A Novel UC Exclusion Diet and Antibiotics for Treatment of Mild to Moderate Pediatric Ulcerative Colitis: A Prospective Open-Label Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
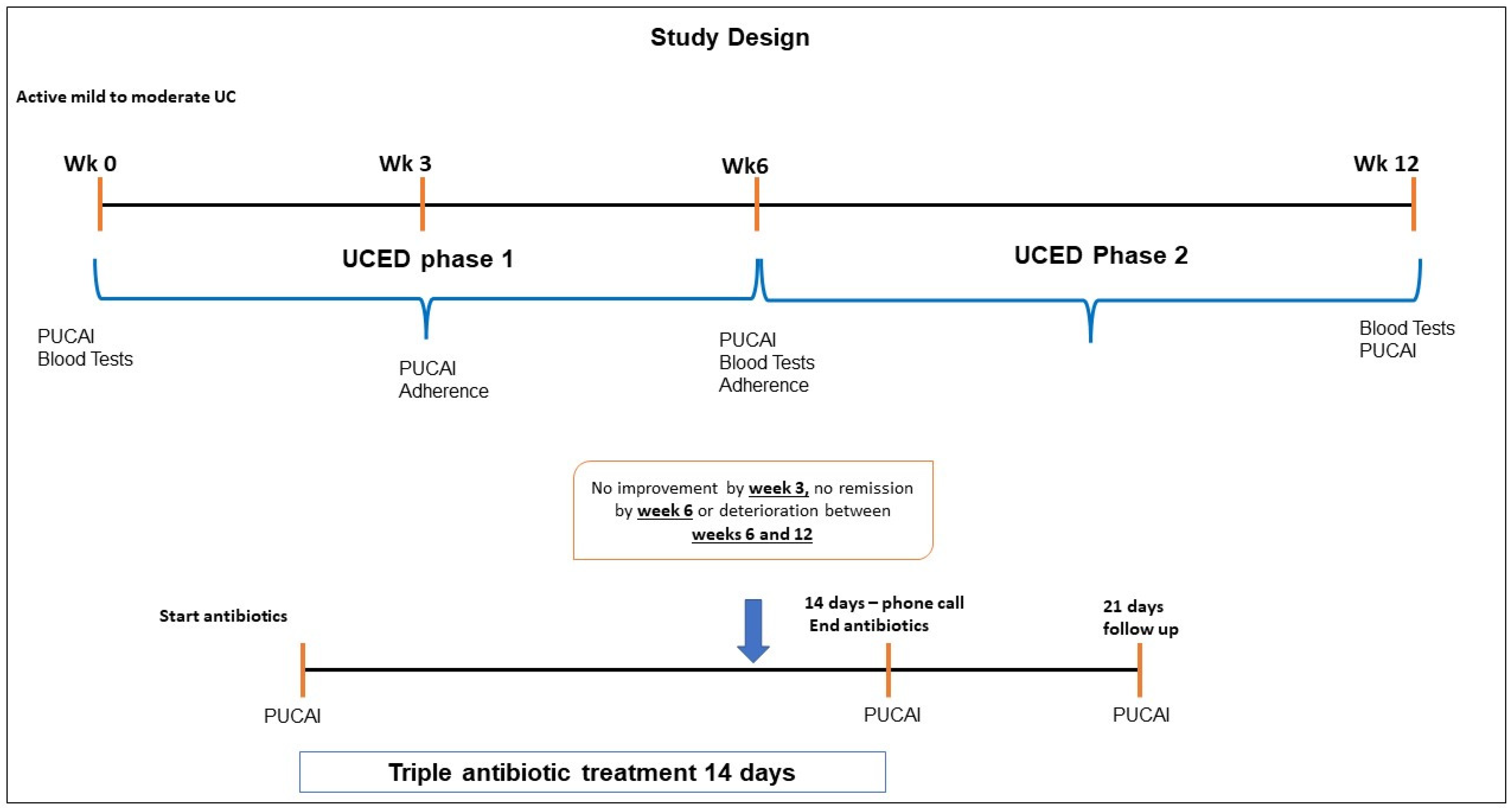
2.1. Study Population and Design
2.2. The Ulcerative Colitis Diet Intervention
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection and Dietary Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Response to UCED Exclusively by Week 6
3.3. Sustained Remission with UCED at Week 12
3.4. Response to ADM after UCED Failure
3.5. Tolerance and Adherence
3.6. Nutritional Outcomes
3.7. Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UC | Ulcerative Colitis |
| UCED | Ulcerative Colitis Exclusion Diet |
| PUCAI | Pediatric UC Activity Index |
| ITT | Intention to Treat |
| IBD | Inflammatory Bowel Disease |
| 5ASA | 5-Aminosalicylic Acid |
| IMM | Immune Modulator |
| FC | Fecal Calprotectin |
| LOCF | Last Observation Carried Forward |
| IQR | Interquartile Range |
| AMD | Amoxycillin, Metronidazole and Doxycycline |
| SAAs | Sulfated Amino Acids |
References
- Lönnfors, S.; Vermeire, S.; Greco, M.; Hommes, D.; Bell, C.; Avedano, L. IBD and health-related quality of life—Discovering the true impact. J. Crohn’s Coliti 2014, 8, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal fibre usage in UC in remission. Gut 2014, 64, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.A.; Walton, G.E.; Gibson, G.R.; Tuohy, K.M.; Andrews, S.C. In vitrobatch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int. J. Food Sci. Nutr. 2013, 65, 79–88. [Google Scholar] [CrossRef]
- Turner, D.; Bishai, J.; Reshef, L.; Abitbol, G.; Focht, G.; Marcus, D.; Ledder, O.; Lev-Tzion, R.; Orlanski-Meyer, E.; Yerushalmi, B.; et al. Antibiotic Cocktail for Pediatric Acute Severe Colitis and the Microbiome: The PRASCO Randomized Controlled Trial. Inflamm. Bowel Dis. 2019, 26, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Soo, W.; Bryant, R.V.; Jairath, V.; Hart, A.L.; Andrews, J.M. Systematic review with meta-analysis: Faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 213–224. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.; Xia, L.; Xu, H.; Ghishan, F.K.; A Carvalho, F.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2013, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- van der Post, S.; Jabbar, K.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef] [Green Version]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.-F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar] [CrossRef]
- Puértolas-Balint, F.; Schroeder, B.O. Does an Apple a Day Also Keep the Microbes Away? The Interplay Between Diet, Microbiota, and Host Defense Peptides at the Intestinal Mucosal Barrier. Front. Immunol. 2020, 11, 1164. [Google Scholar] [CrossRef]
- Zaidi, D.; Huynh, H.Q.; Carroll, M.W.; Mandal, R.; Wishart, D.S.; Wine, E. Gut Microenvironment and Bacterial Invasion in Paediatric Inflammatory Bowel Diseases. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarbagili-Shabat, C.; Sigall-Boneh, R.; Levine, A. Nutritional therapy in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2015, 31, 303–308. [Google Scholar] [CrossRef]
