Adherence to a Mediterranean-Style Dietary Pattern and Cancer Risk in a Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessment and Adherence to the MSDP
2.3. Cancer Outcomes
2.4. Potential Confounders and Effect Modifiers
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorectal Cancer. Available online: Dietandcancerreport.Org (accessed on 1 October 2019).
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean Diet and Cancer: Epidemiological Evidence and Mechanism of Selected Aspects. BMC Surg. 2013, 13, S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontou, N.; Psaltopoulou, T.; Panagiotakos, D.; Dimopoulos, M.A.; Linos, A. The Mediterranean Diet in Cancer Prevention: A Review. J. Med. Food 2011, 14, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Chao, X.; Su, T.; Fu, X.; Tse, A.K.W.; Fong, W.F.; Yu, Z.-L. The Anticancer and Antiobesity Effects of Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2017, 57, 82–94. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; De Pergola, G.; Silvestris, F. Mediterranean Diet and Cancer Risk: An Open Issue. Int. J. Food Sci. Nutr. 2016, 67, 593–605. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean Diet on Metabolic Syndrome, Cancer and Longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Rosato, V.; Guercio, V.; Bosetti, C.; Negri, E.; Serraino, D.; Giacosa, A.; Montella, M.; La Vecchia, C.; Tavani, A. Mediterranean Diet and Colorectal Cancer Risk: A Pooled Analysis of Three Italian Case–Control Studies. Br. J. Cancer 2016, 115, 862–865. [Google Scholar] [CrossRef] [Green Version]
- van den Brandt, P.A.; Schulpen, M. Mediterranean Diet Adherence and Risk of Post-menopausal Breast Cancer: Results of a Cohort Study and Meta-Analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef]
- Buckland, G.; Travier, N.; Cottet, V.; González, C.A.; Luján-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the Mediterranean Diet and Risk of Breast Cancer in the European Prospective Investigation into Cancer and Nutrition Cohort Study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef]
