Impact of Controlling a Nutritional Status Score on Wound Healing in Patients with Chronic Limb-Threatening Ischemia after Endovascular Treatment
Abstract
:1. Introduction
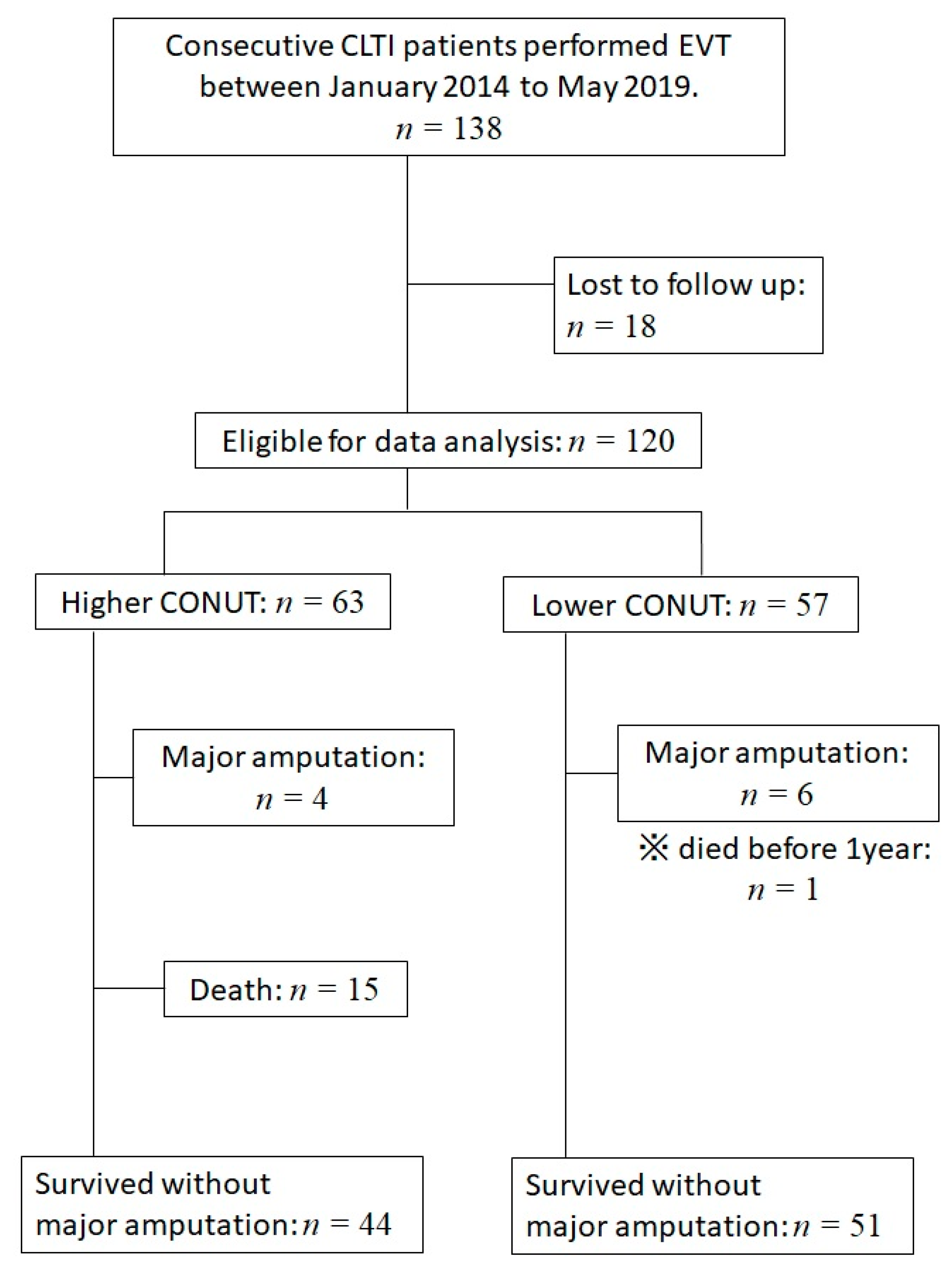
2. Materials and Methods
2.1. Population
2.2. Follow-Up
2.3. EVT Procedure
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes at 12 Months
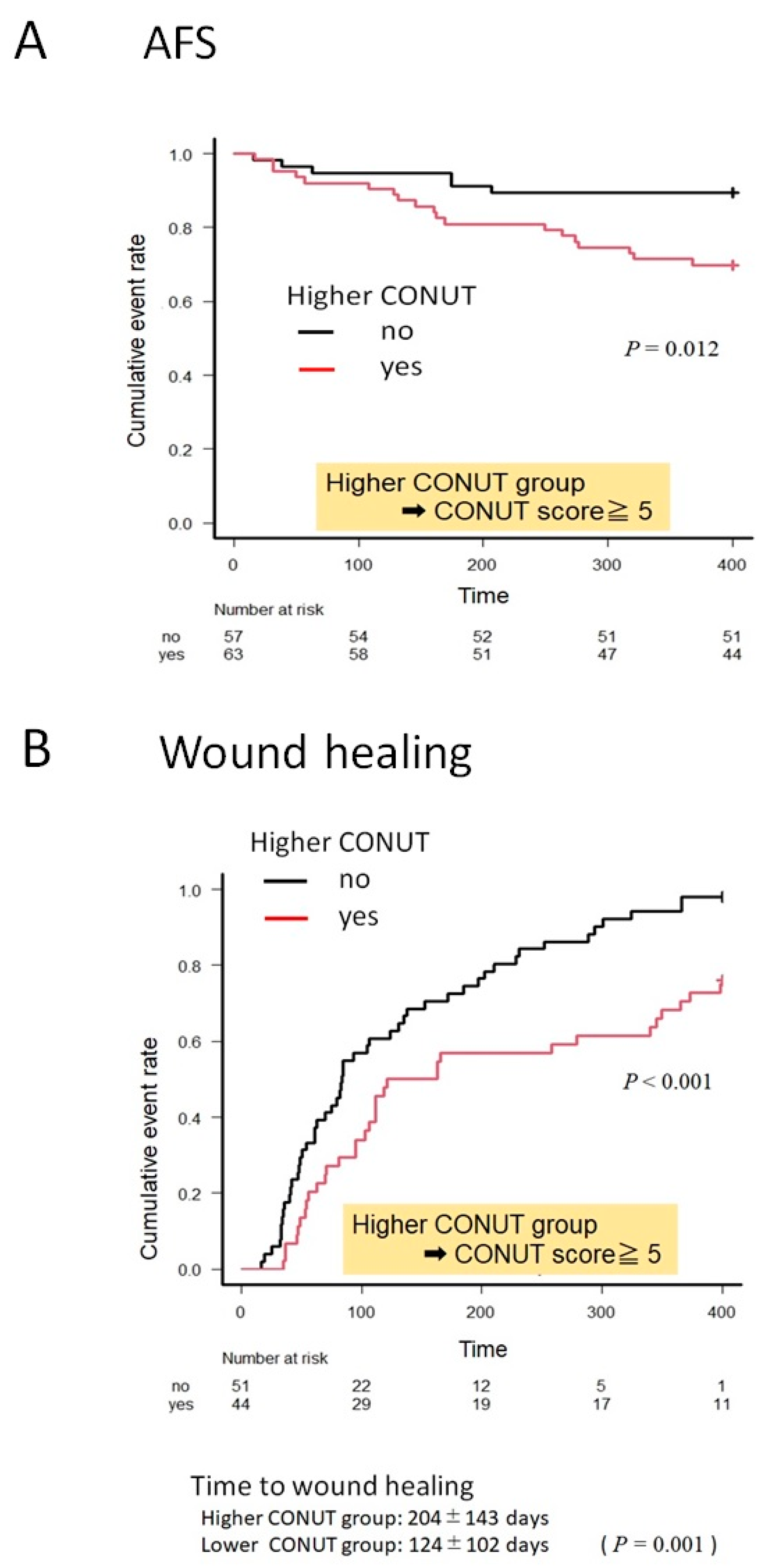
3.3. Kaplan–Meier Curves for AFS and Wound Healing in the Higher and Lower CONUT Groups
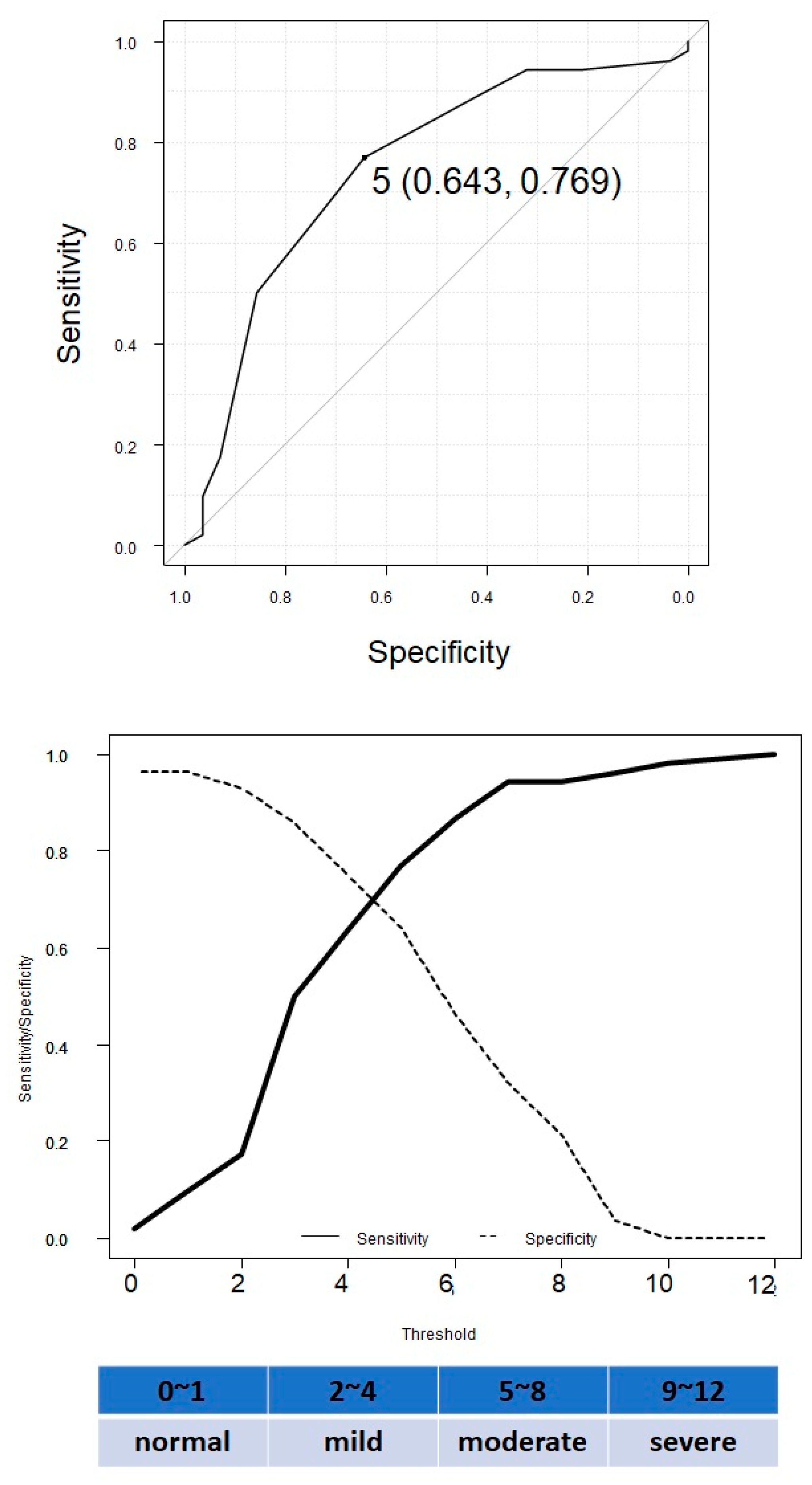
3.4. ROC Curve Analysis to Determine the Cut-Off Levels of the CONUT Score to Distinguish between the Presence and Absence of MALE
3.5. Predictors for AFS and Delayed Wound Healing
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: A companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: The European Stroke Organisation (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, e35–e41. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Bell, K.; Caporusso, J.; Durand-Zaleski, I.; Komori, K.; et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33 (Suppl. 1), S1–S75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawarada, O.; Fujihara, M.; Higashimori, A.; Yokoi, Y.; Honda, Y.; Fitzgerald, P.J. Predictors of adverse clinical outcomes after successful infrapopliteal intervention. Catheter. Cardiovasc. Interv. 2012, 80, 861–871. [Google Scholar] [CrossRef]