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary Guidance from the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Yi, J.; Liu, Y.; Yu, Z.; Chen, S.; Liu, X. Increased mucin-degrading bacteria by high protein diet leads to thinner mucus layer and aggravates experimental colitis. J. Gastroenterol. Hepatol. 2021, 36, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Kato, K.; Ohkusa, T.; Sugitani, M.; Ishii, Y.; Nemoto, N.; Moriyama, M. Efficacy of antibiotic combination therapy in patients with active ulcerative colitis, including refractory or steroid-dependent cases. J. Gastroenterol. Hepatol. 2010, 25, S62–S66. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Kato, K.; Terao, S.; Chiba, T.; Mabe, K.; Murakami, K.; Mizokami, Y.; Sugiyama, T.; Yanaka, A.; Takeuchi, Y.; et al. Newly Developed Antibiotic Combination Therapy for Ulcerative Colitis: A Double-Blind Placebo-Controlled Multicenter Trial. Am. J. Gastroenterol. 2010, 105, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Tan, C.K.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary Protein and Amino Acid Supplementation in Inflammatory Bowel Disease Course: What Impact on the Colonic Mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The Colonic Microbiome and Epithelial Transcriptome Are Altered in Rats Fed a High-Protein Diet Compared with a Normal-Protein Diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Leu, R.K.; Young, G.P.; Hu, Y.; Winter, J.; Conlon, M.A. Dietary Red Meat Aggravates Dextran Sulfate Sodium-Induced Colitis in Mice Whereas Resistant Starch Attenuates Inflammation. Dig. Dis. Sci. 2013, 58, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Nestor, M.; Onyewadume, L.; de Silva, P.S.; Korzenik, J.R.; Aguilar, H.; Bailen, L.; Berman, A.; Bhaskar, S.K.; Brown, M.; et al. High Dietary Intake of Specific Fatty Acids Increases Risk of Flares in Patients with Ulcerative Colitis in Remission during Treatment with Aminosalicylates. Clin. Gastroenterol. Hepatol. 2017, 15, 1390–1396.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Van De Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Alvarado, D.M.; Chen, B.; Iticovici, M.; Thaker, A.I.; Dai, N.; VanDussen, K.L.; Shaikh, N.; Lim, E.; Guillemin, G.; Tarr, P.I.; et al. Epithelial Indoleamine 2,3-Dioxygenase 1 Modulates Aryl Hydrocarbon Receptor and Notch Signaling to Increase Differentiation of Secretory Cells and Alter Mucus-Associated Microbiota. Gastroenterology 2019, 157, 1093–1108.e11. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; Berg, M.V.D.; Scheurink, A.; Schols, H.A.; et al. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2–1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Giudici, F.; Fiorindi, C.; Ficari, F.; Scaringi, S.; Amedei, A. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increasedE coliin CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2013, 63, 116–124. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-Y.; Cevallos, S.A.; Byndloss, M.X.; Tiffany, C.R.; Olsan, E.E.; Butler, B.P.; Young, B.M.; Rogers, A.W.; Nguyen, H.; Kim, K.; et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis that Exacerbates Pre-inflammatory Bowel Disease. Cell Host Microbe 2020, 28, 273–284.e6. [Google Scholar] [CrossRef] [PubMed]
- E Jakobsson, H.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2014, 16, 164–177. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Walker, R.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sittipo, P.; Shim, J.-W.; Lee, Y.K. Microbial Metabolites Determine Host Health and the Status of Some Diseases. Int. J. Mol. Sci. 2019, 20, 5296. [Google Scholar] [CrossRef] [Green Version]