- Jones, P.; Cade, J.E.; Evans, C.E.; Hancock, N.; Greenwood, D.C. The Mediterranean Diet and Risk of Colorectal Cancer in the UK Women’s Cohort Study. Int. J. Epidemiol. 2017, 46, 1786–1796. [Google Scholar] [CrossRef] [Green Version]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian Mediterranean Index and Risk of Colorectal Cancer in the Italian Section of the EPIC Cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, G.; Biondi, F.; et al. Inflammaging and Cancer: A Challenge for the Mediterranean Diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetou, V.; Trichopoulou, A.; Orfanos, P.; Naska, A.; Lagiou, P.; Boffetta, P.; Trichopoulos, D. Conformity to Traditional Mediterranean Diet and Cancer Incidence: The Greek EPIC Cohort. Br. J. Cancer 2008, 99, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjønneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean Dietary Pattern and Cancer Risk in the EPIC Cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean Diet and Survival: EPIC-Elderly Prospective Cohort Study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef] [Green Version]
- Rumawas, M.E.; Dwyer, J.T.; Mckeown, N.M.; Meigs, J.B.; Rogers, G.; Jacques, P.F. The Development of the Mediterranean-Style Dietary Pattern Score and Its Application to the American Diet in the Framingham Offspring Cohort. J. Nutr. 2009, 139, 1150–1156. [Google Scholar] [CrossRef]
- Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J.; Colditz, G.A.; Litin, L.B.; Willett, W.C. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. Am. J. Epidemiol. 1992, 135, 1114–1126. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare Supreme Scientific Health Council of Greece. Dietary Guidelines for Adults in Greece. Arch. Hell. Med. 1999, 16, 516–524. [Google Scholar]
- Kreger, B.E.; Splansky, G.L.; Schatzkin, A. The Cancer Experience in the Framingham Heart Study Cohort. Cancer 1991, 67, 1–6. [Google Scholar] [CrossRef]
- Moore, L.L.; Bradlee, M.L.; Singer, M.R.; Splansky, G.L.; Proctor, M.H.; Ellison, R.C.; Kreger, B.E. BMI and Waist Circumference as Predictors of Lifetime Colon Cancer Risk in Framingham Study Adults. Int. J. Obes. 2004, 28, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.L.; Chadid, S.; Singer, M.R.; Kreger, B.E.; Denis, G.V. Metabolic Health Reduces Risk of Obesity-Related Cancer in Framingham Study Adults. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2057–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadid, S.; Singer, M.R.; Kreger, B.E.; Bradlee, M.L.; Moore, L.L. Midlife Weight Gain Is a Risk Factor for Obesity-Related Cancer. Br. J. Cancer 2018, 118, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, J.; Ellison, R.C.; Singer, M.R.; Bradlee, M.L.; Kalesan, B.; Holick, M.F.; Moore, L.L. Dietary Protein and Preservation of Physical Functioning Among Middle-Aged and Older Adults in the Framingham Offspring Study. Am. J. Epidemiol. 