The Impact of Vegan and Vegetarian Diets on Physical Performance and Molecular Signaling in Skeletal Muscle
Abstract
1. Introduction
2. Properties of Vegetarian and Vegan Diets
2.1. Differences in Macronutrients between Diets
2.2. Differences in Micronutrients between Diets
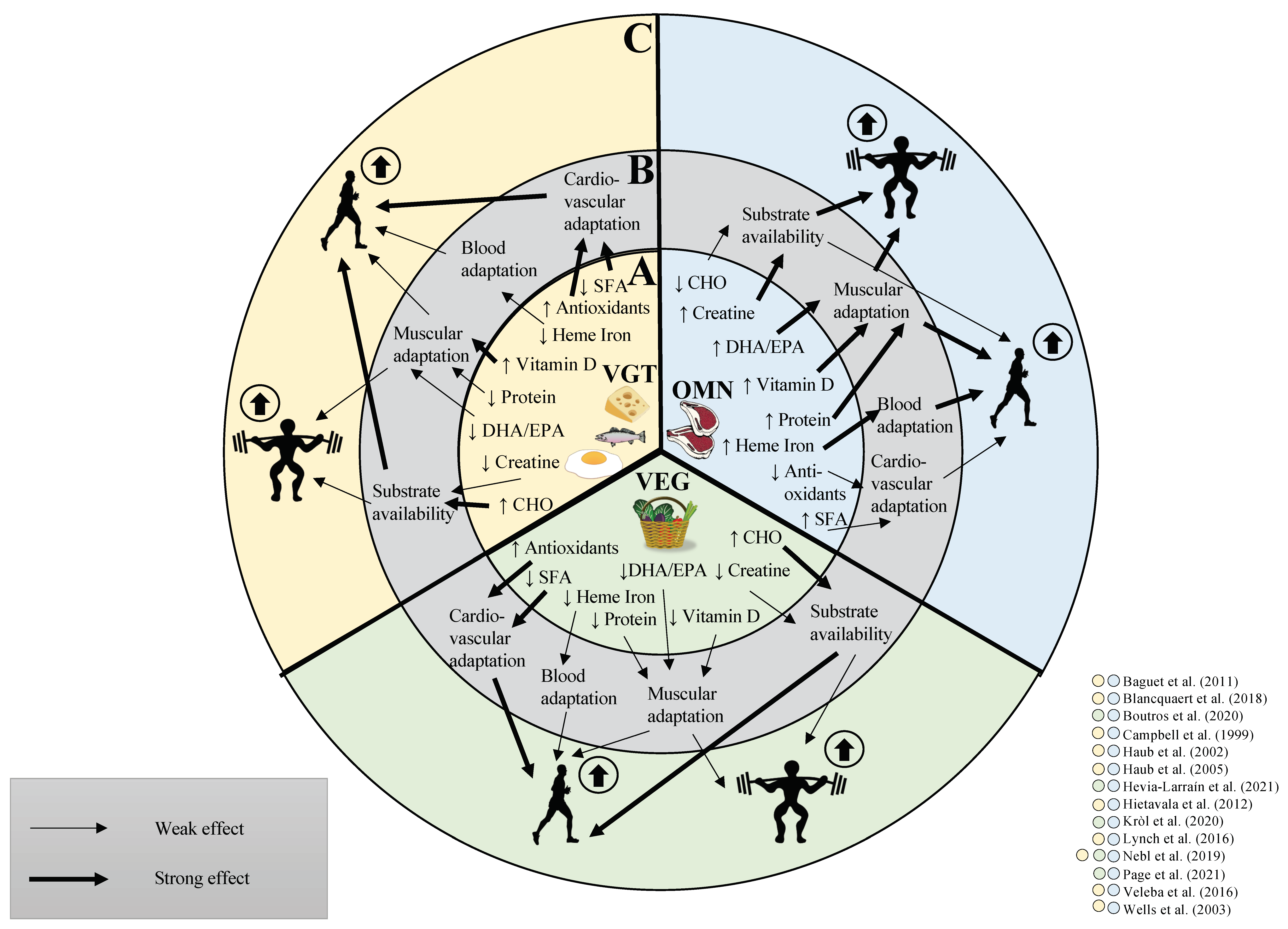
3. Do Vegetarian and Vegan Diets Affect Exercise Performance
4. Vegan and Vegetarian Diet and Endurance Performance
4.1. Factors That May Affect Endurance Performance Differently between Diets
4.2. Differences in Substrate Availability between Vegan or Vegetarian and Omnivorous Diets May Affect Endurance Performance
4.3. Evidences on Vegetarian and Vegan Diets and Endurance Performance
5. Vegan and Vegetarian Diets and Strength Performance
5.1. Properties of Strength Performance
5.2. Nutritional Aspects and Strength Performance
5.3. Vitamin D and Strength Performance
5.4. Evidences on Vegetarian and Vegan Diets and Strength Performance
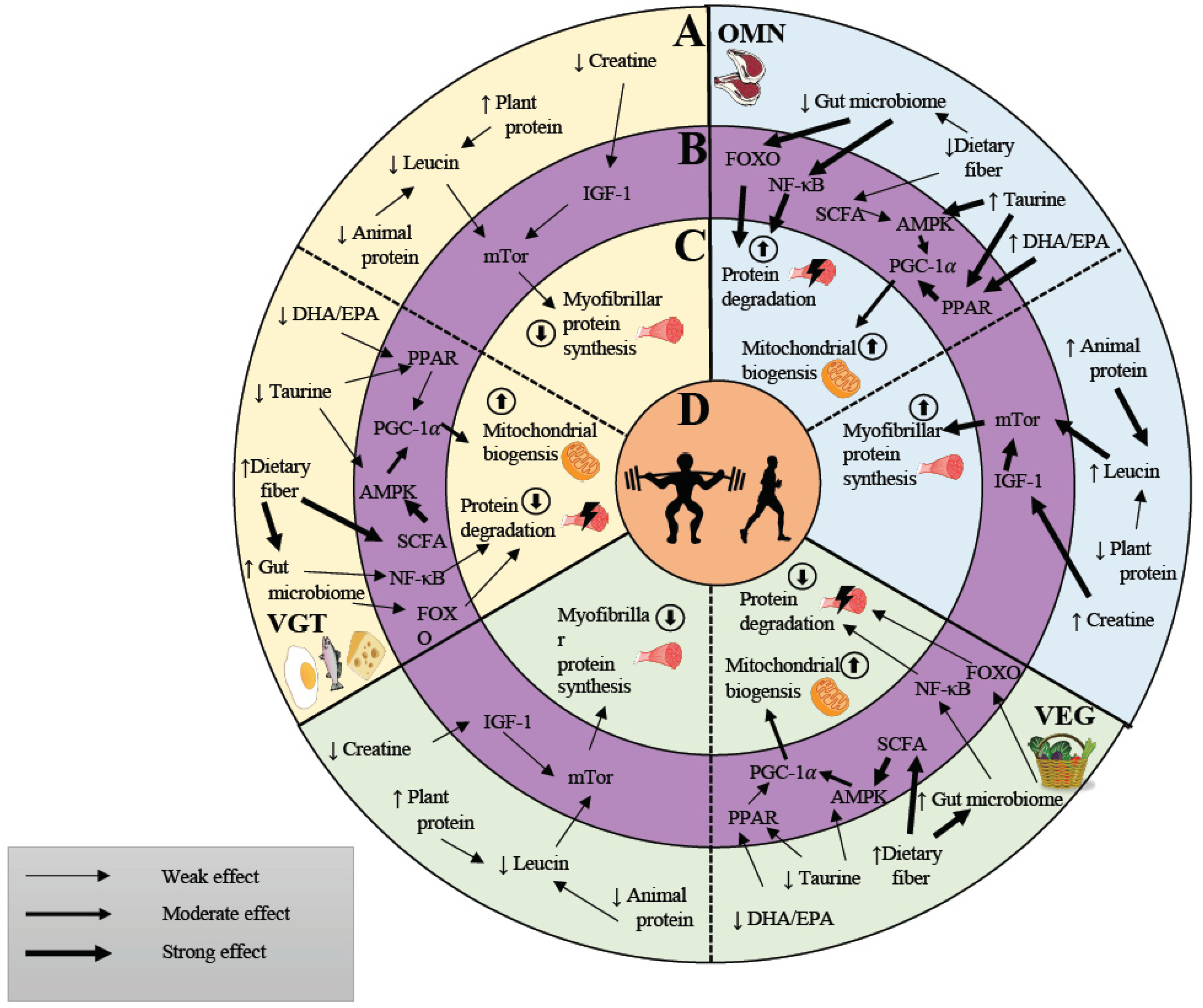
6. Vegan, Vegetarian and Omnivorous Diets May Affect Molecular Regulators of Exercise Adaptation in Human Skeletal Muscle
6.1. Proteins and Amino Acids and Their Impact on Molecular Signaling
6.2. Creatine and Its Impact on Molecular Signaling
6.3. Vitamin D and Its Impact on Molecular Signaling
6.4. Polyunsaturated Fatty Acids May Augment Skeletal Muscle Adaptation in Response to Exercise
7. Influence of Diet on the Microbiome and Its Effect on Exercise Performance and Basal Molecular Signaling
8. Summary and Future Directions of Research
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Lundsgaard, A.-M.; Kiens, B. Dietary Fuels in Athletic Performance. Annu. Rev. Nutr. 2019, 39, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient Profiles of Vegetarian and Nonvegetarian Dietary Patterns. J. Acad. Nutr. Diet 2013, 113, 1610–1619. [Google Scholar] [CrossRef]
- Craddock, J.C.; Probst, Y.C.; Peoples, G.E. Vegetarian and Omnivorous Nutrition—Comparing Physical Performance. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Cock, H.R.; Brex, P.; Giovannoni, G. Vitamin D supplementation. Pract. Neurol. 2018, 18, 35–42. [Google Scholar] [CrossRef]
- Atkinson, S.A.; Fleet, J.C. Canadian recommendations for vitamin D intake for persons affected by multiple sclerosis. J. Steroid Biochem. Mol. Biol. 2020, 199, 105606. [Google Scholar] [CrossRef]
- Zupo, R.; Lampignano, L.; Lattanzio, A.; Mariano, F.; Osella, A.R.; Bonfiglio, C.; Giannelli, G.; De Pergola, G. Association between adherence to the Mediterranean Diet and circulating Vitamin D levels. Int. J. Food Sci. Nutr. 2020, 71, 884–890. [Google Scholar] [CrossRef]
- Kushi, L.H.; Lenart, E.B.; Willett, W.C. Health implications of Mediterranean diets in light of contemporary knowledge. 1. Plant foods and dairy products. Am. J. Clin. Nutr. 1995, 61, 1407S–1415S. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Vu, B.Q.; Lai, T.Q.; Nguyen, N.D.; Nguyen, T.V. Vegetarianism, bone loss, fracture and vitamin D: A longitudinal study in Asian vegans and non-vegans. Eur. J. Clin. Nutr. 2012, 66, 75–82. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC–Oxford:lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Candow, D.G.; Mahoney, D.; Tarnopolsky, M. Effect of Creatine and Weight Training on Muscle Creatine and Performance in Vegetarians. Med. Sci. Sports Exerc. 2003, 35, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef]
- Solis, M.Y.; Painelli, V.D.S.; Artioli, G.G.; Roschel, H.; Otaduy, M.C.; Gualano, B. Brain creatine depletion in vegetarians? A cross-sectional 1H-magnetic resonance spectroscopy (1H-MRS) study. Br. J. Nutr. 2014, 111, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R. American Dietetic Association Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Schuchardt, J.P.; Ströhle, A.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Tegtbur, U.; Hahn, A. Micronutrient Status of Recreational Runners with Vegetarian or Non-Vegetarian Dietary Patterns. Nutrients 2019, 11, 1146. [Google Scholar] [CrossRef]
- Bassett, D.R. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- Montero, D.; Cathomen, A.; Jacobs, R.; Flück, D.; De Leur, J.; Keiser, S.; Bonne, T.; Kirk, N.; Lundby, A.-K.; Lundby, C. Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training. J. Physiol. 2015, 593, 4677–4688. [Google Scholar] [CrossRef]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. Compr. Physiol. 2015, 6, 1–32. [Google Scholar] [CrossRef]
- Gojda, J.; Patková, J.; Jaček, M.; Potočková, J.; Trnka, J.; Kraml, P.; Andel, M. Higher insulin sensitivity in vegans is not associated with higher mitochondrial density. Eur. J. Clin. Nutr. 2013, 67, 1310–1315. [Google Scholar] [CrossRef]
- Fotsis, T.; Pepper, M.; Adlercreutz, H.; Fleischmann, G.; Hase, T.; Montesano, R.; Schweigerer, L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc. Natl. Acad. Sci. USA 1993, 90, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Lundby, C.; Koskolou, M.; Boushel, R. Importance of hemoglobin concentration to exercise: Acute manipulations. Respir. Physiol. Neurobiol. 2006, 151, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Berger, J.; Hines, I. Iron Status of Vegetarian Adults: A Review of Literature. Am. J. Lifestyle Med. 2018, 12, 486–498. [Google Scholar] [CrossRef]
