Effects of Early Weight Gain Velocity, Diet Quality, and Snack Food Access on Toddler Weight Status at 1.5 Years: Follow-Up of a Randomized Controlled Infant Formula Trial
Abstract
1. Introduction
2. Materials and Methods
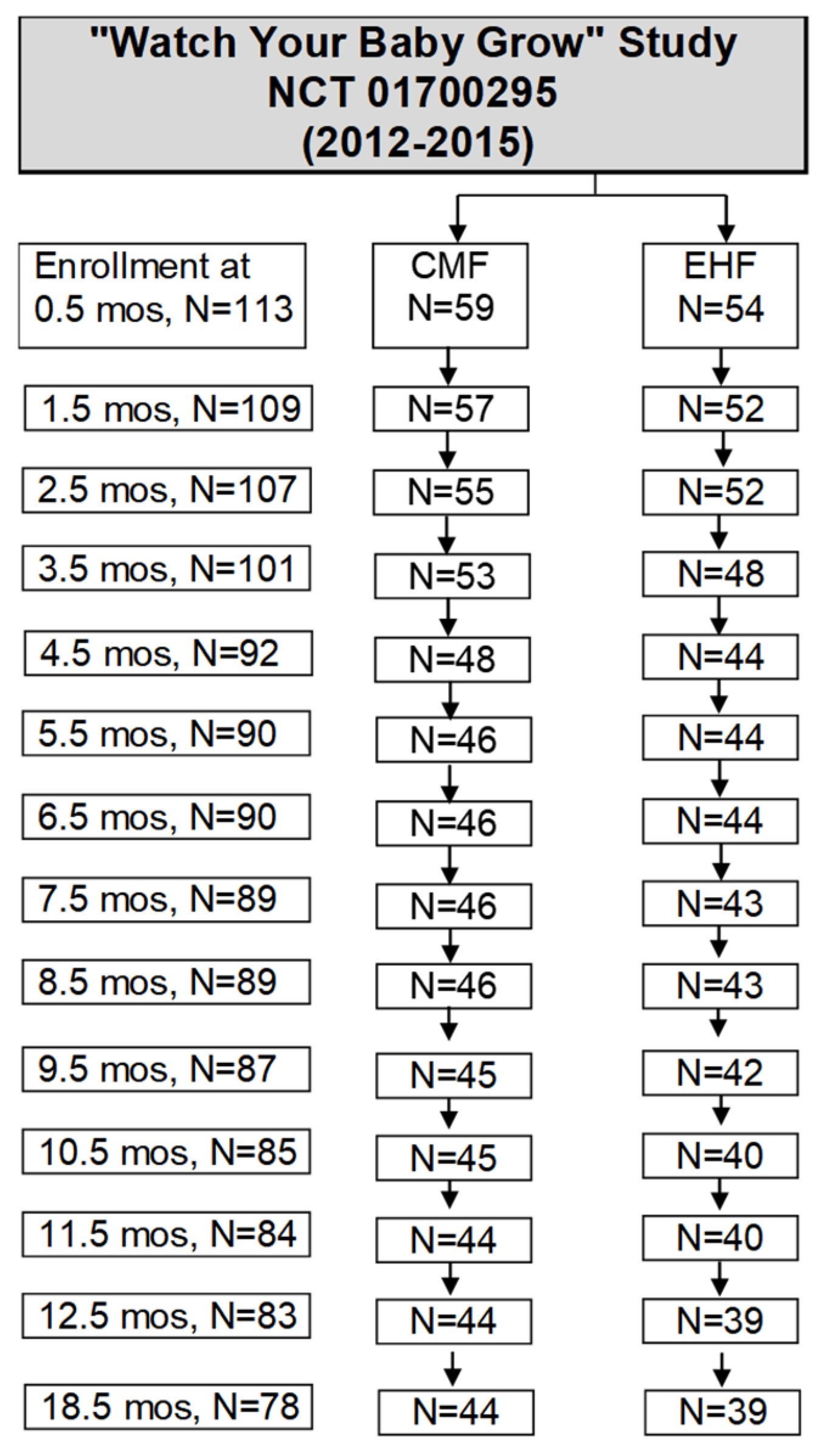
2.1. Participants
2.2. Anthropometry
2.3. Toddler Snack Food Feeding Questionnaire (TSFFQ)
2.4. Dietary Intake
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
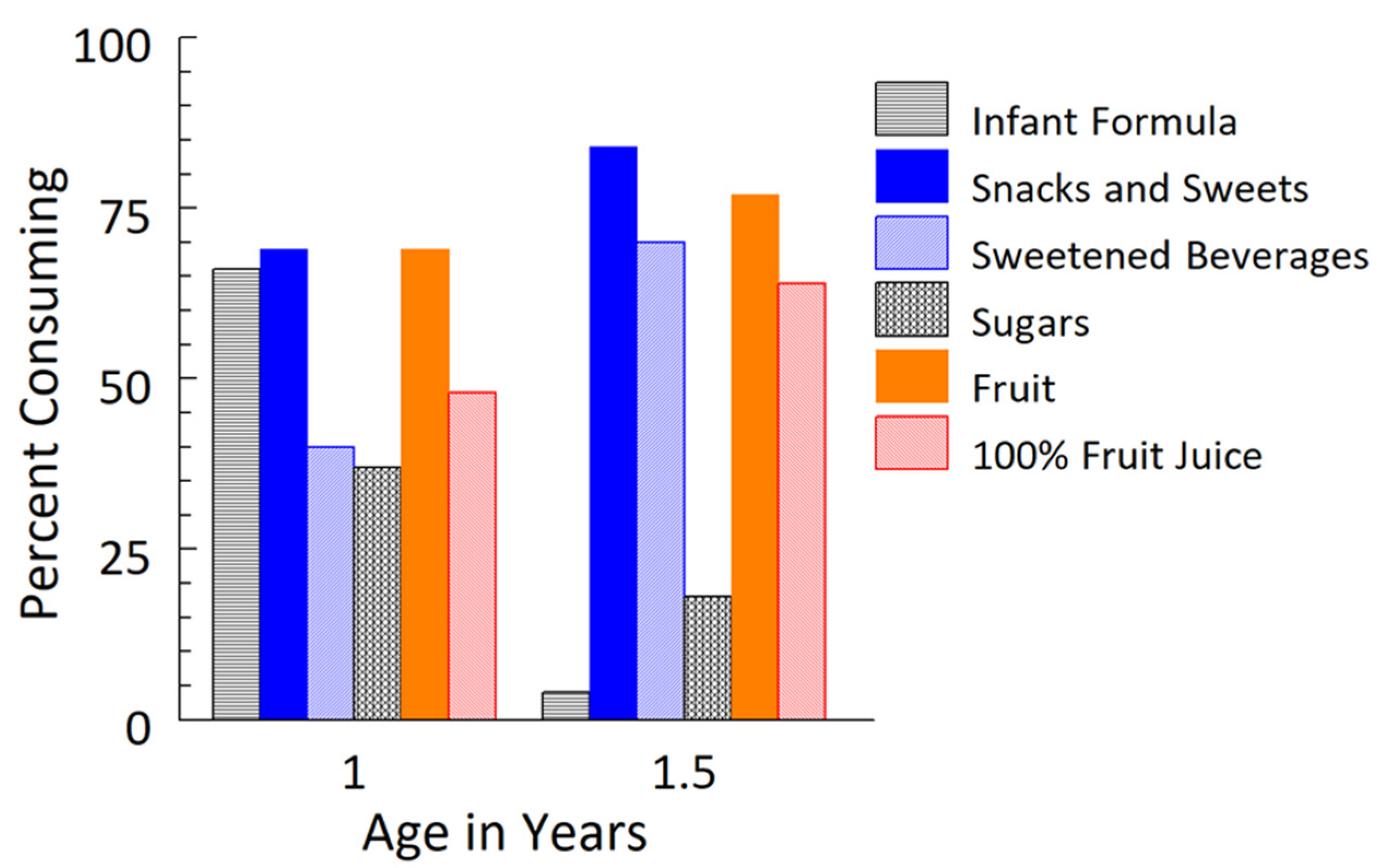
3.2. Dietary Changes from 1 Year to 1.5 Years
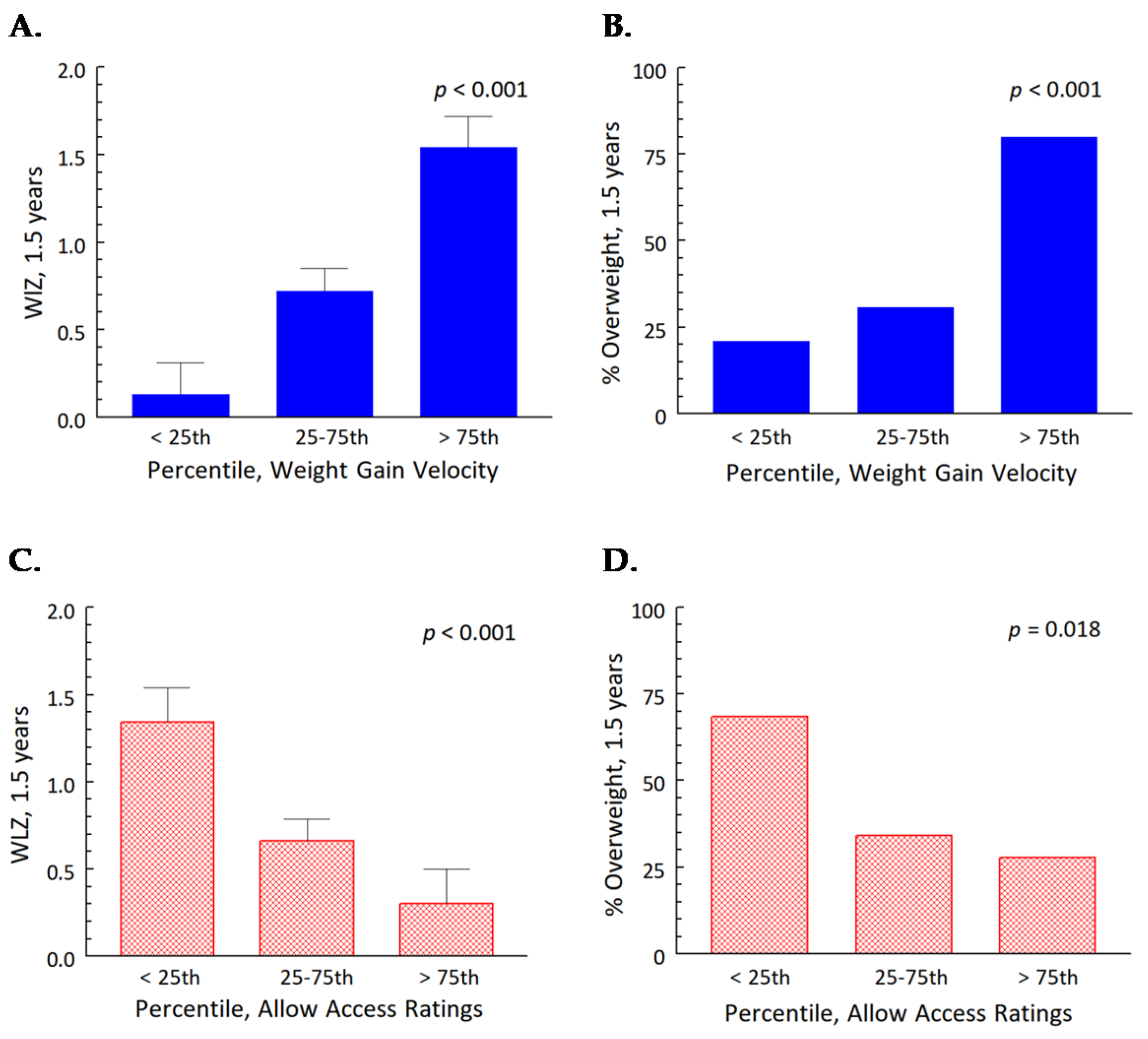
3.3. The General Linear Mixed Effects Model: Independent Effects of Early Weight Gain Velocity and Parenting Specific to Snack