Management of Dietary Habits and Diarrhea in Fap Individuals: A Mediterranean Low-Inflammatory Dietary Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Intervention
2.3. Dietary Questionnaires
- Drinks (including alcohol), milk and dairy products. This group includes also plant-based unsweetened beverages.
- Sweets and confectionery including sugar, whole sugar, honey and malt.
- Bread and grains. This group has items for refined grains, whole grains and grain products.
- Meat, fish, eggs and meat substitutes that include red and processed meat, white meat and meat substitutes made from wheat gluten protein.
- Legumes, vegetables, fresh and dried fruit, nuts, seeds and soy products.
- Sauces, animal and vegetable fats, including also fermented soy condiments (miso and soy sauce).
2.4. Food Categorization
- −
- Vegetable milk, tea, barley coffee, unsweetened yogurt, brown sugar, honey, malt, unsweetened jam, durum wheat bread and pasta, durum wheat pizza, unsweetened muesli, oat flakes, fish, legumes, tofu/tempeh, onions, tomato juice, tomato sauces, green and black olives, cooked vegetables (zucchini, pumpkin etc.), row vegetables, berries, pomegranate, apples, pears, unsweetened fruit juices, other kind of fresh fruits, dried fruits, dried oleaginous fruits (including in unsweetened creams), extra-virgin olive oil, miso, tamari, mushrooms.
- −