- Król, W.; Price, S.; Śliż, D.; Parol, D.; Konopka, M.; Mamcarz, A.; Wełnicki, M.; Braksator, W. A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography. Diagnostics 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.L.; Galeotti, G.G.; Terlizzese, G.; Fabiani, I.; Pugliese, N.R.; Rovai, I. Left Ventricular Mass and Thickness. Hear. Fail. Clin. 2019, 15, 159–166. [Google Scholar] [CrossRef]
- Tuso, P. A Plant-Based Diet, Atherogenesis, and Coronary Artery Disease Prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef]
- Fisher, N.D.; Hurwitz, S.; Hollenberg, N.K. Habitual flavonoid intake and endothelial function in healthy humans. J. Am. Coll. Nutr. 2012, 31, 275–279. [Google Scholar] [CrossRef]
- Rendell, M.; Anderson, E.; Schlueter, W.; Mailliard, J.; Honigs, D.; Rosenthal, R. Determination of hemoglobin levels in the finger using near infrared spectroscopy. Int. J. Lab. Hematol. 2003, 25, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Gregory, I.C. The oxygen and carbon monoxide capacities of foetal and adult blood. J. Physiol. 1974, 236, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S. Iron and the endurance athlete. Appl. Physiol. Nutr. Metab. 2014, 39, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, A.; Luty, J.; Aleksandrowicz-Wrona, E.; Małgorzewicz, S. Iron status and dietary iron intake in vegetarians. Adv. Clin. Exp. Med. 2018, 27, 1383–1389. [Google Scholar] [CrossRef]
- Bouri, S.; Martin, J. Investigation of iron deficiency anaemia. Clin. Med. 2018, 18, 242–244. [Google Scholar] [CrossRef]
- Monsen, E.R. Iron nutrition and absorption: Dietary factors which impact iron bioavailability. J. Am. Diet. Assoc. 1988, 88, 786–790. [Google Scholar] [CrossRef]
- Rana, P.; Marwaha, R.K.; Kumar, P.; Narang, A.; Devi, M.M.; Tripathi, R.P.; Khushu, S. Effect of vitamin D supplementation on muscle energy phospho-metabolites: a31P magnetic resonance spectroscopy-based pilot study. Endocr. Res. 2014, 39, 152–156. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the Vitamin D Status of Vitamin D Deficient Adults Is Associated with Improved Mitochondrial Oxidative Function in Skeletal Muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef] [PubMed]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.; et al. Influence of Vitamin D Supplementation by Sunlight or Oral D3 on Exercise Performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Baguet, A.; Everaert, I.; De Naeyer, H.; Reyngoudt, H.; Stegen, S.; Beeckman, S.; Achten, E.; Vanhee, L.; Volkaert, A.; Petrovic, M.; et al. Effects of sprint training combined with vegetarian or mixed diet on muscle carnosine content and buffering capacity. Eur. J. Appl. Physiol. 2011, 111, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Varanoske, A.; Stout, J.R. Effects of β-Alanine Supplementation on Carnosine Elevation and Physiological Performance. Adv. Food Nutr. Res. 2018, 84, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; Lamacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports Nutr. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Painelli, V.D.S.; de Oliveira, L.F.; Silva, V.D.E.; da Silva, R.P.; Riani, L.; Franchi, M.; Gonçalves, L.; Harris, R.C.; Roschel, H.; et al. Twenty-four Weeks of β-Alanine Supplementation on Carnosine Content, Related Genes, and Exercise. Med. Sci. Sports Exerc. 2017, 49, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Gray, M.; Stewart, R.W.; Moyen, N.E.; Kavouras, S.; DiBrezzo, R.; Turner, R.; Baum, J.I.; Stone, M.S. Effects of 28-Day Beta-Alanine Supplementation on Isokinetic Exercise Performance and Body Composition in Female Masters Athletes. J. Strength Cond. Res. 2016, 30, 200–207. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef]
- Hawley, J.A.; Leckey, J.J. Carbohydrate Dependence During Prolonged, Intense Endurance Exercise. Sports Med. 2015, 45, 5–12. [Google Scholar] [CrossRef]
- Nieman, D.C. Vegetarian dietary practices and endurance performance. Am. J. Clin. Nutr. 1988, 48, 754–761. [Google Scholar] [CrossRef]
- Van Loon, L.J.C.; Greenhaff, P.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Hoppeler, H.; Weibel, E.R. Limits for oxygen and substrate transport in mammals. J. Exp. Biol. 1998, 201, 1051–1064. [Google Scholar] [CrossRef]
- Bergman, B.C.; Butterfield, G.E.; Wolfel, E.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. 1999, 277, E81–E92. [Google Scholar] [CrossRef]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of Acute Carbohydrate Supplementation on Endurance Performance. Sports Med. 2011, 41, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-H.; Paik, I.-Y.; Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar] [PubMed]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Lynch, H.M.; Wharton, C.M.; Johnston, C.S. Cardiorespiratory Fitness and Peak Torque Differences between Vegetarian and Omnivore Endurance Athletes: A Cross-Sectional Study. Nutrients 2016, 8, 726. [Google Scholar] [CrossRef]