Foods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dattilo, A.M.; Birch, L.; Krebs, N.F.; Lake, A.; Taveras, E.M.; Saavedra, J.M. Need for early interventions in the prevention of pediatric overweight: A review and upcoming directions. J. Obes. 2012, 2012, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Forestell, C.A.; Ventura, A.K.; Fisher, J.O. The development of infant feeding. In The Cambridge Handbook of Infant Development; Lockman, J.J., Tamis-LeMonda, C.S., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 263–302. [Google Scholar]
- Raiten, D.J.; Raghavan, R.; Porter, A.; Obbagy, J.E.; Spahn, J.M. Executive summary: Evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“The B-24 Project”. Am. J. Clin. Nutr. 2014, 99, 663S–691S. [Google Scholar] [CrossRef]
- Robinson, S.M. Infant nutrition and lifelong health: Current perspectives and future challenges. J. Dev. Orig. Health Dis. 2015, 6, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escamilla, R.; Segura-Pérez, S.; Moran, V.H. Dietary guidelines for children under 2 years of age in the context of nurturing care. Matern. Child Nutr. 2019, 15, e12855. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture and US Department of Health and Human Services. 2020–2025 Dietary Guidelines for Americans, 9th Edition. Available online: https://www.dietaryguidelines.gov (accessed on 30 August 2021).