2018, 187, 1411–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannel, W.B.; Belanger, A.; D’Agostino, R.; Israel, I. Physical Activity and Physical Demand on the Job and Risk of Cardiovascular Disease and Death: The Framingham Study. Am. Heart J. 1986, 112, 820–825. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S.; Walker, A.M. Concepts of Interaction. Am. J. Epidemiol. 1980, 112, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.J.; VanderWeele, T.J.; Groenwold, R.H.H.; Klungel, O.H.; Rovers, M.M.; Grobbee, D.E. Estimating Measures of Interaction on an Additive Scale for Preventive Exposures. Eur. J. Epidemiol. 2011, 26, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chambless, L. Test for Additive Interaction in Proportional Hazards Models. Ann. Epidemiol. 2007, 17, 227–236. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Gatenby, P.; Gerbi, V.; Janssens, J.; Johnston, B.; Kas, K.; La Vecchia, C.; Mainguet, P.; et al. Cancer Prevention in Europe: The Mediterranean Diet as a Protective Choice. Eur. J. Cancer Prev. 2013, 22, 90. [Google Scholar] [CrossRef]
- Zheng, Y.; Manson, J.E.; Yuan, C.; Liang, M.H.; Grodstein, F.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Associations of Weight Gain from Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA 2017, 318, 255–269. [Google Scholar] [CrossRef]
- Arnold, M.; Jiang, L.; Stefanick, M.L.; Johnson, K.C.; Lane, D.S.; LeBlanc, E.S.; Prentice, R.; Rohan, T.E.; Snively, B.M.; Vitolins, M.; et al. Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States. PLOS Med. 2016, 13, e1002081. [Google Scholar] [CrossRef]
- Bosetti, C.; Turati, F.; Pont, A.D.; Ferraroni, M.; Polesel, J.; Negri, E.; Serraino, D.; Talamini, R.; Vecchia, C.L.; Zeegers, M.P. The Role of Mediterranean Diet on the Risk of Pancreatic Cancer. Br. J. Cancer 2013, 109, 1360–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filomeno, M.; Bosetti, C.; Bidoli, E.; Levi, F.; Serraino, D.; Montella, M.; La Vecchia, C.; Tavani, A. Mediterranean Diet and Risk of Endometrial Cancer: A Pooled Analysis of Three Italian Case-Control Studies. Br. J. Cancer 2015, 112, 