- Animal milk, dairy products, “ricotta”, sugary beverages, alcoholic drinks (wine, beer, spirits), white sugar, artificial sweeteners, sweetened jam, milk chocolate, candies, biscuits, ice creams, brioches, egg noodles, corn flakes, sweetened muesli, red meat, processed meat, potatoes, mashed potatoes, French fries, butter, lard cream, margarine, ready sauces, mayonnaise, ketchup.
- −
- -Café, unsweetened fruit juices, dark chocolate, cookies, ice cream or other desserts without sugar, white bread (and pizza), whole meal flour bread and pizza, white rice, whole rice, rice cakes, other grains, white meat, clams and shellfish, seitan, eggs, artichokes and fennel, cabbage family, peppers and eggplants, citrus fruit, fresh unsweetened fruit juices, seed oil, spices.
2.5. Statistical Analysis
3. Results
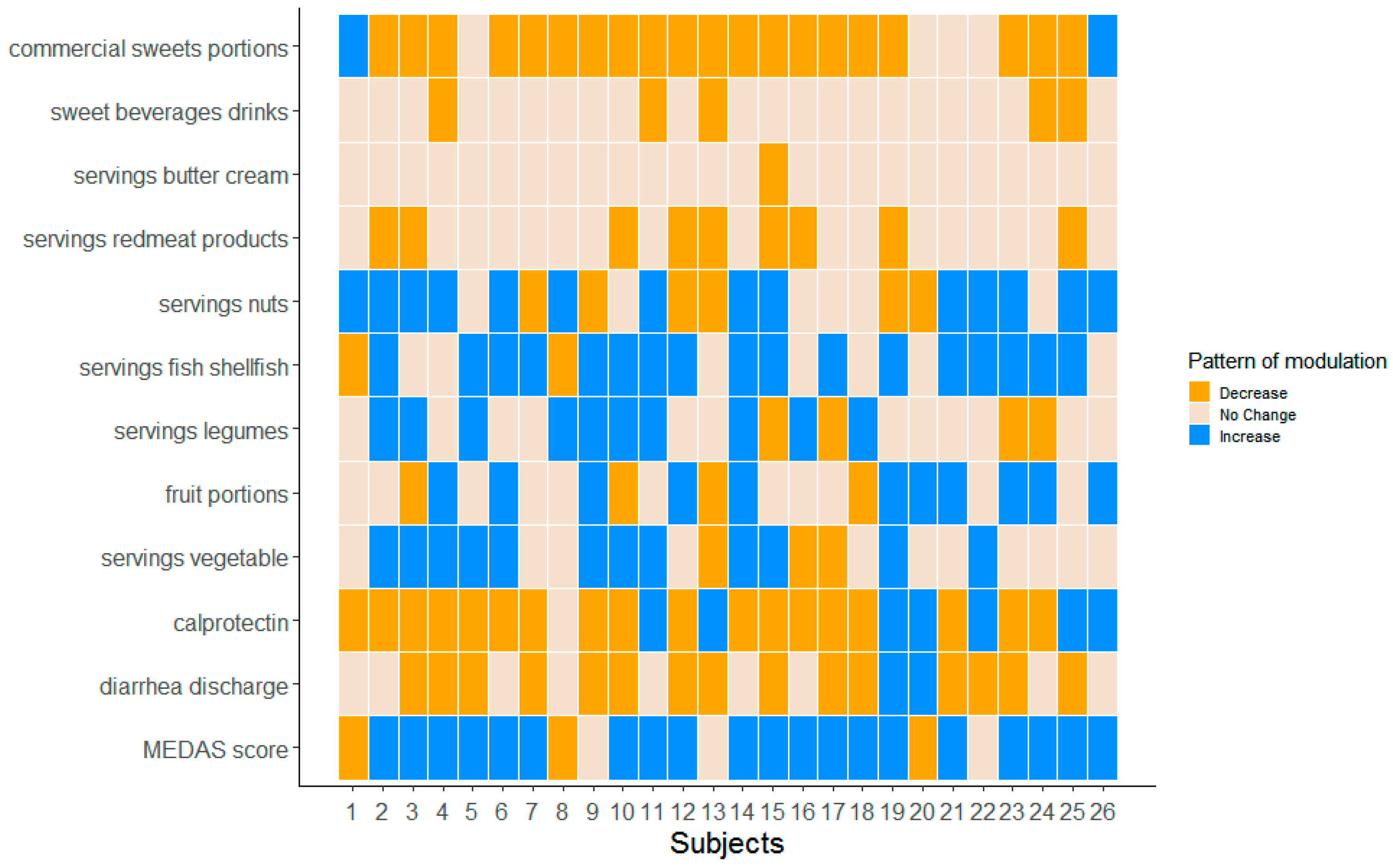
3.1. Food Intake Distributions
3.2. Diarrheal Discharges
3.3. Diarrheal Discharges and Food Intake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasen, H.F.; Möslein, G.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; Capella, G.; et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008, 57, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardoino, I.; Signoroni, S.; Malvicini, E.; Ricci, M.T.; Biganzoli, E.M.; Bertario, L.; Occhionorelli, S.; Vitellaro, M. Long-term survival between total colectomy versus proctocolectomy in patients with FAP: A registry-based, observational cohort study. Tumori 2020, 106, 139–148. [Google Scholar] [CrossRef]