- Koletzko, B.; Hirsch, N.L.; Jewell, J.M.; Dos Santos, Q.; Breda, J.; Fewtrell, M.; Weber, M.W. National recommendations for infant and young child feeding in the World Health Organization European region. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Pannucci, T.; Casavale, K.O.; Davis, T.A.; Donovan, S.M.; Kleinman, R.E.; Taveras, E.M.; Bailey, R.L.; Novotny, R.; Schneeman, B.O.; et al. Development of food pattern recommendations for infants and toddlers 6–24 months of age to support the Dietary Guidelines for Americans, 2020–2025. J. Nutr. 2021, 151, 3113–3124. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G. Growth characteristics of breast-fed compared to formula-fed infants. Biol. Neonate 1998, 74, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Reilly, J.J. Breastfeeding and lowering the risk of childhood obesity. Lancet 2002, 359, 2003–2004. [Google Scholar] [CrossRef]
- Grummer-Strawn, L.M.; Mei, Z. Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics 2004, 113, e81–e86. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Inamdar, L.; Pressman, N.; Schall, J.I.; Papas, M.A.; Schoeller, D.; Stallings, V.A.; Trabulsi, J.C. Type of infant formula increases early weight gain and impacts energy balance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1015–1025. [Google Scholar] [CrossRef]
- German Infant Nutritional Intervention Study Group; Rzehak, P.; Sausenthaler, S.; Koletzko, S.; Reinhardt, D.; Von Berg, A.; Krämer, U.; Berdel, D.; Bollrath, C.; Grübl, A.; et al. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at ≤6 y of age: Results from the German Infant Nutritional Intervention Study. Am. J. Clin. Nutr. 2009, 89, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef]
- Monteiro, P.O.A.; Victora, C. Rapid growth in infancy and childhood and obesity in later life—A systematic review. Obes. Rev. 2005, 6, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk factors for childhood obesity in the first 1,000 days: A systematic review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lamb, K.; Grimes, C.; Laws, R.; Bolton, K.; Ong, K.K.; Campbell, K. Rapid weight gain during infancy and subsequent adiposity: A systematic review and meta-analysis of evidence. Obes. Rev. 2018, 19, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Rossen, L.M.; Simon, A.E.; Herrick, K.A. Types of infant formulas consumed in the United States. Clin. Pediatr. 2016, 55, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Corsini, N.; Wilson, C.; Kettler, L.; Danthiir, V. Development and preliminary validation of the Toddler Snack Food Feeding Questionnaire. Appetite 2010, 54, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Blaine, R.E.; Kachurak, A.; Davison, K.K.; Klabunde, R.; Fisher, J.O. Food parenting and child snacking: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Corsini, N.; Kettler, L.; Danthiir, V.; Wilson, C. Parental feeding practices to manage snack food intake: Associations with energy intake regulation in young children. Appetite 2018, 123, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Sausenthaler, S.; Koletzko, S.; Reinhardt, D.; Von Berg, A.; Krämer, U.; Berdel, D.; Bollrath, C.; Grübl, A.; Bauer, C.P.; et al. Long-term effects of hydrolyzed protein infant formulas on growth—Extended follow-up to 10 y of age: Results from the German Infant Nutritional Intervention (GINI) study. Am. J. Clin. Nutr. 2011, 94, 1803S–1807S. [Google Scholar] [CrossRef]
- Pew Charitable Trusts. Philadelphia 2021: The State of the City. Available online: https://www.pewtrusts.org/en/research-and-analysis/reports/2021/04/philadelphia-2021-state-of-the-city (accessed on 4 September 2021).
- WHO Multicentre Growth Reference Study Group. World Health Organization Child. Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.; Ellis, K.J. Infant Feeding Mode Affects Early Growth and Body Composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.M.; Spivack, J.G.; Faith, M.S.; Chesi, A.; Mitchell, J.A.; Kelly, A.; Grant, S.F.; McCormack, S.E.; Zemel, B.S. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics 2016, 137, e20153492. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.T.; Skinner, A.C.; Yin, H.S.; Rothman, R.L.; Sanders, L.M.; Delamater, A.M.; Perrin, E.M. Bottle size and weight gain in formula-fed infants. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture; Agricultural Research Service. What We Eat in America Food Categories 2017–2018. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/ (accessed on 4 September 2021).
- Herrick, K.A.; Fryar, C.D.; Hamner, H.C.; Park, S.; Ogden, C.L. Added Sugars Intake among US Infants and Toddlers. J. Acad. Nutr. Diet. 2020, 120, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Added Sugars on the New Nutrition Facts Label. Available online: https://www.fda.gov/food/new-nutrition-facts-label/added-sugars-new-nutrition-facts-label (accessed on 4 September 2021).
- US Department of Health & Human Services; Food and Drug Administration; Center for Food Safety and Applied Nutrition. Labeling of Infant Formula: Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-labeling-infant-formula (accessed on 30 September 2021).
