F.; Tuyns, A.J. Diet and cancers of the larynx and hypopharynx: The IARC multi-center study in southwestern Europe. Cancer Causes Control. 1996, 7, 240–252. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. (Eds.) Carotenoids: Handbook; Verlag Birkhäuser: Basel, Switzerland; Berlin, Germany; Boston, MA, USA, 2004. [Google Scholar]
- Khachik, F. Distribution and metabolism of dietary carotenoids in humans as a criterion for development of nutritional supplements. Pure Appl. Chem. 2006, 78, 1551–1557. [Google Scholar] [CrossRef]
- Paiva, S.A.; Russell, R.M. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Mackerras, D.; Buffler, P.A.; Randall, D.E.; Nichaman, M.Z.; Pickle, L.W.; Mason, T.J. Carotene intake and the risk of laryngeal cancer in coastal texas. Am. J. Epidemiol. 1988, 128, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Bidoli, E.; Barón, A.E.; Barra, S.; Talamini, R.; Serraino, D.; La Vecchia, C. Nutrition and cancer of the oral cavity and pharynx in north-east italy. Int. J. Cancer 1991, 47, 20–25. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E.; D’Avanzo, B.; Boyle, P.; Franceschi, S. Dietary Indicators of Oral and Pharyngeal Cancer. Int. J. Epidemiol. 1991, 20, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, S.; Favero, A.; Conti, E.; Talamini, R.; Volpe, R.; Negri, E.; Barzan, L.; La Vecchia, C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br. J. Cancer 1999, 80, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Freudenheim, J.L.; Graham, S.; Byers, T.E.; Marshall, J.R.; Haughey, B.P.; Swanson, M.K.; Wilkinson, G. Diet, smoking, and alcohol in cancer of the larynx: A case-control study. Nutr. Cancer 1992, 17, 33–45. [Google Scholar] [CrossRef]
- Djuric; Ronis; Fowler; Ren; Duffy Levels of Fat-Soluble Micronutrients and 2,6-Cyclolycopene-1,5-Diol in Head and Neck Cancer Patients. Int. J. Vitam. Nutr. Res. 2007, 77, 382–388. [CrossRef]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int. J. Cancer 2007, 122, 2330–2336. [Google Scholar] [CrossRef]
- de Munter, L.; Maasland, D.H.E.; Brandt, P.V.D.; Kremer, B.; Schouten, L.J. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2015, 102, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Maasland, D.H.; Brandt, P.A.V.D.; Kremer, B.; Goldbohm, R.A.; Schouten, L.J. Consumption of vegetables and fruits and risk of subtypes of head-neck cancer in the Netherlands Cohort Study. Int. J. Cancer 2015, 136, E396–E409. [Google Scholar] [CrossRef]
- Arthur, A.E.; Peterson, K.E.; Shen, J.; Djuric, Z.; Taylor, J.M.G.; Hebert, J.R.; Duffy, S.A.; Peterson, L.A.; Bellile, E.L.; Whitfield, J.R.; et al. Diet and proinflammatory cytokine levels in head and neck squamous cell carcinoma. Cancer 2014, 120, 2704–2712. [Google Scholar] [CrossRef] [Green Version]
- Argirion, I.; Arthur, A.E.; Zarins, K.R.; Bellile, E.; Crowder, S.L.; Amlani, L.; Taylor, J.M.; Wolf, G.T.; McHugh, J.; Nguyen, A.; et al. Pretreatment Dietary Patterns, Serum Carotenoids and Tocopherols Influence Tumor Immune Response in Head and Neck Squamous Cell Carcinoma. Nutr. Cancer 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Polesel, J.; Negri, E.; Serraino, D.; Parpinel, M.; Barzan, L.; Libra, M.; Bosetti, C.; Garavello, W.; Montella, M.; La Vecchia, C.; et al. Dietary intakes of carotenoids and other nutrients in the risk of nasopharyngeal carcinoma: A case–control study in Italy. Br. J. Cancer 2012, 107, 1580–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayne, S.T.; Cartmel, B.; Baum, M.; Shor-Posner, G.; Fallon, B.G.; Briskin, K.; Bean, J.; Zheng, T.; Cooper, D.; Friedman, C.; et al. Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. 2001, 61, 11245451. [Google Scholar]
- Mayne, S.T.; Cartmel, B.; Lin, H.; Zheng, T.; Goodwin, W.J., Jr. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J. Am. Coll. Nutr. 2004, 23, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Mercier, J.-P.; Têtu, B.; Harel, F.; Abdous, B.; et al. Randomized Trial of Antioxidant Vitamins to Prevent Acute Adverse Effects of Radiation Therapy in Head and Neck Cancer Patients. J. Clin. Oncol. 2005, 23, 5805–5813. [Google Scholar] [CrossRef]
- Meyer, F.; Bairati, I.; Jobin, E.; Gélinas, M.; Fortin, A.; Nabid, A.; Têtu, B. Acute Adverse Effects of Radiation Therapy and Local Recurrence in Relation to Dietary and Plasma Beta Carotene and Alpha Tocopherol in Head and Neck Cancer Patients. Nutr. Cancer 2007, 59, 29–35. [Google Scholar] [CrossRef]