- Vitellaro, M.; Bonfanti, G.; Sala, P.; Poiasina, E.; Barisella, M.; Signoroni, S.; Mancini, A.; Bertario, L. Laparoscopic colectomy and restorative proctocolectomy for familial adenomatous polyposis. Surg. Endosc. 2011, 25, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Urso, E.D.L.; Celotto, F.; Giandomenico, F.; Gavaruzzi, T.; Del, B.P.; Lotto, L.; Spolverato, G.; Pucciarelli, S.; Bao, Q.R. Analysis of morbidity and mortality, quality of life and bowel function after total colectomy with ileorectal anastomosis versus right and left hemicolectomy: A study to optimise the treatment of lynch syndrome and attenuated polyposis coli. Eur. J. Surg. Oncol. 2020, 46, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Pasanisi, P.; Gariboldi, M.; Verderio, P.; Signoroni, S.; Mancini, A.; Rivoltini, L.; Milione, M.; Masci, E.; Ciniselli, C.M.; Bruno, E.; et al. A Pilot Low-Inflammatory Dietary Intervention to Reduce Inflammation and Improve Quality of Life in Patients with Familial Adenomatous Polyposis: Protocol Description and Preliminary Results. Integr. Cancer Ther. 2019, 18, 1534735419846400. [Google Scholar] [CrossRef]
- Belfiore, A.; Ciniselli, C.M.; Signoroni, S.; Gariboldi, M.; Mancini, A.; Rivoltini, L.; Morelli, D.; Masci, E.; Bruno, E.; Macciotta, A.; et al. Preventive anti-inflammatory diet to reduce gastrointestinal inflammation in familial adenomatous polyposis patients: A prospective pilot study. Cancer Prev. Res. 2021, 14, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Bruno, E.; Krogh, V.; Gargano, G.; Grioni, S.; Bellegotti, M.; Venturelli, E.; Panico, S.; de Magistris, M.S.; Bonanni, B.; Zagallo, E.; et al. Adherence to Dietary Recommendations after One Year of Intervention in Breast Cancer Women: The DIANA-5 Trial. Nutrients 2021, 13, 2990. [Google Scholar] [CrossRef]