- Catalano, P.M.; Farrell, K.; Thomas, A.; Huston-Presley, L.; Mencin, P.; De Mouzon, S.H.; Amini, S.B. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am. J. Clin. Nutr. 2009, 90, 1303–1313. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Berkowitz, R.I.; Stallings, V.A.; Schoeller, D.A. Energy intake, not energy output, is a determinant of body size in infants. Am. J. Clin. Nutr. 1999, 69, 524–530. [Google Scholar] [CrossRef]
- Taveras, E.M.; Rifas-Shiman, S.L.; Belfort, M.B.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009, 123, 1177–1183. [Google Scholar] [CrossRef]
- Mennella, J.A.; Reiter, A.; Brewer, B.; Pohlig, R.T.; Stallings, V.A.; Trabulsi, J.C. Early weight gain forecasts accelerated eruption of deciduous teeth and later overweight status during the first year. J. Pediatr. 2020, 225, 174–181. [Google Scholar] [CrossRef]
- Deming, D.M.; Reidy, K.C.; Fox, M.K.; Briefel, R.R.; Jacquier, E.; Eldridge, A.L. Cross-sectional analysis of eating patterns and snacking in the US Feeding Infants and Toddlers Study 2008. Public Health Nutr. 2017, 20, 1584–1592. [Google Scholar] [CrossRef]
- Ziegler, P.; Hanson, C.; Ponza, M.; Novak, T.; Hendricks, K. Feeding Infants and Toddlers Study: Meal and snack intakes of Hispanic and non-Hispanic infants and toddlers. J. Am. Diet. Assoc. 2006, 106, S107–S123. [Google Scholar] [CrossRef]
- Fisher, J.O.; Birch, L.L. Parents’ restrictive feeding practices are associated with young girls’ negative self-evaluation of eating. J. Am. Diet. Assoc. 2000, 100, 1341–1346. [Google Scholar] [CrossRef]
- Beckers, D.; Karssen, L.T.; Vink, J.M.; Burk, W.J.; Larsen, J.K. Food parenting practices and children’s weight outcomes: A systematic review of prospective studies. Appetite 2021, 158, 105010. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet. Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- Fisher, J.O.; Butte, N.F.; Mendoza, P.M.; Wilson, T.A.; Hodges, E.A.; Reidy, K.C.; Deming, D. Overestimation of infant and toddler energy intake by 24-h recall compared with weighed food records. Am. J. Clin. Nutr. 2008, 88, 407–415. [Google Scholar] [CrossRef]
- Fisher, J.; Wright, G.; Herman, A.; Malhotra, K.; Serrano, E.; Foster, G.; Whitaker, R. “Snacks are not food”. Low-income, urban mothers’ perceptions of feeding snacks to their preschool-aged children. Appetite 2015, 84, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.M.; Jonnalagadda, S.S.; Slavin, J.L. What is a snack, why do we snack, and how can we choose better snacks? A review of the definitions of snacking, motivations to snack, contributions to dietary intake, and recommendations for improvement. Adv. Nutr. 2016, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Anderson, G.H. Snacking definitions: Impact on interpretation of the literature and dietary recommendations. Crit. Rev. Food Sci. Nutr. 2010, 50, 848–871. [Google Scholar] [CrossRef]
- Younginer, N.A.; Blake, C.E.; Davison, K.K.; Blaine, R.E.; Ganter, C.; Orloski, A.; Fisher, J.O. “What do you think of when I say the word ‘snack’?” Towards a cohesive definition among low-income caregivers of preschool-age children. Appetite 2016, 98, 35–40. [Google Scholar] [CrossRef]
- Blaine, R.E.; Fisher, J.O.; Taveras, E.M.; Geller, A.C.; Rimm, E.B.; Land, T.; Perkins, M.; Davison, K.K. Reasons low-income parents offer snacks to children: How feeding rationale influences snack frequency and adherence to dietary recommendations. Nutrients 2015, 7, 5982–5999. [Google Scholar] [CrossRef]