- Sakhi, A.K.; Russnes, K.M.; Thoresen, M.; Bastani, N.E.; Karlsen, A.; Smeland, S.; Blomhoff, R. Pre-radiotherapy plasma carotenoids and markers of oxidative stress are associated with survival in head and neck squamous cell carcinoma patients: A prospective study. BMC Cancer 2009, 9, 458–511. [Google Scholar] [CrossRef] [Green Version]
- Sakhi, A.K.; Bøhn, S.K.; Smeland, S.; Thoresen, M.; Smedshaug, G.B.; Tausjø, J.; Svilaas, A.; Karlsen, A.; Russnes, K.M.; Svilaas, T.; et al. Postradiotherapy Plasma Lutein, α-Carotene, and β-Carotene Are Positively Associated With Survival in Patients With Head and Neck Squamous Cell Carcinoma. Nutr. Cancer 2010, 62, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Prod. Bioprospecting 2020, 10, 109–117. [Google Scholar] [CrossRef]
- Allore, T.; Lemieux, S.; Vohl, M.-C.; Couture, P.; Lamarche, B.; Couillard, C. Correlates of the difference in plasma carotenoid concentrations between men and women. Br. J. Nutr. 2018, 121, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Couillard, C.; Lemieux, S.; Vohl, M.-C.; Couture, P.; Lamarche, B. Carotenoids as biomarkers of fruit and vegetable intake in men and women. Br. J. Nutr. 2016, 116, 1206–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, K.; Idris, A.; Yusof, N.M. Novel protocol optimized for microalgae lutein used as food additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules 2021, 26, 5333. [Google Scholar] [CrossRef] [PubMed]
- Belter, A.; Giel-Pietraszuk, M.; Oziewicz, S.; Chomczyński, P.; Barciszewski, J. Likopen—Wystepowanie, właściwości oraz potencjalne zastosowanie [Lycopene—Occurrence, properties and applications]. Postepy Biochem. 2011, 57, 372–380. (In Polish) [Google Scholar] [PubMed]
| Authors | Year of Publication | Type of Study (Number of Patients) | Types of Carotenoids | Results/Conclusions |
|---|---|---|---|---|
| Systematic reviews and meta-analyses | ||||
| Leoncini et al. [1] | 2015 | Meta-analysis of 16 articles | Total carotenoids α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, | Reduction of HNC risk by carotenoids |
| Leoncini et al. [2] | 2015 | Pooled analysis of 10 articles (18,207 patients) | Total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin | Reduction of HNC risk by carotenoids |
| Studies investigating the relationship between dietary carotenoid consumption and HNC risk | ||||
| Prospective cohort studies | ||||
| Freedman et al. [35] | 2008 | Prospective cohort study (490,802 patients) | Total carotenoids | Significant inverse relationship between cancer risk and carotenoid intake |
| de Munter et al. [36] | 2015 | Prospective cohort study (450 HNC patients) | α-carotene, β-carotene, lutein, zeaxanthin, lycopene, β-cryptoxanthin | No significant relationships between carotenoids and HNC risk. |
| Maasland et al. [37] | 2015 | Prospective cohort study (120,852 patients) | Total carotenoids | Significant inverse relationship between total vegetable and fruit intake and overall HNC risk |
| Case-control studies | ||||
| Mackerras et al. [29] | 1988 | Case-control study (151 patients, 198 controls) | Carotene | Significant inverse relationship between carotene intake and LC risk |
| Franceschi et al. [30] | 1991 | Case-control study (302 patients, 699 controls) | Total carotenoids | Significant inverse relationship between carotenoid intake and OCC and PC risk |
| La Vecchia et al. [31] | 1991 | Case-control study (105 patients, 1169 controls) | Total carotenoids | Significant inverse relationship between carotenoid intake and OCC and OPC risk |
| Freudenheim et al. [33] | 1991 | Case-control study (250 patients, 250 controls) | Total carotenoids | Significant inverse relationship between carotenoid intake and LC risk |
| Arthur et al. [38] | 2014 | Cross-sectional study (160 patients) | Total carotenoids | Significant inverse associations between IL-6, TNF-α, and IFN-γ levels and quartiles of total reported carotenoid intake in HNC |
| Studies investigating relationships between the intake and serum concentrations of specific carotenoids and HNC risk | ||||
| Prospective cohort studies | ||||
| Djuric et al. [34] | 2007 | Prospective cohort study (120 patients) | Lycopene | No impact of smoking on lycopene oxidation |