- Bruno, E.; Oliverio, A.; Paradiso, A.V.; Daniele, A.; Tommasi, S.; Tufaro, A.; Terribile, D.A.; Magno, S.; Filippone, A.; Venturelli, E.; et al. A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial. Cancers 2020, 12, 3732. [Google Scholar] [CrossRef]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods; John Wiley and Sons: New York, NY, USA, 1999. [Google Scholar]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- You, Y.N.; Chua, H.K.; Nelson, H.; Hassan, I.; Barnes, S.A.; Harrington, J. Segmental vs. extended colectomy: Measurable differences in morbidity, function, and quality of life. Dis. Colon Rectum 2008, 51, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Haanstra, J.F.; de Vos Tot Nederveen Cappel, W.H.; Gopie, J.P.; Vecht, J.; Vanhoutvin, S.A.; Cats, A.; van der Zaag-Loonen, H.J.; Langers, A.M.; Bergmann, J.H.; van de Meeberg, P.C.; et al. Quality of life after surgery for colon cancer in patients with Lynch syndrome: Partial versus subtotal colectomy. Dis. Colon Rectum 2012, 55, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaronson, N.K.; Muller, M.; Cohen, P.D.; Essink-Bot, M.L.; Fekkes, M.; Sanderman, R.; Sprangers, M.A.; Te Velde, A.; Verrips, E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J. Clin. Epidemiol. 1998, 51, 1055–1068. [Google Scholar] [CrossRef]