- Boutros, G.H.; Landry-Duval, M.-A.; Garzon, M.; Karelis, A.D. Is a vegan diet detrimental to endurance and muscle strength? Eur. J. Clin. Nutr. 2020, 74, 1550–1555. [Google Scholar] [CrossRef]
- Page, J.; Erskine, R.M.; Hopkins, N.D. Skeletal muscle properties and vascular function do not differ between healthy, young vegan and omnivorous men. Eur. J. Sport Sci. 2021, 1–10. [Google Scholar] [CrossRef]
- Blancquaert, L.; Baguet, A.; Bex, T.; Volkaert, A.; Everaert, I.; Delanghe, J.; Petrovic, M.; Vervaet, C.; De Henauw, S.; Constantin-Teodosiu, D.; et al. Changing to a vegetarian diet reduces the body creatine pool in omnivorous women, but appears not to affect carnitine and carnosine homeostasis: A randomised trial. Br. J. Nutr. 2018, 119, 759–770. [Google Scholar] [CrossRef]
- Hietavala, E.-M.; Puurtinen, R.; Kainulainen, H.; Mero, A.A. Low-protein vegetarian diet does not have a short-term effect on blood acid–base status but raises oxygen consumption during submaximal cycling. J. Int. Soc. Sports Nutr. 2012, 9, 50. [Google Scholar] [CrossRef]
- Veleba, J.; Matoulek, M.; Hill, M.; Pelikanova, T.; Kahleova, H. “A Vegetarian vs. Conventional Hypocaloric Diet: The Effect on Physical Fitness in Response to Aerobic Exercise in Patients with Type 2 Diabetes.” A Parallel Randomized Study. Nutrients 2016, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Hevia-Larraín, V.; Gualano, B.; Longobardi, I.; Gil, S.; Fernandes, A.L.; Costa, L.A.R.; Pereira, R.M.R.; Artioli, G.G.; Phillips, S.M.; Roschel, H. High-Protein Plant-Based Diet Versus a Protein-Matched Omnivorous Diet to Support Resistance Training Adaptations: A Comparison Between Habitual Vegans and Omnivores. Sports Med. 2021, 51, 1317–1330. [Google Scholar] [CrossRef]
- Wells, A.M.; Haub, M.D.; Fluckey, J.; Williams, D.K.; Chernoff, R.; Campbell, W.W. Comparisons of vegetarian and beef-containing diets on hematological indexes and iron stores during a period of resistive training in older men. J. Am. Diet Assoc. 2003, 103, 594–601. [Google Scholar] [CrossRef]
- Haub, M.D.; Wells, A.M.; Campbell, W.W. Beef and soy-based food supplements differentially affect serum lipoprotein-lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive-train. Metabolism 2005, 54, 769–774. [Google Scholar] [CrossRef]
- Haub, M.D.; Wells, A.M.; Tarnopolsky, M.A.; Campbell, W.W. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 2002, 76, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Barton, M.L.; Cyr-Campbell, D.; Davey, S.L.; Beard, J.L.; Parise, G.; Evans, W.J. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am. J. Clin. Nutr. 1999, 70, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Haufe, S.; Eigendorf, J.; Wasserfurth, P.; Tegtbur, U.; Hahn, A. Exercise capacity of vegan, lacto-ovo-vegetarian and omnivorous recreational runners. J. Int. Soc. Sports Nutr. 2019, 16, 23. [Google Scholar] [CrossRef]
- Bruce, R.A.; Blackmon, J.R.; Jones, J.W.; Strait, G. Exercising Testing in Adult Normal Subjects and Cardiac Patients. Ann. Noninvasive Electrocardiol. 2004, 9, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Stone, M.H. The Importance of Muscular Strength in Athletic Performance. Sports Med. 2016, 46, 1419–1449. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 1–13. [Google Scholar] [CrossRef]
- Grassi, B. Delayed Metabolic Activation of Oxidative Phosphorylation in Skeletal Muscle at Exercise Onset. Med. Sci. Sports Exerc. 2005, 37, 1567–1573. [Google Scholar] [CrossRef]
- Gastin, P.B. Energy System Interaction and Relative Contribution During Maximal Exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Karatzaferi, C.; De Haan, A.; Ferguson, R.; Van Mechelen, W.; Sargeant, A. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflügers Arch. 2001, 442, 467–474. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Daniels, J.; Evans, W.; Fink, W.; Krahenbuhl, G.; Saltin, B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J. Appl. Physiol. 1976, 40, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.; Karandreas, N.; Spengos, K.; Zaras, N.; Stasinaki, A.-N.; Terzis, G. Muscle Fiber Conduction Velocity, Muscle Fiber Composition, and Power Performance. Med. Sci. Sports Exerc. 2016, 48, 1761–1771. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Horio, Y.; Sakuma, K.; Katsuta, S. The effect of nutrition on the size and proportion of muscle fibre types during growth. J. Anat. 1993, 182, 29–36. [Google Scholar] [PubMed]
- Pensini, M.; Martin, A.; Maffiuletti, N.A. Central Versus Peripheral Adaptations Following Eccentric Resistance Training. Int. J. Sports Med. 2002, 23, 567–574. [Google Scholar] [CrossRef]
- Stragier, S.; Baudry, S.; Poortmans, J.; Duchateau, J.; Carpentier, A. Leucine-enriched protein supplementation does not influence neuromuscular adaptations in response to a 6-month strength training programme in older adults. Exp. Gerontol. 2016, 82, 58–66. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 1–21. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 1–57. [Google Scholar] [CrossRef]