1816–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtaugh, M.A.; Sweeney, C.; Giuliano, A.R.; Herrick, J.S.; Hines, L.; Byers, T.; Baumgartner, K.B.; Slattery, M.L. Diet Patterns and Breast Cancer Risk in Hispanic and Non-Hispanic White Women: The Four-Corners Breast Cancer Study. Am. J. Clin. Nutr. 2008, 87, 978–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- Demetriou, C.A.; Hadjisavvas, A.; Loizidou, M.A.; Loucaides, G.; Neophytou, I.; Sieri, S.; Kakouri, E.; Middleton, N.; Vineis, P.; Kyriacou, K. The Mediterranean Dietary Pattern and Breast Cancer Risk in Greek-Cypriot Women: A Case-Control Study. BMC Cancer 2012, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr. Nutr. Rep. 2016, 5, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central Obesity and the Mediterranean Diet: A Systematic Review of Intervention Trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-Analysis Comparing Mediterranean to Low-Fat Diets for Modification of Cardiovascular Risk Factors. Am. J. Med. 2011, 124, 841–851.e2. [Google Scholar] [CrossRef]
- Rumawas, M.E.; Meigs, J.B.; Dwyer, J.T.; McKeown, N.M.; Jacques, P.F. Mediterranean-Style Dietary Pattern, Reduced Risk of Metabolic Syndrome Traits, and Incidence in the Framingham Offspring Cohort123. Am. J. Clin. Nutr. 2009, 90, 1608–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feskanich, D.; Rimm, E.B.; Giovannucci, E.L.; Colditz, G.A.; Stampfer, M.J.; Litin, L.B.; Willett, W.C. Reproducibility and Validity of Food Intake Measurements from a Semiquantitative Food Frequency Questionnaire. J. Am. Diet Assoc. 1993, 93, 790–796. [Google Scholar] [CrossRef]
- Barak, Y.; Fridman, D. Impact of Mediterranean Diet on Cancer: Focused Literature Review. Cancer Genom. Proteom. 2017, 14, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Weir, H.K.; Thompson, T.D.; Soman, A.; Møller, B.; Leadbetter, S.; White, M.C. Meeting the Healthy People 2020 Objectives to Reduce Cancer Mortality, Peer Reviewed: Meeting the Healthy People 2020 Objectives to Reduce Cancer Mortality. Prev. Chronic Dis. 2015, 12, E104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
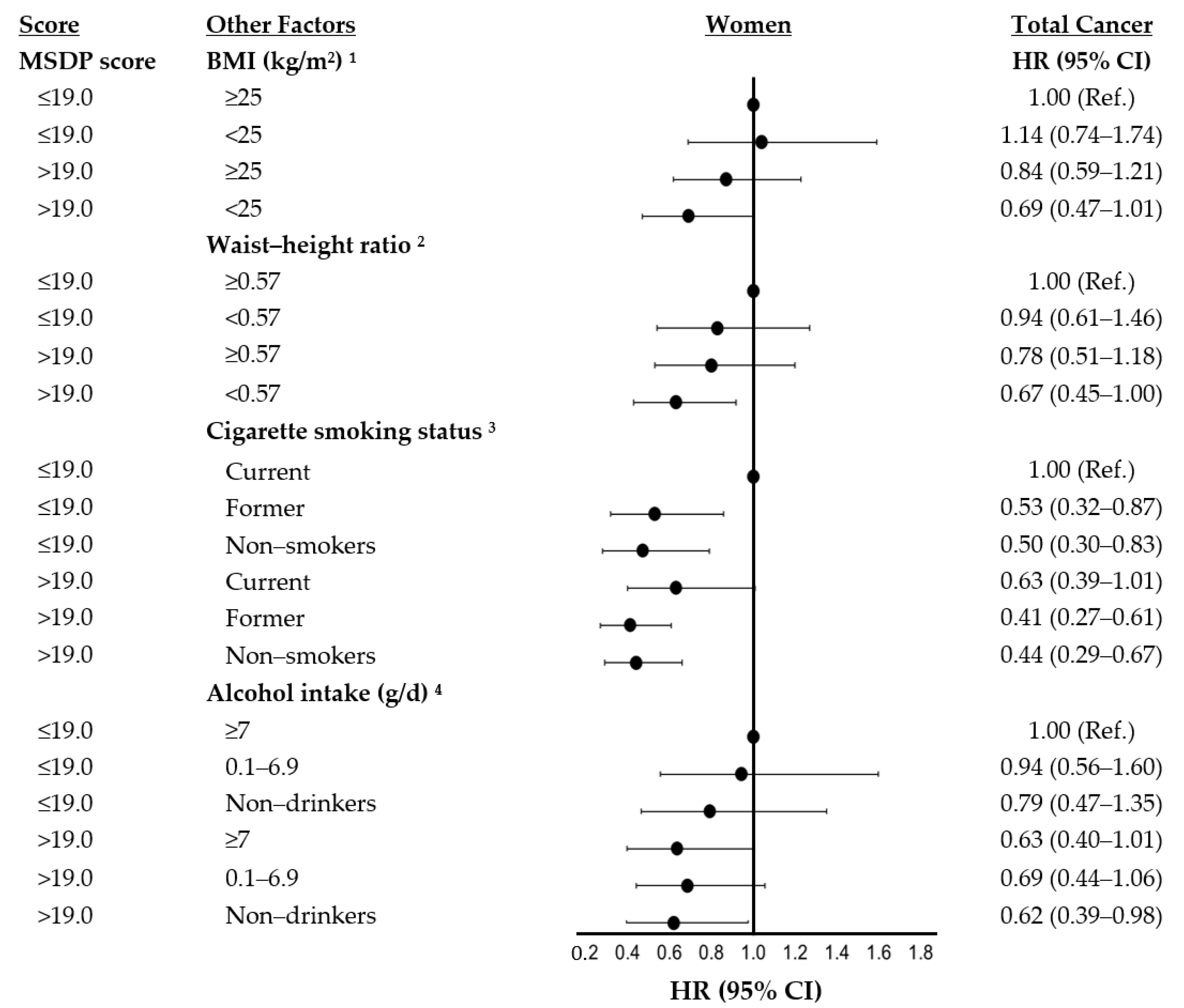
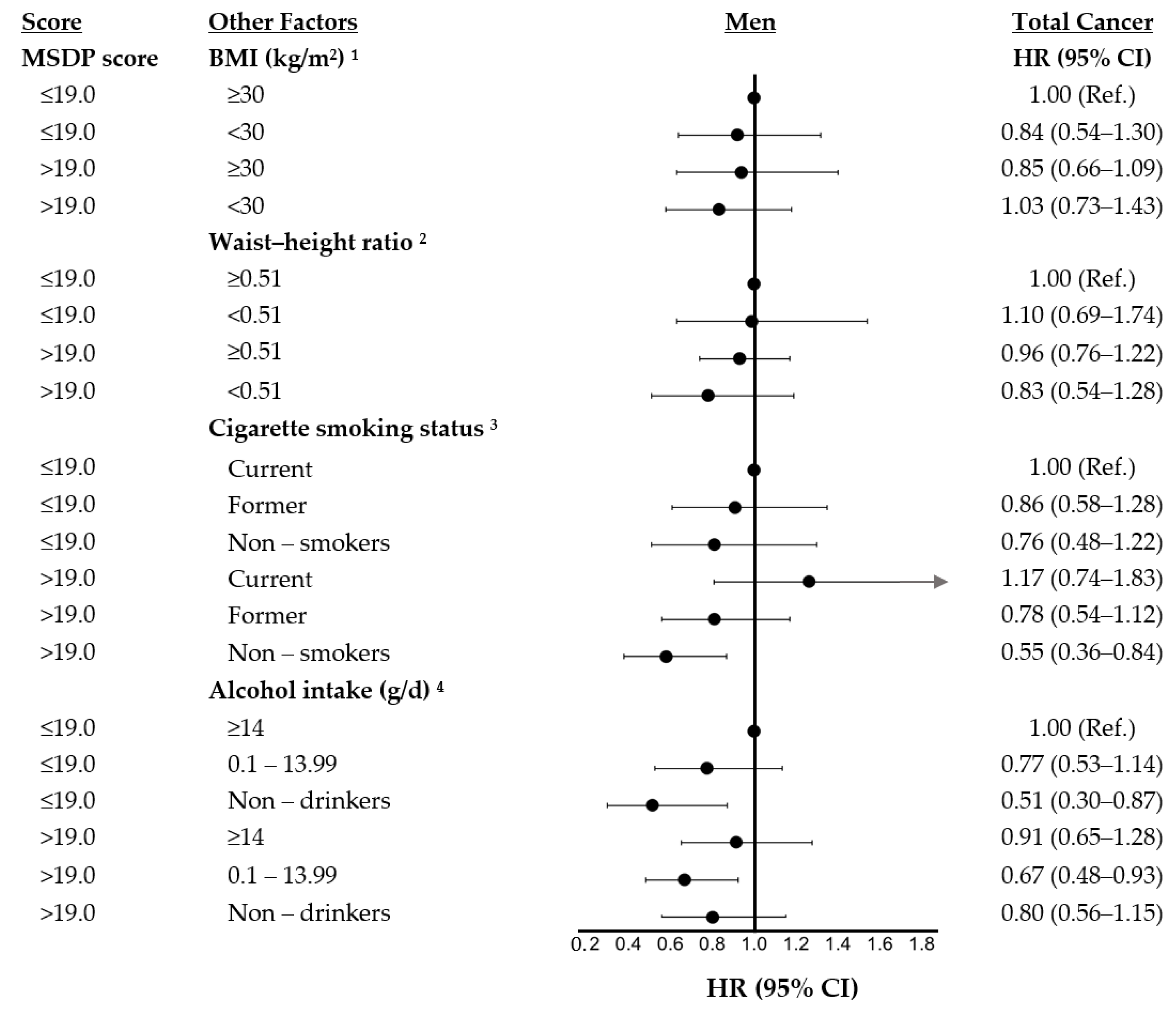
| MSDP Score Categories | |||
| Low | Moderate | High | |
| (4.0–19.0) | (19.1–25.0) | (25.1–50.9) | |
| Characteristic (n = 2966) | n = 995 | n = 951 | n = 1020 |
| Sex, n (%) | |||
| Women | 443 (45) | 505 (53) | 630 (62) |