| Argirion et al. [39] | 2020 | Prospective cohort study (116 patients) | Total carotenoids, xanthophylls lycopene | Significant inverse association between total carotenoids, xanthophylls, lycopene, and CD68 in HNC |
| Case-control studies | ||||
| Schantz et al. [21] | 1997 | Case-control study (167 patients, 177 controls) | α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin | Significant protective association between β-cryptoxanthin intake and HNC risk |
| Negri et al. [12] | 2000 | Case-control study (754 patients, 1775 controls) | Carotene, lycopene | Significant protective impact of carotenoids on OCC and OPC risk |
| De Stefani et al. [17] | 2000 | Case-control study (230 patients, 491 controls) | Lycopene | Significant inverse relationship between lycopene and HNC risk |
| Bidoli et al. [18] | 2003 | Case-control study (230 patients, 491 controls) | α-carotene, β-carotene, lutein, zeaxanthin | Significant inverse relationship between carotenoid intake and LC risk |
| Gallus et al. [24] | 2003 | Case-control study (68 patients, 340 controls) | Carotene | Nonsignificant inverse association between carotene intake and LC risk |
| Polesel et al. [40] | 2012 | Case-control study (198 patients, 594 controls) | Total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene | Significant inverse relationship between total carotenoids, α-carotene, β-carotene, and NPC risk |
| Bravi et al. [20] | 2013 | Case-control study (768 patients, 2078 controls) | α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin | Significant inverse relationship between carotenoids and OC and PC risk |
| Interventional studies on the relationship between carotenoids and HNC | ||||
| Mayne et al. [41] | 2001 | Randomized, placebo-controlled, double-blinded clinical trial (264 patients) | β-carotene | Possible decrease in second HNC risk in supplementation |
| Mayne et al. [42] | 2004 | Randomized, placebo-controlled, double-blinded clinical trial (259 patients) | Total carotenoids α-carotene, β-carotene, lycopene, lutein, zeaxanthin | Inverse association between lycopene level and mortality, inverse association between lycopene, α-carotene, total carotenoids, and mortality in non-smokers |
| Bairati et al. [43] | 2005 | Randomized, placebo-controlled, double-blinded clinical trial (540 patients) | β-carotene | Reduction of adverse RT effects and possible reduction RT efficacy |
| Studies on the association between carotenoids and survival rates in HNC patients | ||||
| Prospective cohort studies | ||||
| Meyer et al. [44] | 2007 | Prospective cohort study (540 patients) | β-carotene | Reduction of adverse RT effects and better progression-free survival in patients with higher carotenoid levels |
| Case-control studies | ||||
| Sakhi et al. [45] | 2009 | Case-control study (78 HNC patients, 100 controls) | Total carotenoids, α-carotene, β-carotene, lutein, zeaxanthin, lycopene | Better progression-free survival in patients with higher carotenoid levels |
| Sakhi et al. [46] | 2010 | Case-control study (29 HNC patients, 51 controls) | Total carotenoids, α-carotene, β-carotene, lutein, zeaxanthin, lycopene | Possible better survival in patients with increasing levels of carotenoids before RT and increasing oxidative stress during RT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewczyński, A.; Jabłońska, B.; Kentnowski, M.; Mrowiec, S.; Składowski, K.; Rutkowski, T. The Association between Carotenoids and Head and Neck Cancer Risk. Nutrients 2022, 14, 88. https://doi.org/10.3390/nu14010088
Brewczyński A, Jabłońska B, Kentnowski M, Mrowiec S, Składowski K, Rutkowski T. The Association between Carotenoids and Head and Neck Cancer Risk. Nutrients. 2022; 14(1):88. https://doi.org/10.3390/nu14010088
Chicago/Turabian StyleBrewczyński, Adam, Beata Jabłońska, Marek Kentnowski, Sławomir Mrowiec, Krzysztof Składowski, and Tomasz Rutkowski. 2022. "The Association between Carotenoids and Head and Neck Cancer Risk" Nutrients 14, no. 1: 88. https://doi.org/10.3390/nu14010088
APA StyleBrewczyński, A., Jabłońska, B., Kentnowski, M., Mrowiec, S., Składowski, K., & Rutkowski, T. (2022). The Association between Carotenoids and Head and Neck Cancer Risk. Nutrients, 14(1), 88. https://doi.org/10.3390/nu14010088






