Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Ethical Consideration
2.4. Depression Assessment
2.5. Markers of Inflammation
2.6. Markers of Gut Permeability
2.7. Fecal Sample Collection
2.8. 16S rRNA Gene Amplification Sequences
2.9. Genera Selection Process for Correlation Heatmaps
2.10. Statistical Analysis
3. Results
3.1. Changes of Clinical Measurements and Serum Biomarkers
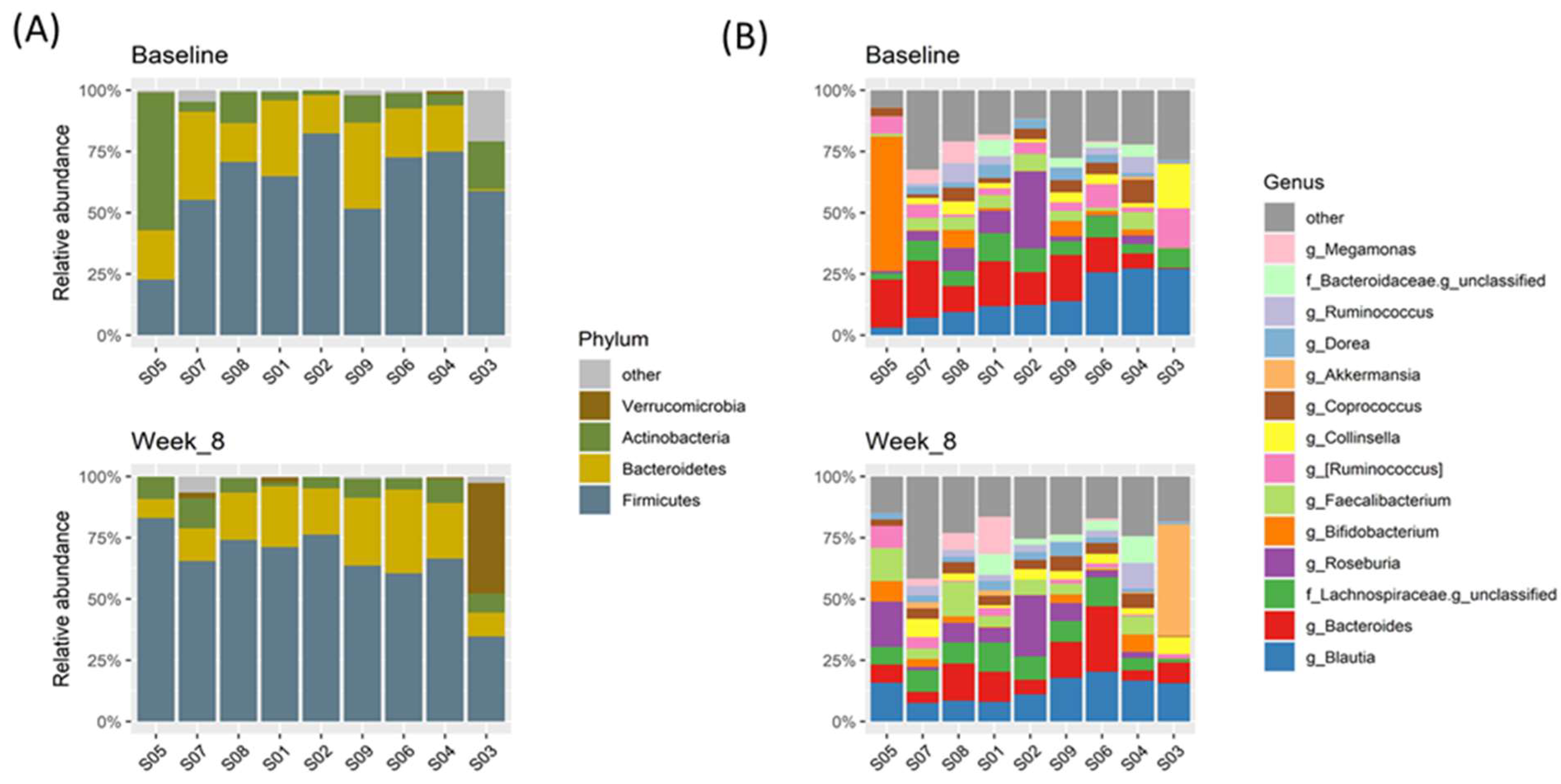
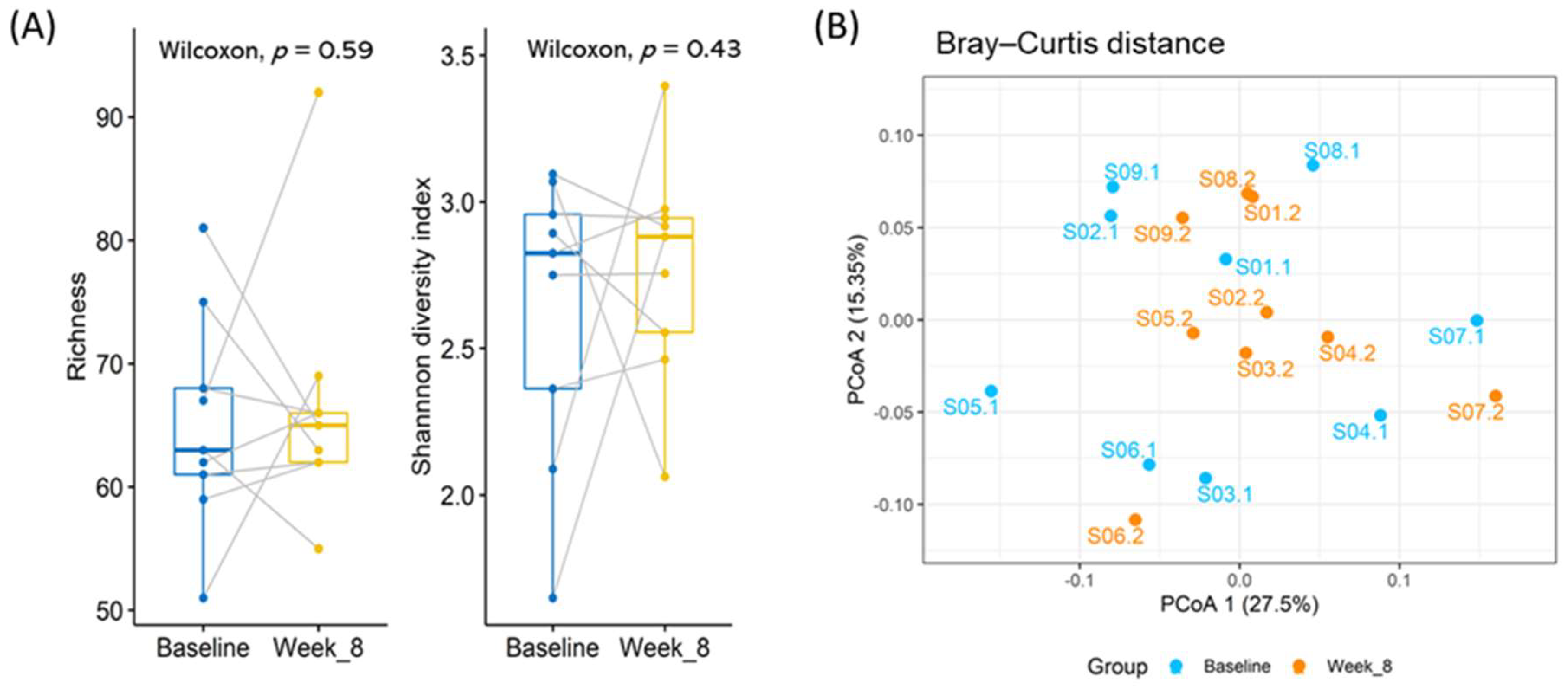
3.2. Differences in the Microbiota Composition of the Study Population
3.3. The Degree of Microbial Co-Occurrence within Specific Genera Changed after Consumption of Probiotics
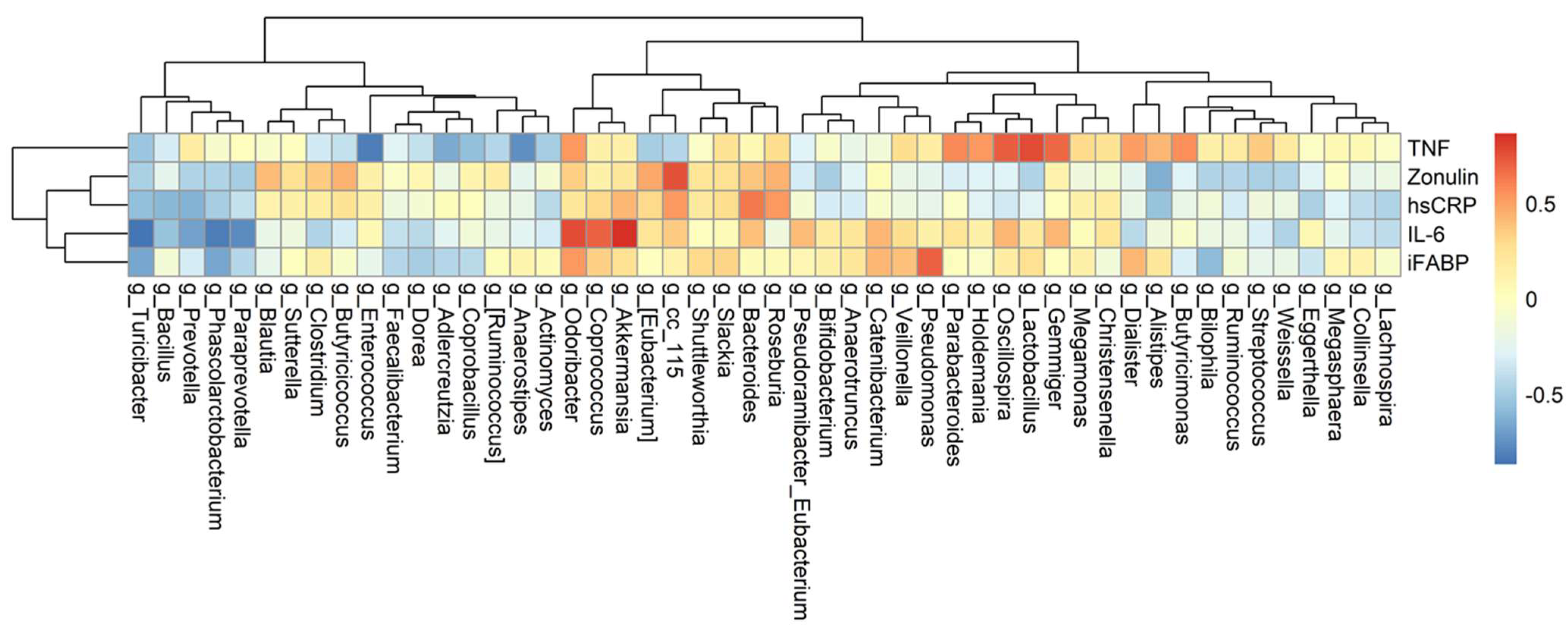
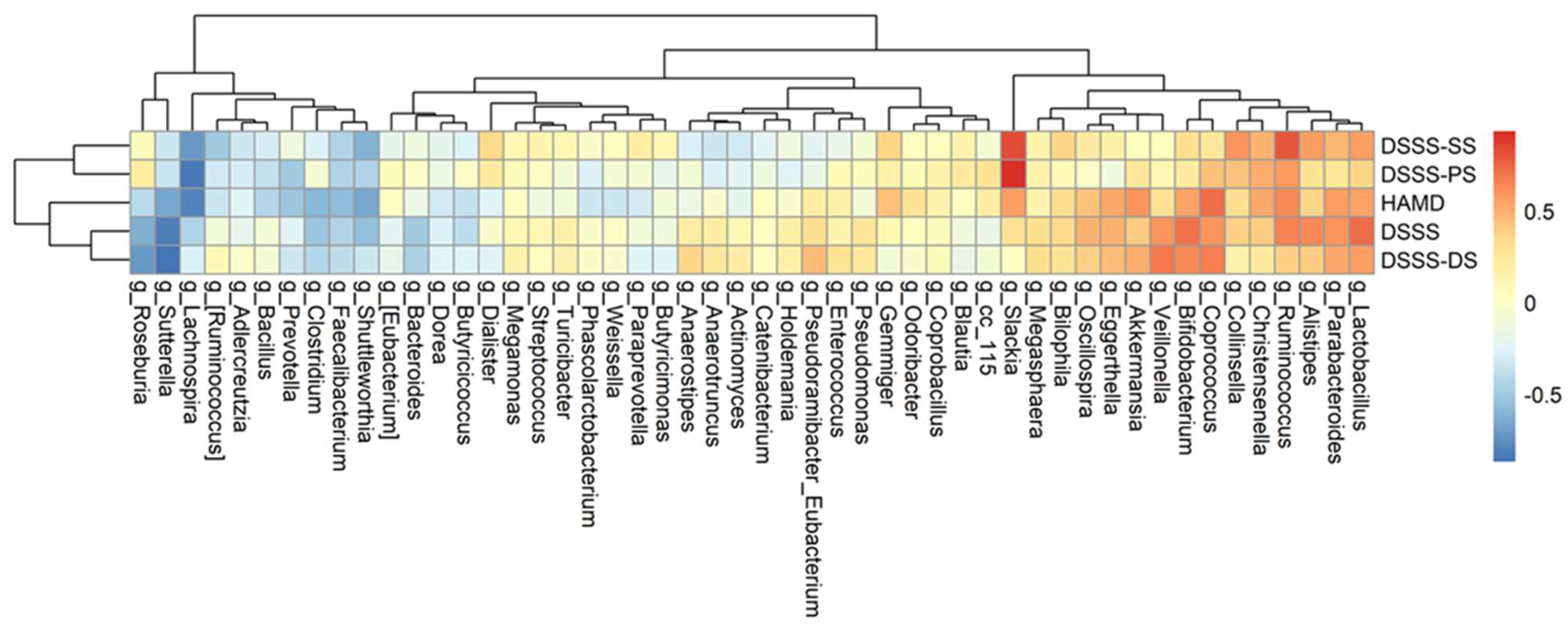
3.4. The Association among Changes of Symptoms, Serum Biomarkers, and Gut Microbiota
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlson, F.J.; Coates, M.; Coggeshall, M.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef] [Green Version]
- Karling, P.; Maripuu, M.; Wikgren, M.; Adolfsson, R.; Norrback, K.-F. Association between gastrointestinal symptoms and affectivity in patients with bipolar disorder. World J. Gastroenterol. 2016, 22, 8540–8548. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, T.P. Depressive disorders: Treatment failures and poor prognosis over the last 50 years. Pharmacol. Res. Perspect. 2019, 7, e00472. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Pelsers, M.M.; Namiot, Z.; Kisielewski, W.; Namiot, A.; Januszkiewicz, M.; Hermens, W.T.; Glatz, J.F. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 2003, 36, 529–535. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019, 139, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Slawik, S.; Staufenbiel, I.; Schilke, R.; Nicksch, S.; Weinspach, K.; Stiesch, M.; Eberhard, J. Probiotics affect the clinical inflammatory parameters of experimental gingivitis in humans. Eur. J. Clin. Nutr. 2011, 65, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef]
- Montassier, E.; Valdés-Mas, R.; Batard, E.; Zmora, N.; Dori-Bachash, M.; Suez, J.; Elinav, E. Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner. Nat. Microbiol. 2021, 6, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Kobayashi, R.; Sakai, F.; Hosoya, T.; Yamamoto, M.; Kurita-Ochiai, T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 2017, 7, 545. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Langen, M.L.-V.; Madsen, K.L. Secreted bioactive factors fromBifidobacterium infantisenhance epithelial cell barrier function. Am. J. Physiol. Liver Physiol. 2008, 295, G1025–G1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, K.K.; Liu, Y.-W.; Kuo, P.-H.; Chung, Y.-C.E.; Lu, M.-L.; Chen, C.-H. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef]
- Chao, S.-H.; Wu, R.-J.; Watanabe, K.; Tsai, Y.-C. Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 2009, 135, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M. Development of a Rating Scale for Primary Depressive Illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef]
- Baumeister, D.; Ciufolini, S.; Mondelli, V. Effects of psychotropic drugs on inflammation: Consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology 2016, 233, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-I.; Weng, L.-J.; Su, Y.-J.; Liu, C.-Y. Depression and somatic symptoms scale: A new scale with both depression and somatic symptoms emphasized. Psychiatry Clin. Neurosci. 2006, 60, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Adriaanse, M.P.M.; Tack, G.J.; Passos, V.L.; Damoiseaux, J.G.M.C.; Schreurs, M.W.J.; Van Wijck, K.; Riedl, R.G.; Masclee, A.A.M.; Buurman, W.A.; Mulder, C.J.J.; et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment. Pharmacol. Ther. 2013, 37, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.E.; Chen, H.