| Men | 592 (56) | 446 (47) | 390 (38) |
| Age (years) | 52.8 (± 9.5) | 55.0 (± 9.9) | 55.2 (± 9.3) |
| Age at diagnosis (years) | 70.3 (± 8.9) | 72.5 (± 9.4) | 73.0 (± 8.9) |
| Education, n (%) | |||
| ≤High School | 445 (44.7) | 351 (36.9) | 326 (32.0) |
| Some college | 265 (26.6) | 283 (29.8) | 316 (31.0) |
| College, Graduate degree | 285 (28.6) | 317 (33.3) | 378 (37.1) |
| BMI (kg/m2) | 27.4 (± 5.0) | 27.4 (± 4.8) | 27.1 (± 5.0) |
| Waist (cm) | 93.8 (± 14.2) | 93.0 (± 14.1) | 91.1 (± 14.3) |
| Waist-to-height ratio | 0.55 (± 0.08) | 0.55 (± 0.08) | 0.55 (± 0.08) |
| Cigarette smoking, n (%) | |||
| Never | 311 (31.3) | 325 (34.3) | 416 (40.8) |
| Former | 406 (40.8) | 450 (47.4) | 478 (46.9) |
| Current | 378 (27.9) | 174 (18.3) | 126 (12.3) |
| Pack years of smoking 1 | 20.0 (± 0.9) | 16.1 (± 0.8) | 12.0 (± 0.6) |
| Energy intake (kcals/day) | 1741 (± 650) | 1885 (± 611) | 1963 (± 580) |
| Alcohol (g/day) 1 | 8.0 (± 0.4) | 7.3 (± 0.3) | 6.7 (± 0.3) |
| Supplement use, n (%) | |||
| No | 735 (73.9) | 680 (71.5) | 636 (62.4) |
| Yes | 242 (24.3) | 250 (26.3) | 367 (36.0) |
| Physical activity (METs/day) | 14.3 (± 9.2) | 14.5 (± 8.1) | 15.1 (± 8.0) |
| Prevalent diabetes, n (%) | 59 (5.9) | 65 (6.8) | 79 (7.8) |
| Estrogen use, n (%) 2 | |||
| Never | 379 (85.8) | 426 (84.4) | 507 (80.1) |
| Ever | 63 (14.2) | 79 (15.6) | 122 (19.4) |
| Food Groups Considered in the MSDP Score | Median Intakes (5%–95%) | Median Scores (5%–95%) 1 |
|---|---|---|
| Servings/day | ||
| Whole grains | 0.90 (0.00–3.60) | 1.13 (0.00–4.50) |
| Fruit | 1.50 (0.20–4.40) | 4.67 (0.33–9.33) |
| Vegetables | 2.40 (0.80–5.80) | 4.00 (1.17–8.50) |
| Dairy | 1.20 (0.20–4.10) | 5.00 (0.00–9.00) |
| Wine: Men | 0.10 (0.00–0.90) | 0.33 (0.00–3.00) |
| Women | 0.10 (0.00–0.90) | 0.67 (0.00–5.33) |
| Servings/week | ||
| Olives, pulses, and nuts | 1.20 (0.00–5.10) | 3.00 (0.00–9.25) |
| Potatoes | 3.50 (0.50–7.60) | 5.00 (0.00–10.00) |
| Poultry | 5.00 (0.80–9.90) | 5.75 (0.00–8.25) |
| Eggs | 0.50 (0.00–3.00) | 1.67 (0.00–10.00) |
| Meat | 4.00 (0.50–11.90) | 0.00 (0.00–8.00) |
| Sweets | 13.80 (1.90–45.00) | 0.00 (0.00–7.67) |
| Fish | 2.30 (0.50–7.00) | 3.73 (0.67–9.00) |
| Olive oil use score 2 | 0.00 (0.00–10.00) |
| Subjects | Cases/PY | Rate/10,000 py | HR (95% CI) 1 | HR (95% CI) 2 | |
|---|---|---|---|---|---|
| All subjects (n = 2966) | |||||
| MSDP score | |||||