- Gibson, E.L.; Androutsos, O.; Moreno, L.; Flores-Barrantes, P.; Socha, P.; Iotova, V.; Cardon, G.; De Bourdeaudhuij, I.; Koletzko, B.; Skripkauskaite, S.; et al. Influences of parental snacking-related attitudes, behaviours and nutritional knowledge on young children’s healthy and unhealthy snacking: The ToyBox study. Nutrients 2020, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F. Meals and snacking, diet quality and energy balance. Physiol. Behav. 2014, 134, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.W.; Kay, M.C.; Jacquier, E.; Catellier, D.; Hampton, J.; Anater, A.S.; Story, M. Trends in food consumption patterns of US infants and toddlers from Feeding Infants and Toddlers Studies (FITS) in 2002, 2008, 2016. Nutrients 2019, 11, 2807. [Google Scholar] [CrossRef] [PubMed]
- Shriver, L.H.; Marriage, B.; Bloch, T.D.; Spees, C.K.; Ramsay, S.A.; Taylor, C.A. Nutritional composition and the contribution of snacks to the dietary intakes of 2–5 year old children. J. Acad. Nutr. Diet. 2015, 115, A79. [Google Scholar] [CrossRef]
- Liu, J.; Lee, Y.; Micha, R.; Li, Y.; Mozaffarian, D. Trends in junk food consumption among US children and adults, 2001–2018. Am. J. Clin. Nutr. 2021, 114, 1039–1048. [Google Scholar] [CrossRef]
- Vos, M.B.; Kaar, J.L.; Welsh, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Munos, J.C.; Krebs, N.F.; Xanthakos, S.A.; et al. Added sugars and cardiovascular disease risk in children: A scientific statement from the American Heart Association. Circulation 2016, 135, e1017–e1034. [Google Scholar] [CrossRef]
- Roess, A.A.; Jacquier, E.F.; Catellier, D.J.; Carvalho, R.; Lutes, A.C.; Anater, A.S.; Dietz, W.H. Food consumption patterns of infants and toddlers: Findings from the Feeding Infants and Toddlers Study (FITS) 2016. J. Nutr. 2018, 148, 1525S–1535S. [Google Scholar] [CrossRef]
- Maalouf, J.; Cogswell, M.E.; Yuan, K.; Martin, C.; Gunn, J.P.; Pehrsson, P.; Merritt, R.; Bowman, B. Top sources of dietary sodium from birth to age 24 mo, United States, 2003–2010. Am. J. Clin. Nutr. 2015, 101, 1021–1028. [Google Scholar] [CrossRef]
- Mennella, J.A.; Reed, D.R.; Roberts, K.M.; Mathew, P.S.; Mansfield, C.J. Age-related differences in bitter taste and efficacy of bitter blockers. PLoS ONE 2014, 9, e103107. [Google Scholar] [CrossRef]
- Mennella, J.A.; Finkbeiner, S.; Lipchock, S.V.; Hwang, L.D.; Reed, D.R. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS ONE 2014, 9, e92201. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C. Consumption of added sugars among US children and adults by food purchase location and food source. Am. J. Clin. Nutr. 2014, 100, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Alexy, U.; Sichert-Hellert, W.; Kersting, M. Associations between intake of added sugars and intakes of nutrients and food groups in the diets of German children and adolescents. Br. J. Nutr. 2003, 90, 441–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meleleo, D.; Susca, G.; Andrulli Buccheri, V.; Lamanna, G.; Cassano, L.; De Chirico, V.; Mustica, S.; Caroli, M.; Bartolomeo, N. Effectiveness of an innovative sensory approach to improve children’s nutritional choices. Int. J. Environ. Res. Public Health 2021, 18, 6462. [Google Scholar] [CrossRef]
- Larsen, J.K.; Hermans, R.C.; Sleddens, E.F.; Engels, R.C.; Fisher, J.O.; Kremers, S.P. How parental dietary behavior and food parenting practices affect children’s dietary behavior. Interacting sources of influence? Appetite 2015, 89, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, J.C.; Smethers, A.D.; Eosso, J.R.; Papas, M.A.; Stallings, V.A.; Mennella, J.A. Impact of early rapid weight gain on odds for overweight at one year differs between breastfed and formula-fed infants. Pediatr. Obes. 2020, 15, e12688. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.; Fisher, D.; Lucas, P.; Kleijnen, J.; Roberts, H.; Law, C. Being big or growing fast: Systematic review of size and growth in infancy and later obesity. BMJ 2005, 331, 929. [Google Scholar] [CrossRef]