- Sprangers, M.A.; Te Velde, A.; Aaronson, N.K. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur. J. Cancer 1999, 35, 238–247. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Hedin, C.R.; McCarthy, N.E.; Louis, P.; Farquharson, F.M.; McCartney, S.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. Prebiotic fructans have greater impact on luminal microbiology and CD3+ T cells in healthy siblings than patients with Crohn’s disease: A pilot study investigating the potential for primary prevention of inflammatory bowel disease. Clin. Nutr. 2021, 40, 5009–5019. [Google Scholar] [CrossRef]
- Valcheva, R.; Koleva, P.; Martinez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes 2019, 10, 334–357. [Google Scholar] [CrossRef]
- Ricciardiello, L.; Ahnen, D.J.; Lynch, P.M. Chemoprevention of hereditary colon cancers: Time for new strategies. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 352–361. [Google Scholar] [CrossRef]
- Luceri, C.; Femia, A.P.; Tortora, K.; D’Ambrosio, M.; Fabbri, S.; Fazi, M.; Caderni, G. Supplementation with phytoestrogens and insoluble fibers reduces intestinal carcinogenesis and restores ER-β expression in Apc-driven colorectal carcinogenesis. Eur. J. Cancer Prev. 2020, 29, 27–35. [Google Scholar] [CrossRef]