- Salvador, A.F.; Askow, A.T.; Mckenna, C.F.; Fang, H.-Y.; Burke, S.K.; Li, Z.; Ulanov, A.V.; Paluska, S.A.; Petruzzello, S.J.; Boppart, M.D.; et al. Resistance Exercise–induced Regulation of Muscle Protein Synthesis to Intraset Rest. Med. Sci. Sports Exerc. 2020, 52, 1022–1030. [Google Scholar] [CrossRef]
- Joanisse, S.; Lim, C.; McKendry, J.; McLeod, J.C.; Stokes, T.; Phillips, S.M. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Research 2020, 9, 141. [Google Scholar] [CrossRef]
- Pennings, B.; Koopman, R.; Beelen, M.; Senden, J.M.G.; Saris, W.H.M.; van Loon, L.J. Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am. J. Clin. Nutr. 2011, 93, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Church, D.D.; Azhar, G.; Schutzler, S.E.; Ferrando, A.A.; Wolfe, R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.; Ferrando, A.A.; Phillips, S.; Doyle, D.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. Metab. 1999, 276, E628–E634. [Google Scholar] [CrossRef]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.; Senden, J.M.; Gijsen, A.P.; Verdijk, L.; van Loon, L.J. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef]
- Symons, T.B.; Schutzler, S.E.; Cocke, T.L.; Chinkes, D.L.; Wolfe, R.R.; Paddon-Jones, D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007, 86, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Symonsi, T.B.; Sheffield-Moore, M.; Mamerow, M.M.; Wolfe, R.R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Moore, D.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.; Tipton, K.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. Ser. Boil. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Ciuris, C.; Lynch, H.M.; Wharton, C.; Johnston, C.S. A Comparison of Dietary Protein Digestibility, Based on DIAAS Scoring, in Vegetarian and Non-Vegetarian Athletes. Nutrients 2019, 11, 3016. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.M.; Trommelen, J.; Snijders, T.; van Loon, L.J.C. The Anabolic Response to Plant-Based Protein Ingestion. Sports Med. 2021, 1–16. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Horstman, A.M.H.; Franssen, R.; Crombag, J.J.R.; Langer, H.; Bierau, J.; Respondek, F.; Van Loon, L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef]
- Chesdachai, S.; Tangpricha, V. Treatment of vitamin D deficiency in cystic fibrosis. J. Steroid Biochem. Mol. Biol. 2016, 164, 36–39. [Google Scholar] [CrossRef]
- Capiati, D.; Benassati, S.; Boland, R.L. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J. Cell. Biochem. 2002, 86, 128–135. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.; Hamrick, M.W.; Holick, M.; Gunton, J.E. The Roles of Vitamin D in Skeletal Muscle: Form, Function, and Metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Gerdhem, P.; Ringsberg, K.A.M.; Obrant, K.J.; Akesson, K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos. Int. 2005, 16, 1425–1431. [Google Scholar] [CrossRef]
- Marantes, I.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J.; Amin, S. Is vitamin D a determinant of muscle mass and strength? J. Bone Miner. Res. 2011, 26, 2860–2871. [Google Scholar] [CrossRef]
- Han, Q.; Li, X.; Tan, Q.; Shao, J.; Yi, M. Effects of vitamin D3 supplementation on serum 25(OH)D concentration and strength in athletes: A systematic review and meta-analysis of randomized controlled trials. J. Int. Soc. Sports Nutr. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Quan, M.; Cao, Z.-B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Freyssenet, D.; Berthon, P.; Denis, C. Mitochondrial Biogenesis in Skeletal Muscle in Response to Endurance Exercises. Arch. Physiol. Biochem. 1996, 104, 129–141. [Google Scholar] [CrossRef]
- Gavin, T.P. Basal and Exercise-Induced Regulation of Skeletal Muscle Capillarization. Exerc. Sport Sci. Rev. 2009, 37, 86–92. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, C.K.; Kim, H.J. The effect of resistance and endurance exercise training on muscle proteome expression in human skeletal muscle. Biol. Sport 2012, 29, 121–127. [Google Scholar] [CrossRef]
- Huffman, K.M.; Koves, T.; Hubal, M.; Abouassi, H.; Beri, N.; Bateman, L.A.; Stevens, R.D.; Ilkayeva, O.R.; Hoffman, E.; Muoio, D.; et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 2014, 57, 2282–2295. [Google Scholar] [CrossRef]
- Morville, T.; Sahl, R.E.; Moritz, T.; Helge, J.W.; Clemmensen, C. Plasma Metabolome Profiling of Resistance Exercise and Endurance Exercise in Humans. Cell Rep. 2020, 33, 108554. [Google Scholar] [CrossRef]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.B.; Puig, L.S.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Ho, J.T.K.; Chan, G.C.F.; Li, J.C.B. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 2015, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.-E.; Kang, D.-W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Aguirre, N.; van Loon, L.J.C.; Baar, K. The Role of Amino Acids in Skeletal Muscle Adaptation to Exercise. Nestle Nutr. Inst. Workshop Ser. 2013, 76, 85–102. [Google Scholar] [CrossRef]