- Ong, K.K.; Emmett, P.; Northstone, K.; Golding, J.; Rogers, I.; Ness, A.R.; Wells, J.C.; Dunger, D.B. Infancy weight gain predicts childhood body fat and age at menarche in girls. J. Clin. Endocrinol. Metab. 2009, 94, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Salgin, B.; Norris, S.A.; Prentice, P.; Pettifor, J.; Richter, L.M.; Ong, K.K.; Dunger, P.D. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int. J. Obes. 2015, 39, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.X.-L.; Goh, A.; Aris, I.B.M.; Teoh, O.H.; Shek, L.P.-C.; Lee, B.W.; Chan, Y.H.; Tint, M.T.; Soh, S.-E.; Saw, S.-M.; et al. Effects of infant weight gain on subsequent allergic outcomes in the first 3 years of life. BMC Pediatr. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rothbart, M.K.; Ellis, L.K.; Posner, M.I. Temperament and self-regulation. In Handbook of Self-Regulation: Research, Theory and Applications; Baumeister, R.F., Vohs, K.D., Eds.; Guilford: New York, NY, USA, 2011; pp. 357–370. [Google Scholar]
- Spill, M.K.; Callahan, E.H.; Shapiro, M.J.; Spahn, J.M.; Wong, Y.P.; Benjamin-Neelon, S.E.; Birch, L.; Black, M.M.; Cook, J.T.; Faith, M.S.; et al. Caregiver feeding practices and child weight outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109, 990S–1002S. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.K.; Blake, C.E.; Blaine, R.E.; Younginer, N.A.; Orloski, A.; Hamtil, H.A.; Ganter, C.; Bruton, Y.P.; Vaughn, A.E.; Fisher, J.O. Parenting around child snacking: Development of a theoretically-guided, empirically informed conceptual model. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, J.C.; Mennella, J.A. Diet, sensitive periods in flavour learning, and growth. Int. Rev. Psychiatry 2012, 24, 219–230. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Formula Treatment Group | p-Value 1 | |
|---|---|---|---|
| CMF | EHF | ||
| Number of dyads | 44 | 39 | |
| Infants | |||
| Female | 21 (48%) | 20 (51%) | 0.75 |
| Race | 0.15 | ||
| Black | 25 (57%) | 22 (56%) | |
| White | 14 (32%) | 7 (18%) | |
| Other/more than one race | 5 (11%) | 10 (26%) | |
| Anthropometry, Z-scores | |||
| Weight for age | −0.24 ± 0.13 | −0.24 ± 0.13 | 1.00 |
| Length for age | −0.37 ± 0.16 | −0.43 ± 0.17 | 0.76 |
| Weight for length | −0.23 ± 0.14 | −0.14 ± 0.14 | 0.66 |
| Mothers | |||
| Age, years | 27.9 ± 0.9 | 27.3 ± 0.9 | 0.66 |
| Body Mass Index, kg/m2 | 31.6 ± 1.2 | 31.0 ± 1.3 | 0.73 |
| Parity, primiparous | 9 (20%) | 8 (21%) | 0.99 |
| Household income | 0.83 | ||
| <$35,000 | 32 (73%) | 26 (67%) | |
| $35,000–75,000 | 4 (9%) | 4 (10%) | |
| >$75,000 | 8 (18%) | 9 (23%) | |
| Education level | 0.14 | ||
| Primary school | 6 (14%) | 1 (3%) | |
| High school/technical school | 26 (59%) | 29 (74%) | |
| College degree or higher | 12 (27%) | 9 (23%) | |
| Data presented as n (%) or as mean ± standard error of mean (SEM). 1 p-values for main effect of infant formula treatment (CMF, cow milk formula, EHF, extensive protein hydrolysate formula) group. | |||
| Outcomes | Formula Treatment Group | p-Value 1 | |
|---|---|---|---|
| CMF | EHF | ||
| From 0.5 to 4.5 months | n = 44 | n = 39 | |
| Weight gain velocity (g/day) | 29.2 ± 0.9 | 26.1 ± 1.0 | 0.02 |
| Length gain velocity (cm/day) | 0.102 ± 0.002 | 0.104 ± 0.002 | 0.62 |
| From 4.5 months to 1 year | |||
| Weight gain velocity (g/day) | 12.5 ± 0.4 | 12.7 ± 0.5 | 0.71 |
| Length gain velocity (cm/day) | 0.050 ± 0.001 | 0.047 ± 0.001 | 0.14 |
| At 1 year | n = 44 | n = 39 | |
| Weight for Length Z (WLZ) | 0.78 ± 0.14 | 0.51 ± 0.17 | 0.20 |
| At 1.5 years | n = 40 | n = 38 | |
| Weight for Length Z (WLZ) | 0.80 ± 0.14 | 0.68 ± 0.17 | 0.57 |
| Body weight status 2 | 0.10 | ||
| Not overweight | 20 (50%) | 26 (68%) | |
| With overweight | 20 (50%) | 12 (32%) | |
| Data presented as mean ± standard error of mean (SEM) or as n (%). 1 p-values for main effect of infant formula treatment (CMF, cow milk formula; EHF, extensive protein hydrolysate) group for the children who remained in the trial until 1 year (n = 83) and then 1.5 years (n = 78). 2 Categories based on age- and sex-specific WLZ percentiles, which defines overweight as values greater than the 85th percentile (WLZ > 1.0364). | |||