| Low (4.0–19.0) | 995 | 226/15,964 | 1416 | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (19.1–25.0) | 951 | 191/15,407 | 1240 | 0.82 (0.67–0.99) | 0.85 (0.70–1.04) |
| High (25.1–50.9) | 1020 | 194/16,717 | 1160 | 0.79 (0.65–0.96) | 0.84 (0.69–1.03) |
| P-trend | 0.02 | 0.09 | |||
| Women (n = 1578) | |||||
| MSDP score | |||||
| Low (4.0–19.0) | 443 | 88/7333 | 1200 | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (19.1–25.0) | 505 | 74/8552 | 865 | 0.86 (0.50–0.93) | 0.71 (0.52–0.97) |
| High (25.1–50.9) | 630 | 97/10,718 | 905 | 0.70 (0.53–0.94) | 0.74 (0.55–0.99) |
| P-trend | 0.02 | 0.05 | |||
| Men (n = 1388) | |||||
| MSDP score | |||||
| Low (4.0–19.0) | 552 | 138/8632 | 1599 | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (19.1–25.0) | 446 | 117/6856 | 1707 | 0.90 (0.70–1.16) | 0.95 (0.74–1.22) |
| High (25.1–47.7) | 390 | 97/5999 | 1617 | 0.83 (0.64–1.08) | 0.91 (0.70–1.20) |
| P-trend | 0.17 | 0.51 |
| Food Groups Considered in the MSDP Score (servings/day) | Women | Men |
|---|---|---|
| HR (95% CI) 1 | HR (95% CI) 1 | |
| Whole grains | ||
| Low (<0.5) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (0.5–<1.0) | 0.90 (0.62–1.31) | 1.21 (0.88–1.66) |
| High (≥1) | 1.11 (0.79–1.55) | 1.10 (0.83–1.46) |
| P-trend | 0.39 | 0.66 |
| Fruit | ||
| Low (<0.75) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (0.75–<2.5) | 0.62 (0.44–0.88) | 1.10 (0.81–1.48) |
| High (≥2.5) | 0.87 (0.56–1.34) | 1.02 (0.70–1.48) |
| P-trend | 0.92 | 0.93 |
| Vegetables | ||
| Low (<1.5) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (1.5–<3) | 0.99 (0.67–1.44) | 0.95 (0.71–1.27) |
| High (≥3) | 0.81 (0.52–1.26) | 1.08 (0.75–1.55) |
| P-trend | 0.22 | 0.59 |
| Dairy | ||
| Low (<0.5) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (0.5–<1.5) | 0.67 (0.47–0.96) | 0.69 (0.51–0.94) |
| High (≥1.5) | 0.73 (0.49–1.08) | 1.02 (0.74–1.40) |
| P-trend | 0.43 | 0.17 |
| Wine | ||
| Non–drinkers | 1.00 (Ref.) | 1.00 (Ref.) |
| Drinkers | 1.07 (0.81–1.41) | 1.12 (0.89–1.42) |
| P-trend | 0.64 | 0.34 |
| Servings/week | ||
| Olives, pulses & nuts | ||
| Low (<0.5) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (0.5–<1.5) | 1.09 (0.74–1.63) | 0.80 (0.54–1.17) |
| High (≥1.5) | 0.89 (0.58–1.37) | 0.89 (0.59–1.34) |
| P-trend | 0.32 | 0.82 |
| Potatoes | ||
| Low (<1.5) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (1.5–<3.5) | 0.73 (0.50–1.08) | 0.72 (0.51–1.01) |