- Kellingray, L.; Tapp, H.S.; Saha, S.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Consumption of a diet rich in Brassica vegetables is associated with a reduced abundance of sulphate-reducing bacteria: A randomised crossover study. Mol. Nutr. Food Res. 2017, 61, 1600992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- A Daley, C.; Abbott, A.; Doyle, P.S.; A Nader, G.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, T. Relapse Prevention by Plant-Based Diet Incorporated into Induction Therapy for Ulcerative Colitis: A Single-Group Trial. Perm. J. 2019, 23, 18–220. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, P.; Yilmaz, B.; Rossel, J.-B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boneh, R.S.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients with Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 19, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.; Gustafsson, J.K.; McDonald, K.G.; Kulkarni, D.H.; Kassel, R.; Newberry, R.D. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 2017, 8, 400–411. [Google Scholar] [CrossRef]
- Shabat, C.S.; Scaldaferri, F.; Zittan, E.; Hirsch, A.; Mentella, M.C.; Musca, T.; Cohen, N.A.; Ron, Y.; Isakov, N.F.; Pfeffer, J.; et al. Use of Fecal transplantation with a novel diet for mild to moderate active ulcerative colitis: The CRAFT UC randomized controlled trial. J. Crohn’s Coliti 2021, jjab165. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Total (n = 24) |
|---|---|
| Female gender, n (%) | 12 (50.0) |
| Age (years), mean (SD) | 15.3 (2.9) |
| Disease duration (years), median (IQR) | 1.0 (0.4–1.9) |
| Family history of IBD, n (%) | 5 (20.8) |
| Fecal calprotectin, μg/g, (n = 18), median (IQR) | 818.5 (630–1880) |
| CRP, mg/dL, median (IQR) | 0.5 (0.3–0.5) |
| PUCAI | |
| Mean (SD) | 34.0 (10.0) |
| Median (IQR) | 35 (30–40) |
| Disease severity, PUCAI, n (%) | |
| Mild (10–30) | 7 (29.2) |
| Range of mild disease | 15–30 |
| Moderate (35–45) | 17 (70.8) |
| Range of moderate disease | 35–45 |
| Severe | 0 (0) |
| Disease location, n (%) | |
| Pancolitis | 4 (16.7) |
| Extensive | 1 (4.2) |
| Left sided | 14 (58.3) |
| Proctitis | 5 (20.8) |
| Current treatment, n (%) | |
| 5-ASA (oral or oral and topical) | 20 (83.3) |
| None | 3 (12.5) |
| Immunomodulators | 2 (8.3) |
| Steroids * | 1 (4.2) |
| Height (cm), mean (SD) | 161.7 (13.0) |
| Weight (kg), median (IQR) | 58.4 (43.8–64.9) |
| Weight z-score, mean (SD) | 0.04 (1.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbagili-Shabat, C.; Albenberg, L.; Van Limbergen, J.; Pressman, N.; Otley, A.; Yaakov, M.; Wine, E.; Weiner, D.; Levine, A. A Novel UC Exclusion Diet and Antibiotics for Treatment of Mild to Moderate Pediatric Ulcerative Colitis: A Prospective Open-Label Pilot Study. Nutrients 2021, 13, 3736. https://doi.org/10.3390/nu13113736
Sarbagili-Shabat C, Albenberg L, Van Limbergen J, Pressman N, Otley A, Yaakov M, Wine E, Weiner D, Levine A. A Novel UC Exclusion Diet and Antibiotics for Treatment of Mild to Moderate Pediatric Ulcerative Colitis: A Prospective Open-Label Pilot Study. Nutrients. 2021; 13(11):3736. https://doi.org/10.3390/nu13113736
Chicago/Turabian StyleSarbagili-Shabat, Chen, Lindsey Albenberg, Johan Van Limbergen, Naomi Pressman, Anthony Otley, Michal Yaakov, Eytan Wine, Dror Weiner, and Arie Levine. 2021. "A Novel UC Exclusion Diet and Antibiotics for Treatment of Mild to Moderate Pediatric Ulcerative Colitis: A Prospective Open-Label Pilot Study" Nutrients 13, no. 11: 3736. https://doi.org/10.3390/nu13113736
APA StyleSarbagili-Shabat, C., Albenberg, L., Van Limbergen, J., Pressman, N., Otley, A., Yaakov, M., Wine, E., Weiner, D., & Levine, A. (2021). A Novel UC Exclusion Diet and Antibiotics for Treatment of Mild to Moderate Pediatric Ulcerative Colitis: A Prospective Open-Label Pilot Study. Nutrients, 13(11), 3736. https://doi.org/10.3390/nu13113736