| Category | Age of Toddlers | p-Value 1 | |
|---|---|---|---|
| 1 Year | 1.5 Years | ||
| Total daily intake | |||
| Energy, kcal/day | 1169 ± 49 | 1367 ± 57 | <0.001 |
| Sodium, mg/day | 1411 ± 74 | 2209 ± 120 | <0.001 |
| Added sugar 2, kcal/day | 64 ± 8 | 129 ± 13 | <0.001 |
| WWEIA food categories 3, kcal/day | |||
| Infant formula | 399 ± 42 | 9 ± 9 | <0.001 |
| Snacks and sweets | 67 ± 12 | 183 ± 27 | <0.001 |
| Sweetened beverages | 21 ± 5 | 77 ± 12 | <0.001 |
| Sugars | 9 ± 3 | 6 ± 12 | 0.34 |
| Fruit | 45 ± 6 | 66 ± 2 | 0.02 |
| 100% fruit juice | 39 ± 9 | 58 ± 8 | 0.07 |
| Percent daily energy, % | |||
| Added sugar | 5 ± 1 | 9 ± 15 | <0.001 |
| WWEIA food categories 2 | |||
| Infant formula | 33 ± 3 | 1 ± 1 | <0.001 |
| Snacks and sweets | 5 ± 1 | 12 ± 1 | <0.001 |
| Sweetened beverages | 2 ± 1 | 5 ± 1 | <0.001 |
| Sugars | 0.7 ± 0.2 | 0.4 ± 0.2 | 0.28 |
| Fruit | 4 ± 1 | 5 ± 1 | 0.30 |
| 100% fruit juice | 3 ± 1 | 4 ± 1 | 0.15 |
| Data presented as mean ± standard error of mean (SEM). 1 p-values for main effect of age; t-test for dependent samples. Number enrolled was n = 83 at 1 year and n = 78 at 1.5 years. Diet records were available for n = 67 toddlers at 1 year and n = 73 at 1.5 years. 2 Labeling requirement for “added sugars” is not required for infant formula, which has specific labeling regulations in 21 CFR 107.100 [32]. 3 Selected food categories from What We Eat in America (WWEIA) [29]. Snacks and sweets category includes baby-food snacks and sweets and fruit category includes baby-food fruit. | |||
| Model | Coefficient ± SEM | p-Value |
|---|---|---|
| Weight gain velocity, 0.5–4 months | 0.09 ± 0.02 | <0.001 |
| Allow Access 1 | −0.25 ± 0.27 | 0.35 |
| Visit | 0.67 ± 0.15 | <0.001 |
| Formula treatment group 2 | −0.09 ± 1.30 | 0.95 |
| Weight gain velocity, 0.5–4 months × visit | −0.01 ± 0.00 | <0.001 |
| Allow Access × visit | −0.09 ± 0.04 | <0.001 |
| Formula treatment group × visit | −0.01 ± 0.04 | 0.83 |
| Weight gain velocity × formula treatment group | 0.01 ± 0.03 | 0.64 |
| Allow Access × formula treatment group | −0.08 ± 0.33 | 0.82 |
| 1 The Allow Access construct of the Toddler Snack Food Feeding Questionnaire measures how much mothers allow their children access to sweets and snacks [20]. 2 Reference group, CMF (cow milk formula) treatment group. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennella, J.A.; Smethers, A.D.; Decker, J.E.; Delahanty, M.T.; Stallings, V.A.; Trabulsi, J.C. Effects of Early Weight Gain Velocity, Diet Quality, and Snack Food Access on Toddler Weight Status at 1.5 Years: Follow-Up of a Randomized Controlled Infant Formula Trial. Nutrients 2021, 13, 3946. https://doi.org/10.3390/nu13113946
Mennella JA, Smethers AD, Decker JE, Delahanty MT, Stallings VA, Trabulsi JC. Effects of Early Weight Gain Velocity, Diet Quality, and Snack Food Access on Toddler Weight Status at 1.5 Years: Follow-Up of a Randomized Controlled Infant Formula Trial. Nutrients. 2021; 13(11):3946. https://doi.org/10.3390/nu13113946
Chicago/Turabian StyleMennella, Julie A., Alissa D. Smethers, Jessica E. Decker, Michelle T. Delahanty, Virginia A. Stallings, and Jillian C. Trabulsi. 2021. "Effects of Early Weight Gain Velocity, Diet Quality, and Snack Food Access on Toddler Weight Status at 1.5 Years: Follow-Up of a Randomized Controlled Infant Formula Trial" Nutrients 13, no. 11: 3946. https://doi.org/10.3390/nu13113946
APA StyleMennella, J. A., Smethers, A. D., Decker, J. E., Delahanty, M. T., Stallings, V. A., & Trabulsi, J. C. (2021). Effects of Early Weight Gain Velocity, Diet Quality, and Snack Food Access on Toddler Weight Status at 1.5 Years: Follow-Up of a Randomized Controlled Infant Formula Trial. Nutrients, 13(11), 3946. https://doi.org/10.3390/nu13113946