- Rindom, E.; Vissing, K. Mechanosensitive Molecular Networks Involved in Transducing Resistance Exercise-Signals into Muscle Protein Accretion. Front. Physiol. 2016, 7, 547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- How the Amino Acid Leucine Activates the Key Cell-Growth Regulator mTOR. Available online: https://www.nature.com/articles/d41586-021-01943-7 (accessed on 28 July 2021).
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef]
- Roberson, P.A.; Mobley, C.B.; Romero, M.A.; Haun, C.T.; Osburn, S.C.; Mumford, P.W.; Vann, C.G.; Greer, R.A.; Ferrando, A.A.; Roberts, M.D. LAT1 Protein Content Increases Following 12 Weeks of Resistance Exercise Training in Human Skeletal Muscle. Front. Nutr. 2021, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Hartman, J.W.; Wilkinson, S.B. Dietary Protein to Support Anabolism with Resistance Exercise in Young Men. J. Am. Coll. Nutr. 2005, 24, 134S–139S. [Google Scholar] [CrossRef]
- Koopman, R.; van Loon, L.J. Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Bopp, M.J.; Houston, D.K.; Lenchik, L.; Easter, L.; Kritchevsky, S.B.; Nicklas, B.J. Lean Mass Loss Is Associated with Low Protein Intake during Dietary-Induced Weight Loss in Postmenopausal Women. J. Am. Diet Assoc. 2008, 108, 1216–1220. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef]
- Mettler, S.; Mitchell, N.; Tipton, K. Increased Protein Intake Reduces Lean Body Mass Loss during Weight Loss in Athletes. Med. Sci. Sports Exerc. 2010, 42, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Guerra, V.; Donghia, R.; Bortone, I.; Griseta, C.; Lampignano, L.; Dibello, V.; Lozupone, M.; Coelho-Júnior, H.J.; et al. Associations between Nutritional Frailty and 8-Year All-Cause Mortality in Older Adults: The Salus in Apulia Study. J. Intern. Med. 2021, 290, 1071–1082. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Bortone, I.; Griseta, C.; Sardone, R.; Lampignano, L.; Lozupone, M.; Solfrizzi, V.; Castellana, M.; Giannelli, G.; et al. Nutritional domains in frailty tools: Working towards an operational definition of nutritional frailty. Ageing Res. Rev. 2020, 64, 101148. [Google Scholar] [CrossRef]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Phillips, S.M. Dietary protein requirements and adaptive advantages in athletes. Br. J. Nutr. 2012, 108, S158–S167. [Google Scholar] [CrossRef]
- Phillips, S.M.; van Loon, L.J. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29, S29–S38. [Google Scholar] [CrossRef]
- Kerksick, C.; Jagim, A.; Hagele, A.; Jäger, R. Plant Proteins and Exercise: What Role Can Plant Proteins Have in Promoting Adaptations to Exercise? Nutrients 2021, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.; Soeters, P.B.; Boirie, Y.; Deutz, N.E. Differential metabolic effects of casein and soy protein meals on skeletal muscle in healthy volunteers. Clin. Nutr. 2011, 30, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein After Workout on Body Composition and Muscle Performance. J. Strength Cond. Res. 2018, 32, 2233–2242. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E.P.; Jäkel, M.; Soeters, P.B. Casein and Soy Protein Meals Differentially Affect Whole-Body and Splanchnic Protein Metabolism in Healthy Humans. J. Nutr. 2005, 135, 1080–1087. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.; Macdonald, J.R.; Armstrong, D.; Phillips, S. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Yuan, L.-J.; Yang, Y.; Zhang, M.; Chen, W.-F. IGF-1 inhibits MPTP/MPP+-induced autophagy on dopaminergic neurons through the IGF-1R/PI3k-Akt-mTOR pathway and GPER. Am. J. Physiol. Metab. 2020, 319, E734–E743. [Google Scholar] [CrossRef]
- Louis, M.; Van Beneden, R.; Dehoux, M.; Thissen, J.P.; Francaux, M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C2C12cells. FEBS Lett. 2004, 557, 243–247. [Google Scholar] [CrossRef]
- Deldicque, L.; Louis, M.; Theisen, D.; Nielens, H.; Dehoux, M.; Thissen, J.-P.; Rennie, M.J.; Francaux, M. Increased IGF mRNA in Human Skeletal Muscle after Creatine Supplementation. Med. Sci. Sports Exerc. 2005, 37, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; Candow, D.G.; Chilibeck, P.D.; MacNeil, L.G.; Roy, B.D.; Tarnopolsky, M.A.; Ziegenfuss, T. Effect of Creatine Supplementation and Resistance-Exercise Training on Muscle Insulin-Like Growth Factor in Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 389–398. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef]
- Simpson, R.U.; Thomas, G.A.; Arnold, A.J. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985, 260, 8882–8891. [Google Scholar] [CrossRef]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; Pablo, G.S. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Zhao, M.; Nie, Y.; Liu, P.; Zhu, Y.; Zhang, X. Skeletal Muscle Lipid Droplets and the Athlete’s Paradox. Cells 2019, 8, 249. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.P.; Frais, F.F. Changes in Plasmalogen Content of Human Heart and Skeletal Muscle with Age and Development. Nature 1967, 215, 993–994. [Google Scholar] [CrossRef]