- Benito, I.; Encío, I.J.; Milagro, F.I.; Alfaro, M.; Martinez-Peñuela, A.; Barajas, M.; Marzo, F. Microencapsulated Bifidobacterium bifidum and Lactobacillus gasseri in Combination with Quercetin Inhibit Colorectal Cancer Development in Apc (Min/+) Mice. Int J. Mol. Sci. 2021, 22, 4906. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Tutino, V.; Caruso, M.G.; Francavilla, A. N-3 polyunsaturated fatty acids reverse the development of polyps in Apc (Min/+) transgenic mice. Oncol. Rep. 2016, 35, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Donovan, M.G.; Doetschman, T.C.; Selmin, O.I. N-6 Linoleic Acid Induces Epigenetics Alterations Associated with Colonic Inflammation and Cancer. Nutrients 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, C.; Rizzello, F.; Gionchetti, P.; Calafiore, A.; Pagano, N.; De, F.L.; Valerii, M.C.; Cavazza, E.; Strillacci, A.; Comelli, M.C.; et al. Can supplementation of phytoestrogens/insoluble fibers help the management of duodenal polyps in familial adenomatous polyposis? Carcinogenesis 2016, 37, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
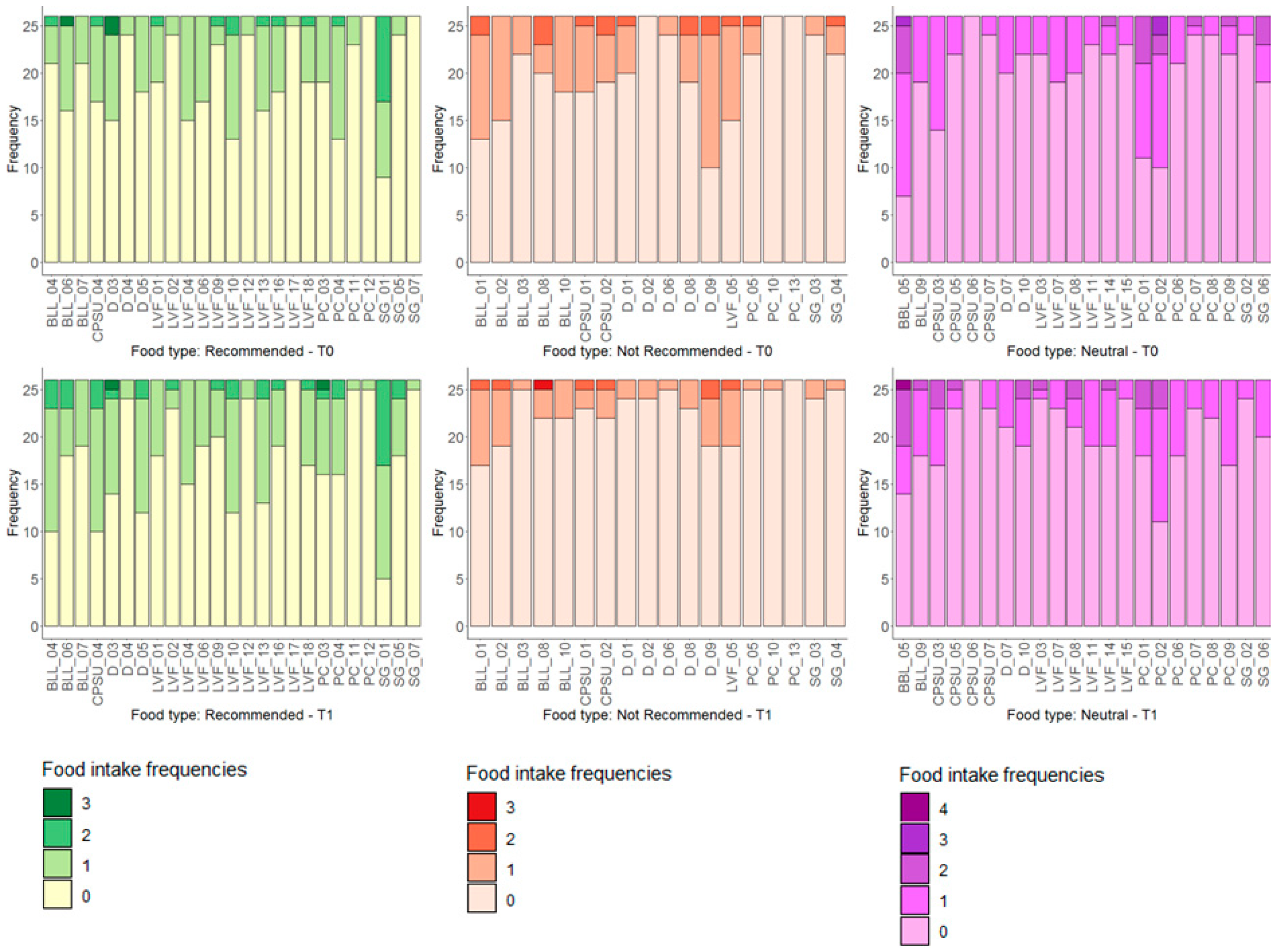
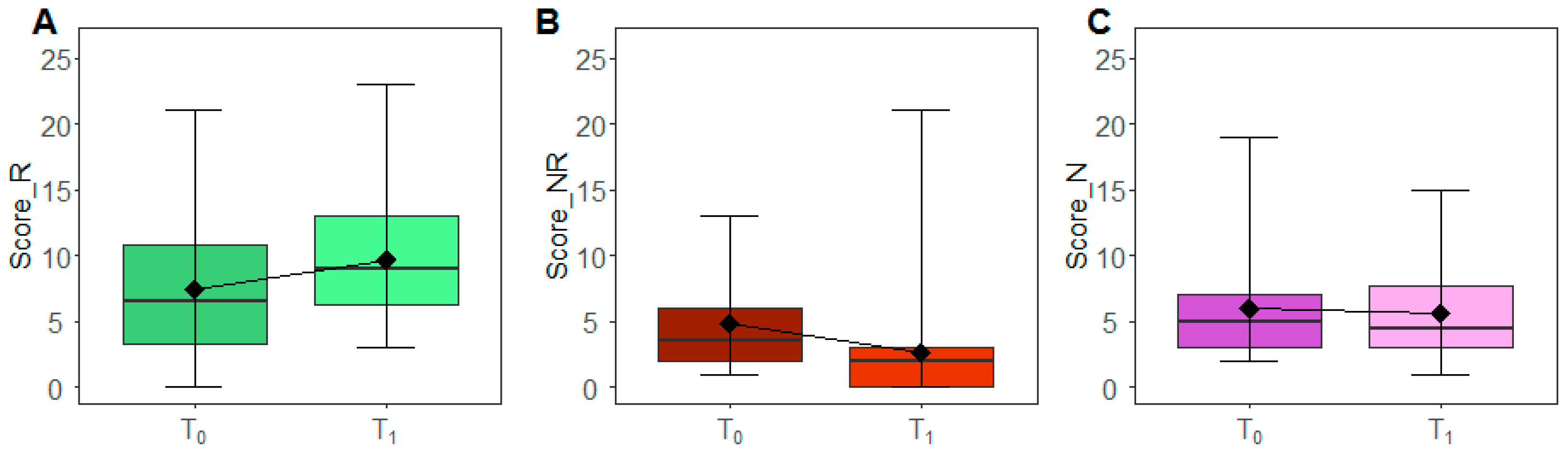
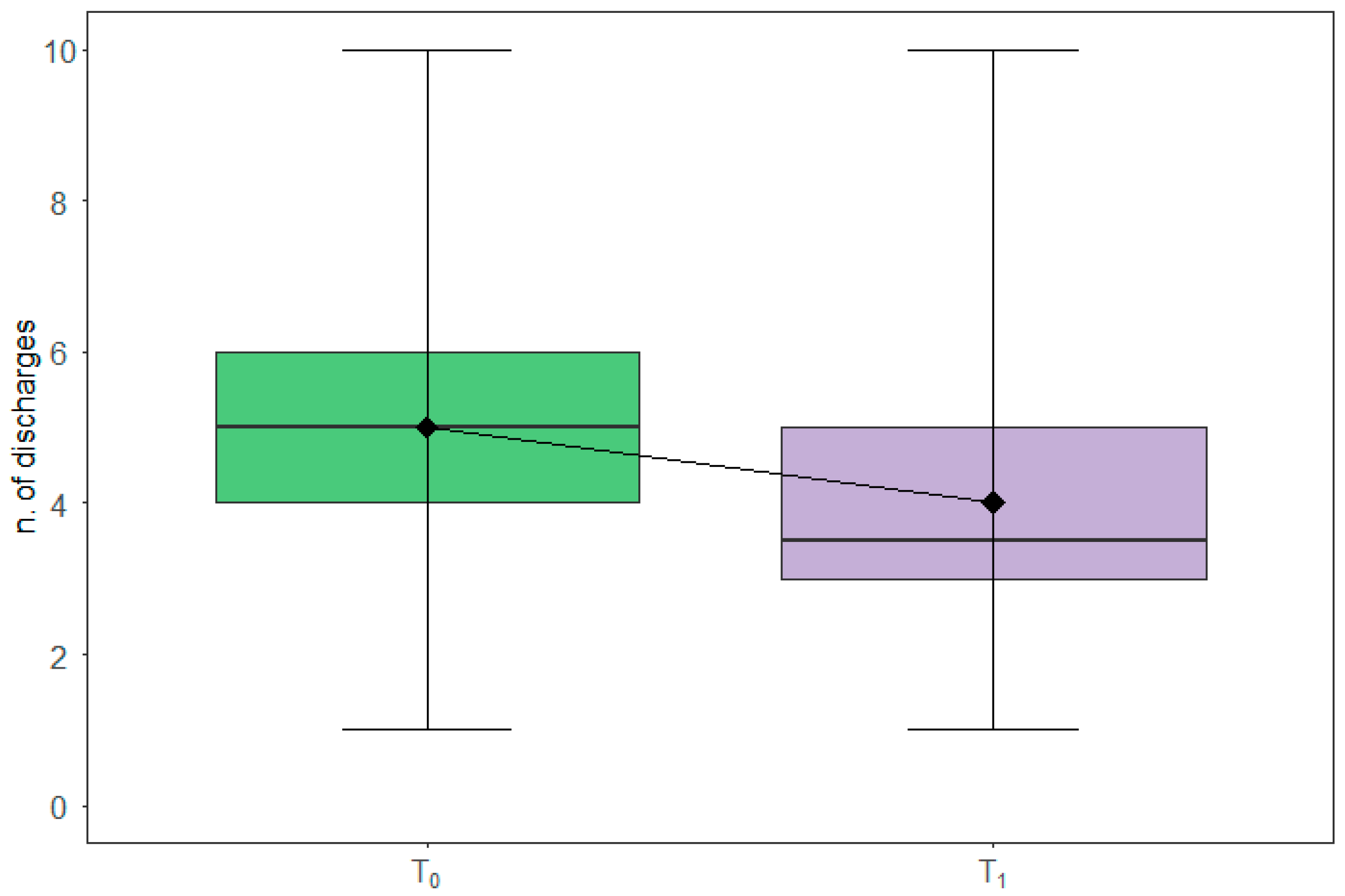
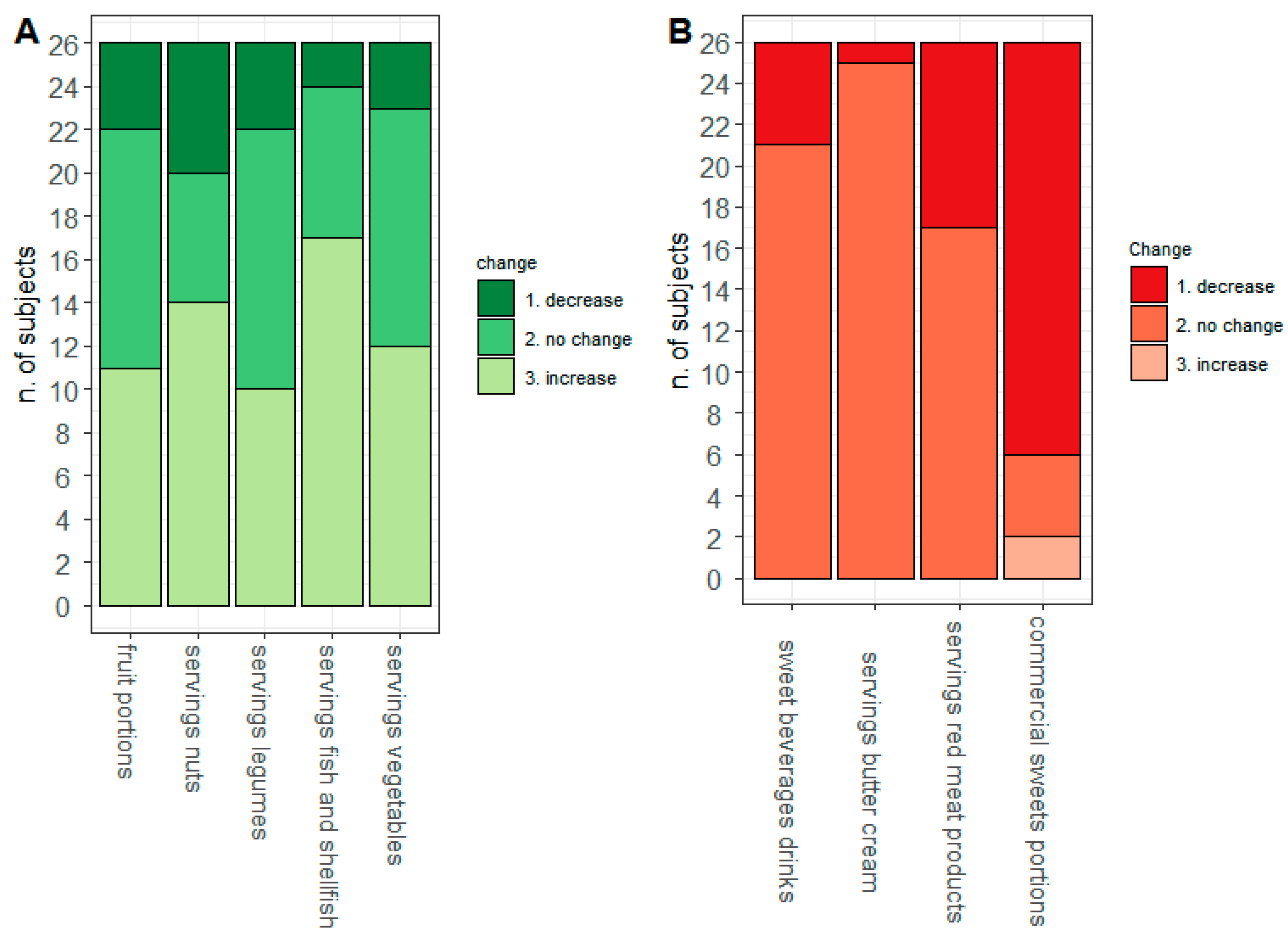
| Variables | n | min | 25th Centile | Median | 75th Centile | Max | IQR | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Recommended (score_R) | ||||||||
| T0 | 26 | 0 | 3 | 6.5 | 11 | 21 | 8 | 0.075 |
| T1 | 26 | 3 | 6 | 9 | 13 | 22 | 7 | |
| Not Recommended (score_NR) | ||||||||
| T0 | 26 | 1 | 2 | 3.5 | 6 | 13 | 4 | 0.002 |
| T1 | 26 | 0 | 0 | 2 | 3 | 21 | 3 | |
| Neutral (score_N) | ||||||||
| T0 | 26 | 2 | 3 | 5 | 7 | 19 | 4 | 0.977 |