| High (≥3.5) | 0.76 (0.52–1.12) | 0.87 (0.63–1.20) |
| P-trend | 0.39 | 0.95 |
| Poultry | ||
| Low (<2) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (2–<5) | 1.04 (0.66–1.64) | 0.76 (0.54–1.08) |
| High (≥5) | 1.19 (0.86–1.65) | 0.88 (0.67–1.15) |
| P-trend | 0.28 | 0.45 |
| Meat | ||
| Low (<2) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (2–<5) | 1.22 (0.85–1.75) | 1.63 (1.11–2.42) |
| High (≥5) | 1.14 (0.75–1.74) | 1.46 (0.95–2.26) |
| P-trend | 0.81 | 0.67 |
| Sweets | ||
| Low (<7) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (7–<21) | 0.92 (0.67–1.27) | 0.96 (0.70–1.34) |
| High (≥21) | 0.79 (0.50–1.27) | 0.94 (0.64–1.37) |
| P-trend | 0.34 | 0.75 |
| Fish & other seafood | ||
| Low (<2) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (2–<4) | 0.84 (0.61–1.16) | 1.12 (0.86–1.45) |
| High (≥4) | 1.18 (0.83–1.67) | 0.95 (0.69–1.32) |
| P-trend | 0.41 | 0.85 |
| Eggs | ||
| Low (0) | 1.00 (Ref.) | 1.00 (Ref.) |
| Moderate (0.5–<1.5) | 0.69 (0.48–0.98) | 0.99 (0.70–1.40) |
| High (≥1.5) | 0.82 (0.58–1.15) | 0.97 (0.70–1.34) |
| P-trend | 0.89 | 0.85 |
| Olive oil | ||
| No use of olive oil | 1.00 (Ref.) | 1.00 (Ref.) |
| Olive oil use | 0.71 (0.49–1.01) | 0.89 (0.67–1.20) |
| Olive oil and Vegetable oil use | 0.93 (0.47–1.84) | 1.37 (0.69–2.70) |
| P-trend | 0.06 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiannakou, I.; Singer, M.R.; Jacques, P.F.; Xanthakis, V.; Ellison, R.C.; Moore, L.L. Adherence to a Mediterranean-Style Dietary Pattern and Cancer Risk in a Prospective Cohort Study. Nutrients 2021, 13, 4064. https://doi.org/10.3390/nu13114064
Yiannakou I, Singer MR, Jacques PF, Xanthakis V, Ellison RC, Moore LL. Adherence to a Mediterranean-Style Dietary Pattern and Cancer Risk in a Prospective Cohort Study. Nutrients. 2021; 13(11):4064. https://doi.org/10.3390/nu13114064
Chicago/Turabian StyleYiannakou, Ioanna, Martha R. Singer, Paul F. Jacques, Vanessa Xanthakis, R. Curtis Ellison, and Lynn L. Moore. 2021. "Adherence to a Mediterranean-Style Dietary Pattern and Cancer Risk in a Prospective Cohort Study" Nutrients 13, no. 11: 4064. https://doi.org/10.3390/nu13114064
APA StyleYiannakou, I., Singer, M. R., Jacques, P. F., Xanthakis, V., Ellison, R. C., & Moore, L. L. (2021). Adherence to a Mediterranean-Style Dietary Pattern and Cancer Risk in a Prospective Cohort Study. Nutrients, 13(11), 4064. https://doi.org/10.3390/nu13114064