- Khalil, M.B.; Hou, W.; Zhou, H.; Elisma, F.; Swayne, L.A.; Blanchard, A.P.; Yao, Z.; Bennett, S.A.; Figeys, D. Lipidomics era: Accomplishments and challenges. Mass Spectrom. Rev. 2010, 29, 877–929. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, C.G. Obese super athletes: Fat-fueled migration in birds and bats. J. Exp. Biol. 2018, 221, jeb165753. [Google Scholar] [CrossRef] [PubMed]
- Driedzic, W.R.; Crowe, H.L.; Hicklin, P.W.; Sephton, D.H. Adaptations in pectoralis muscle, heart mass, and energy metabolism during premigratory fattening in semipalmated sandpipers (Calidris pusilla). Can. J. Zool. 1993, 71, 1602–1608. [Google Scholar] [CrossRef]
- Nagahuedi, S.; Popesku, J.T.; Trudeau, V.L.; Weber, J.M. Mimicking the natural doping of migrant sandpipers in sedentary quails: Effects of dietary n-3 fatty acids on muscle membranes and PPAR expression. J. Exp. Biol. 2009, 212, 1106–1114. Available online: https://journals.biologists.com/jeb/article/212/8/1106/19107/Mimicking-the-natural-doping-of-migrant-sandpipers (accessed on 28 July 2021). [CrossRef] [PubMed]
- Weber, J.-M.; Cruz, S.; Shiao, J.-C.; Liao, B.-K.; Huang, C.-J.; Hwang, P.-P. The physiology of long-distance migration: Extending the limits of endurance metabolism. J. Exp. Biol. 2009, 212, 593–597. [Google Scholar] [CrossRef]
- Phua, W.W.T.; Wong, M.X.Y.; Liao, Z.; Tan, N.S. An aPPARent Functional Consequence in Skeletal Muscle Physiology via Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2018, 19, 1425. [Google Scholar] [CrossRef]
- Ehrenborg, E.; Krook, A. Regulation of Skeletal Muscle Physiology and Metabolism by Peroxisome Proliferator-Activated Receptor δ. Pharmacol. Rev. 2009, 61, 373–393. [Google Scholar] [CrossRef]
- Hondares, E.; Torra, I.P.; Iglesias, R.; Staels, B.; Villarroya, F.; Giralt, M. PPARδ, but not PPARα, activates PGC-1α gene transcription in muscle. Biochem. Biophys. Res. Commun. 2007, 354, 1021–1027. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, Y.; Zhang, Y.; Zhao, J.; Chang, F.; Lu, T.; Zhang, R.; Li, Q.; Hu, X.; Li, N. Different effects of omega-3 fatty acids on the cell cycle in C2C12 myoblast proliferation. Mol. Cell. Biochem. 2012, 367, 165–173. [Google Scholar] [CrossRef]
- Hsueh, T.-Y.; Baum, J.I.; Huang, Y. Effect of Eicosapentaenoic Acid and Docosahexaenoic Acid on Myogenesis and Mitochondrial Biosynthesis during Murine Skeletal Muscle Cell Differentiation. Front. Nutr. 2018, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, Y.; Hu, X.; Peng, X.; Wei, H.; Peng, J.; Jiang, S. Activation of PPARγ2 by PPARγ1 through a functional PPRE in transdifferentiation of myoblasts to adipocytes induced by EPA. Cell Cycle 2015, 14, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, L.M.; De Carvalho, S.C.; Neto, H.S.; Marques, M.J. Long-Term Therapy With Omega-3 Ameliorates Myonecrosis and Benefits Skeletal Muscle Regeneration inMdxMice. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2015, 298, 1589–1596. [Google Scholar] [CrossRef]
- Saini, A.; Sharples, A.P.; Al-Shanti, N.; Stewart, C.E. Omega-3 fatty acid EPA improves regenerative capacity of mouse skeletal muscle cells exposed to saturated fat and inflammation. Biogerontology 2017, 18, 109–129. [Google Scholar] [CrossRef]
- Isesele, P.O.; Mazurak, V.C. Regulation of Skeletal Muscle Satellite Cell Differentiation by Omega-3 Polyunsaturated Fatty Acids: A Critical Review. Front. Physiol. 2021, 12, 682091. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016, 4, e12715. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. [Google Scholar] [CrossRef]
- Boss, A.; Lecoultre, V.; Ruffieux, C.; Tappy, L.; Schneiter, P. Combined effects of endurance training and dietary unsaturated fatty acids on physical performance, fat oxidation and insulin sensitivity. Br. J. Nutr. 2010, 103, 1151–1159. [Google Scholar] [CrossRef]
- Raastad, T.; Hastmark, A.T.; Strømme, S.B. Omega-3 fatty acid supplementation does not improve maximal aerobic power, anaerobic threshold and running performance in well-trained soccer players. Scand. J. Med. Sci. Sports 1997, 7, 25–31. [Google Scholar] [CrossRef]
- Buckley, J.D.; Burgess, S.; Murphy, K.J.; Howe, P.R. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J. Sci. Med. Sport 2009, 12, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshikawa, N.; Schaffer, S.W.; Azuma, J. Tissue Taurine Depletion Alters Metabolic Response to Exercise and Reduces Running Capacity in Mice. J. Amino Acids 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Borck, P.C.; Vettorazzi, J.F.; Branco, R.C.S.; Batista, T.M.; Santos-Silva, J.C.; Nakanishi, V.Y.; Boschero, A.C.; Ribeiro, R.A.; Carneiro, E.M. Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 2018, 50, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Hood, D.A. Mechanisms of Exercise-Induced Mitochondrial Biogenesis in Skeletal Muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 465–472. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.M.; Kennedy, R.G. Gastrointestinal Problems in Runners. Curr. Sports Med. Rep. 2004, 3, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal Muscle FOXO1 (FKHR) Transgenic Mice Have Less Skeletal Muscle Mass, Down-regulated Type I (Slow Twitch/Red Muscle) Fiber Genes, and Impaired Glycemic Control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Reed, S.A.; Sandesara, P.B.; Senf, S.M.; Judge, A.R. Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J. 2012, 26, 987–1000. [Google Scholar] [CrossRef]