| T1 | 26 | 1 | 3 | 5 | 8 | 16 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maura, C.C.; Eleonora, B.; Andreina, O.; Ivan, B.; Marta, P.; Stefano, S.; Marco, V.; Teresa, R.M.; Massimo, M.; Laura, C.; et al. Management of Dietary Habits and Diarrhea in Fap Individuals: A Mediterranean Low-Inflammatory Dietary Intervention. Nutrients 2021, 13, 3988. https://doi.org/10.3390/nu13113988
Maura CC, Eleonora B, Andreina O, Ivan B, Marta P, Stefano S, Marco V, Teresa RM, Massimo M, Laura C, et al. Management of Dietary Habits and Diarrhea in Fap Individuals: A Mediterranean Low-Inflammatory Dietary Intervention. Nutrients. 2021; 13(11):3988. https://doi.org/10.3390/nu13113988
Chicago/Turabian StyleMaura, Ciniselli Chiara, Bruno Eleonora, Oliverio Andreina, Baldassari Ivan, Pastori Marta, Signoroni Stefano, Vitellaro Marco, Ricci Maria Teresa, Milione Massimo, Cattaneo Laura, and et al. 2021. "Management of Dietary Habits and Diarrhea in Fap Individuals: A Mediterranean Low-Inflammatory Dietary Intervention" Nutrients 13, no. 11: 3988. https://doi.org/10.3390/nu13113988
APA StyleMaura, C. C., Eleonora, B., Andreina, O., Ivan, B., Marta, P., Stefano, S., Marco, V., Teresa, R. M., Massimo, M., Laura, C., Manuela, G., Andrea, M., Licia, R., Daniele, M., Patrizia, P., & Paolo, V. (2021). Management of Dietary Habits and Diarrhea in Fap Individuals: A Mediterranean Low-Inflammatory Dietary Intervention. Nutrients, 13(11), 3988. https://doi.org/10.3390/nu13113988