-C.; Chou, H.-C.L.; Chen, I.-M.; Lee, M.-S.; Chuang, L.-C.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H.; Wu, C.-S.; et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Xu, Z.Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an in-creased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, R89-15. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Van Baarlen, P.; Hooiveld, G.; Norin, E.; Müller, M.; de Vos, W.M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front. Microbiol. 2011, 2, 166. [Google Scholar] [CrossRef] [Green Version]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [Green Version]

| Baseline | Week 2 | Week 4 | Week 8 | P a | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| HAMD-17 | 20.1 (5.6) | 12 (4.2) | 16.2 (5.8) | 12.0 (6.1) | 0.01 a |
| DSSS, total score | 31.0 (4.5) | 20.2 (3.8) | 22.2 (3.6) | 18.4 (3.5) | <0.001 a |
| DSSS, depressive | 19.2 (9.0) | 12.6 (8.3) | 13.8 (7.6) | 11.1 (6.9) | <0.001 a |
| DSSS, somatic | 11.8 (6.9) | 7.6 (5.6) | 8.4 (5.6) | 7.3 (6.6) | 0.001 a |
| DSSS, pain | 5.6 (3.5) | 3.7 (2.9) | 4.0 (2.7) | 3.5 (3.4) | 0.006 a |
| hs-CRP, n = 10 | 3.1 (3.7) | - | - | 4.1 (6.5) | 0.71 |
| IL-6, ng/mL | 35.7 (10.1) | - | - | 35.0 (11.9) | 0.61 |
| TNF-α, pg/mL | 886.9 (248.8) | - | - | 961.5 (304.1) | 0.13 |
| Zonulin, mg/mL | 0.94 (0.48) | - | - | 0.92 (0.54) | 0.92 |
| I-FABP, pg/mL | 1.67 (11.2) | - | - | 1.46 (1.02) | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-M.; Kuo, P.-H.; Hsu, C.-Y.; Chiu, Y.-H.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. https://doi.org/10.3390/nu13113731
Chen H-M, Kuo P-H, Hsu C-Y, Chiu Y-H, Liu Y-W, Lu M-L, Chen C-H. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients. 2021; 13(11):3731. https://doi.org/10.3390/nu13113731
Chicago/Turabian StyleChen, Hui-Mei, Po-Hsiu Kuo, Chia-Yueh Hsu, Yi-Hung Chiu, Yen-Wenn Liu, Mong-Liang Lu, and Chun-Hsin Chen. 2021. "Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial" Nutrients 13, no. 11: 3731. https://doi.org/10.3390/nu13113731
APA StyleChen, H.-M., Kuo, P.-H., Hsu, C.-Y., Chiu, Y.-H., Liu, Y.-W., Lu, M.-L., & Chen, C.-H. (2021). Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients, 13(11), 3731. https://doi.org/10.3390/nu13113731