- Cai, D.; Frantz, J.; Tawa, N.E.; Melendez, P.A.; Oh, B.-C.; Lidov, H.G.; Hasselgren, P.-O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKβ/NF-κB Activation Causes Severe Muscle Wasting in Mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.L.; Bs, B.J.G.; Senf, S.M.; Hain, B.; Judge, S. Ros-mediated activation of NF-κB and Foxo during muscle disuse. Muscle Nerve 2010, 41, 110–113. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Witte, A.V. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.; Macdonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Hills, J.R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Jandhyala, S.M. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2018, 58, 2895–2910. [Google Scholar] [CrossRef] [PubMed]
| Type of Diet | Foods Included |
|---|---|
| Omnivorous | Eats red meat, poultry, fish, dairy and eggs |
| Semi vegetarian | Eats dairy, eggs and some red meat, poultry and fish ≥1 time/month but <1 time/week |
| Lacto-vegetarian | Eats dairy, but no red meat, poultry, fish or eggs |
| Ovo-vegetarian | Eats eggs but no red meat, poultry, fish or dairy |
| Pesco-vegetarian | Eats fish, but no red meat, poultry, dairy or eggs |
| Lacto-ovo-vegetarian | Eats dairy and eggs but no red meat, poultry or fish |
| Pesco-lacto-ovo-vegetarian | Eats fish, dairy and eggs but no red meat or poultry |
| Vegan | Eats only plant-based foods (no red meat, poultry, fish, dairy or eggs) |
| Nutrient | Omnivorous | Semi Vegetarian | Pesco-Vegetarian | Lacto-Ovo-Vegetarian | Vegan | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Caloric intake (kcal/day) | 1890 | 4 | 1713 | 12 | 1937 | 9 | 1899 | 5 | 1894 | 10 |
| Total carbohydrate (g) | 266 | 0.2 | 283 | 0.7 | 284 | 0.5 | 286 | 0.3 | 309 | 0.6 |
| Carbohydrate (% Energy) | 53.1 | <0.1 | 56.6 | 0.1 | 56.8 | 0.1 | 57.2 | 0.1 | 61.7 | 0.1 |
| Total fiber (g) | 30.4 | <0.1 | 34.9 * | 0.1 | 37.7 * | 0.1 | 37.5 * | 0.1 | 46.7 * | 0.1 |
| Total fat (g) | 78.1 | 0.1 | 74.2 | 0.3 | 73.4 | 0.2 | 73.6 | 0.1 | 66.1 | 0.2 |
| Fat (% Energy) | 35.1 | <0.1 | 33.4 | 0.1 | 33.0 | 0.1 | 33.1 | 0.1 | 29.8 | 0.1 |
| MUFA (g) a | 32.4 | 0.1 | 30.5 | 0.2 | 30.9 | 0.1 | 30.3 | 0.1 | 28.0 | 0.1 |
| SFA (g) b | 19.9 | <0.1 | 17.4 | 0.1 | 15.8 * | 0.1 | 16.0 | 0.1 | 11.6 * | 0.1 |
| DHA (g) c | 182 | 1.2 | 69.8 * | 3.6 | 187 | 2.8 | 33.8 * | 1.5 | 18.2 * | 3 |
| Total protein (g) | 75.8 | 0.1 | 71.8 | 0.2 | 74.3 | 0.2 | 72.0 | 0.1 | 72.3 | 0.2 |
| Protein (% Energy) | 15.2 | <0.1 | 14.4 | <0.1 | 14.9 | <0.1 | 14.4 | 0.1 | 14.5 | <0.1 |
| Animal protein (g) | 31.8 | 0.1 | 17.6 * | 0.2 | 16.0 * | 0.2 | 12.2 * | 0.1 | 3.1 * | 0.2 |
| Animal protein (% Energy) | 6.4 | <0.1 | 3.5 * | <0.1 | 3.2 * | <0.1 | 2.4 * | <0.1 | 0.6 * | <0.1 |
| Plant protein (g) | 43.9 | 0.1 | 54.1 * | 0.2 | 58.2 * | 0.2 | 59.7 * | 0.1 | 69.2 * | 0.2 |
| Plant protein (% Energy) | 8.8 | <0.1 | 10.8 * | <0.1 | 11.6 * | <0.1 | 11.9 * | <0.1 | 13.8 * | <0.1 |
| Vitamin D (μg) | 10.6 | 0.1 | 9.9 | 0.2 | 9.8 | 0.2 | 8.6 | 0.1 | 6.3 * | 0.2 |
| Magnesium (mg) | 509 | 1.3 | 554 | 3.7 | 581 | 2.9 | 567 | 1.6 | 652 * | 3.1 |
| Iron (mg) | 32.9 | 0.3 | 34.1 | 0.9 | 34.6 | 0.7 | 34.1 | 0.4 | 31.6 | 0.8 |
| Authors | Participants | Training Status | Study Design | Nutritional Intervention | Exercise Intervention | Performance Measurements | Outcome and Direction of Outcome |
|---|---|---|---|---|---|---|---|
| Baguet et al. (2011) | Group 1 (n = 10) Age: 21.5 ± 1.7 years Group 2 (n = 10) Age: 20.8 ± 1.4 years | Physically active (2–3 h per week) | Intervention (5 weeks) | Group 1: Mixed diet Group 2: Lacto-ovo vegetarian diet | Sprint training (running and cycling) Week 1–2: 2× week Week 3–5: 3× week | Power output on an electromagnetically braked cycle ergometer | Mean power output: ↑ (Independent of groups) |
| Blanquaert et al. (2018) | Group 1 (n = 10) Age: 25.9 ± 9.0 years Group 2 (n = 15) Age: 25.4 ± 7.1 years Group 3 (n = 14) Age: 25.5 ± 6.6 years | - | Intervention (6 months) | Group 1: Omnivorous diet Group 2: Lacto-ovo vegetarian diet + placebo Group 3: Lacto-ovo vegetarian diet + β-alanine and creatine | - | VO2max (mL/kg/min) via an incremental cycling test | VO2max: → Body weight: → (Independent of groups) |
| Boutros et al. (2020) | n = 56 Age: 25.6 ± 4.1 years 28 vegan 28 omnivorous | 150–200 min aerobic physical activity/week | Cross-sectional | - | - | Estimated VO2max (mL/kg/min) via cycle ergometer Muscle strength (1RM of leg and chest press) | Estimated VO2max in vegans: ↑ Muscle strength: → Body weight: → |
| Campbell et al. (1999) | Group 1 (n = 9) Age: 60 ± 1 years Group 2 (n = 10) Age: 58 ± 2 years | Sedentary | Intervention (12 weeks) | Group 1: Habitual omnivorous diet Group 2: Self-selected lacto-ovo-vegetarian diet | Resistance training (2×/week) | Dynamic muscular strength (1RM) | Dynamic muscular strength: ↑ (Independent of groups) |
| Haub et al. (2002) | Group 1 (n = 10) Age: 63 ± 3 years Group 2 (n = 11) Age: 67 ± 6 years | - | Intervention (12 weeks) | Group 1: Self-selected lacto- ovo-vegetarian diet supplemented with beef Group 2: Self-selected lacto- ovo-vegetarian diet supplemented with vegetable protein (soy) | Resistance training (3×/week) | Muscular strength of the lower and upper body | Lower body strength: ↑ (Independent of groups) Upper body strength: ↑ (Independent of groups) |
| Haub et al. (2005) | Group 1 (n = 10 Group 2 (n = 11) Age: 65 ± 5 years | - | Intervention (14 weeks) | Group 1: Self-selected lacto- ovo-vegetarian diet supplemented with beef Group 2: Self-selected lacto- ovo-vegetarian diet supplemented with vegetable protein (soy) | Resistance training (3×/week) | Muscular strength of the lower and upper body (Three maximum repetitions at 20%, 40%, 60% and 80% of the 1RM at the time of the testing | Lower body strength: ↑ (Independent of groups) Upper body strength: ↑ (Independent of groups) |
| Hevia-Larraín et al. (2021) | n = 38 19 vegan Age: 26 ± 5 years 19 omnivorous Age: 26 ± 4 years | physically active but not involved in resistance training for at least 1 year | Intervention (12 weeks) | - | Resistance training (2×/week) | Leg press 1RM | Lower body strength: ↑ (Independent of groups) |
| Hietavala et al. (2012) | n = 9 Age: 23.5 ± 3.4 years | Recreationally active | Intervention (18–24 days) | Group 1 (n = 5): (1.) 4 d habitual omnivorous diet (2.) 10–16 d wash-out phase (habitual omnivorous diet) (3.) 4 d low-protein vegetarian diet Group 2 (n = 4): (1.) 4 d low-protein vegetarian diet (2.) 10–16 d wash-out phase (habitual omnivorous diet) (3.) 4 d habitual omnivorous diet | - | VO2 (L/min) at 40%, 60% and 80% of VO2max VO2max | After low-protein vegetarian diet: VO2 ↑ (at 40%, 60% and 80% of VO2max) |
| Kròl et al. (2020) | n = 52 22 vegan Age: 32 ± 5 years 30 omnivorous Age: 30 ± 5 years | Physically active (at least 3×/week) | Cross-sectional | - | - | Peak power output (W) VO2max (mL/kg/min) | VO2max in vegans: ↑ Peak power output: → Body weight in vegans: ↓ |
| Lynch et al. (2016) | n = 70 27 vegetarian 43 omnivorous Age: 21–58 years | Competitive club sports team | Cross-sectional | - | - | VO2max (mL/kg/min) Peak torque leg extension | VO2max (mL/kg/min) max in female vegetarians: ↑ VO2max (L/min): → Body weight in female vegetarians: ↑ (n.s.) |
| Nebl et al. (2019) | n = 74 26 omnivorous 24 lacto-ovo vegetarian 24 vegan Age: 18-35 years | Recreational runners | Cross-sectional | - | - | Maximum exercise capacity (Pmax/bodyweight) Power output related to lean body mass (Pmax/LBM) | Maximum exercise capacity: → Power output related to lean body mass: → |
| Page et al. (2021) | n = 25 16omnivorous Age: 21 ± 1 years 9 vegan Age: 24 ± 3 years | No history of resistance or endurance exercise training in the preceding six months | Cross-sectional | - | - | VO2max (ml/kg/min) and (L/min) Maximal voluntary isometric contraction (MVIC) force | VO2max: → MVIC: → |
| Veleba et al. (2016) | Group 1 (n = 7) Age: 57.7 ± 4.9 years Group 2 (n = 37) Age: 54.6 ± 7.8 years | - | Intervention (12 weeks) | Group 1: Hypocaloric (−500 kcal) conventional diet Group 2: Hypocaloric (−500 kcal) vegetarian diet | Aerobic exercise 3×/week | Maximum performance (Wattmax) VO2max (ml/kg/min) | Group 1: Maximum performance: → VO2max: → Group 2: Maximum performance: ↑ VO2max: ↑ |
| Wells et al. (2003) | Group 1 (n = 10) Group 2 (n = 11) Age: 59-78 years | - | Intervention (12 weeks) | Group 1: Self-selected lacto-ovo vegetarian diet + beef protein supplement (0.6 g/kg/day) Group 2: Self-selected lacto-ovo-vegetarian diet + vegan protein supplement (0.6 g/kg/day) | Resistance training (3×/week) | Maximal strength (1RM) | Baseline maximal strength: → Maximal strength after 12 weeks of resistance training: ↑ (independent of group) Strength in knee extension in Group 2 compared to Group 1: ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohl, A.; Schünemann, F.; Bersiner, K.; Gehlert, S. The Impact of Vegan and Vegetarian Diets on Physical Performance and Molecular Signaling in Skeletal Muscle. Nutrients 2021, 13, 3884. https://doi.org/10.3390/nu13113884
Pohl A, Schünemann F, Bersiner K, Gehlert S. The Impact of Vegan and Vegetarian Diets on Physical Performance and Molecular Signaling in Skeletal Muscle. Nutrients. 2021; 13(11):3884. https://doi.org/10.3390/nu13113884
Chicago/Turabian StylePohl, Alexander, Frederik Schünemann, Käthe Bersiner, and Sebastian Gehlert. 2021. "The Impact of Vegan and Vegetarian Diets on Physical Performance and Molecular Signaling in Skeletal Muscle" Nutrients 13, no. 11: 3884. https://doi.org/10.3390/nu13113884
APA StylePohl, A., Schünemann, F., Bersiner, K., & Gehlert, S. (2021). The Impact of Vegan and Vegetarian Diets on Physical Performance and Molecular Signaling in Skeletal Muscle. Nutrients, 13(11), 3884. https://doi.org/10.3390/nu13113884