- Shiraki, T.; Takahara, M.; Iida, O.; Soga, Y.; Kodama, A.; Miyashita, Y.; Shintani, Y.; Endo, M.; Azuma, N. Baseline and updated information on nutritional status in patients with chronic limb threatening ischaemia undergoing revascularization. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 467–472. [Google Scholar] [CrossRef]
- Iida, O.; Nakamura, M.; Yamauchi, Y.; Kawasaki, D.; Yokoi, Y.; Yokoi, H.; the OLIVE Investigators. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia. Circ. Cardiovasc. Interv. 2013, 6, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Nakagomi, A.; Kohashi, K.; Morisawa, T.; Kosugi, M.; Atarashi, H.; Shimizu, W. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J. Atheroscler. Thromb. 2016, 23, 713–727. [Google Scholar] [CrossRef] [Green Version]
- Mizobuchi, K.; Jujo, K.; Minami, Y.; Ishida, I.; Nakaoand, M.; Hagiwara, N. The Baseline Nutritional Status Predicts Long-Term Mortality in Patients Undergoing Endovascular Therapy. Nutrients 2019, 11, 1745. [Google Scholar] [CrossRef] [Green Version]
- Ignacio, I.; de Ulı´barri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, F.; Schuetz, P.; Bounoure, L.; Austin, P.; Ballesteros-Pomar, M.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; Poulia, K.A. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin. Nutr. 2018, 37, 336–353. [Google Scholar] [CrossRef] [Green Version]
- Mueller, C.; Compher, C.; Ellen, D.M. ASPEN clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN 2011, 35, 16–24. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Tribolet, P.; Bregenzer, T.; Braun, N.; Hoess, C.; et al. Individualized nutritional support in medical inpatients at nutritional risk: A randomized clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Chan, C.; Puckridge, P.; Ullah, S.; Delaney, C.; Spark, J.I. Neutrophil-lymphocyte ratio as a prognostic marker of outcome in infrapopliteal percutaneous interventions for critical limb ischemia. J. Vasc. Surg. 2014, 60, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teperman, J.; Carruthers, D.; Guo, Y.; Barnett, M.P.; Harris, A.A.; Sedlis, S.P.; Pillinger, M.; Babaev, A.; Staniloae, C.; Attubato, M.; et al. Relationship between neutrophil-lymphocyte ratio and severity of lower extremity peripheral artery disease. Int. J. Cardiol. 2017, 228, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.B.; Moughan, P.J.; Wood, L.G.; Singh, H.; Garg, M.L. Postprandial lipemia: Factoring in lipemic response for ranking foods for their healthiness. Lipids Health Dis. 2017, 16, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; Dehghan, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Li, W.; Chen, H.; Yi, S.; et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 2017, 5, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Shiraki, T.; Iida, O.; Takahara, M.; Soga, Y.; Yamauchi, Y.; Hirano, K.; Kawasaki, D.; Fujihara, M.; Utsunomiya, M.; Tazaki, J.; et al. Predictors of delayed wound healing after endovascular therapy of isolated infrapopliteal lesions underlying critical limb ischemia in patients with high prevalence of diabetes mellitus and hemodialysis. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Azuma, N.; Uchida, H.; Kokubo, T.; Koya, A.; Akasaka, N.; Sasajima, T. Factors influencing wound healing of critical limb ischemia foot after bypass surgery is the angiosome important in selecting bypass target artery. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Iida, O.; Nakamura, M.; Yamauchi, Y.; Fukunaga, M.; Yokoi, Y.; Yokoi, H.; Soga, Y.; Zen, K.; Suematsu, N.; Inoue, N.; et al. 3-Year outcomes of the OLIVE registry, a prospective multicenter study of patients with critical limb ischemia. J. Am. Coll. Cardiol. Intv. 2015, 8, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Iida, O.; Soga, Y.; Yamauchi, Y.; Hirano, K.; Kawasaki, D.; Yamaoka, T.; Takahara, M.; Uematsu, M. Clinical efficacy of endovascular therapy for patients with critical limb ischemia attributable to pure isolated infrapopliteal lesions. J. Vasc. Surg. 2013, 57, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewoude, M.F.; Alish, C.J.; Sauer, A.C.; Hegazi, R.A. Malnutrition sarcopenia syndrome: Is this the future of nutrition screening and assessment for older adults? J. Aging Res. 2012, 2012, 651570. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall (n = 120) | Higher CONUT (n = 63) | Lower CONUT (n = 57) | p |
|---|---|---|---|---|
| Patient | ||||
| Age, years | 73.2 ± 10.4 | 73.1 ± 9.9 | 73.3 ± 10.9 | 0.886 |
| Male sex | 84 (70) | 41 (65.1) | 43 (75.4) | 0.237 |
| Body mass index, kg/m2 | 21.6 ± 3.7 | 21.3 ± 3.5 | 22.0 ± 3.9 | 0.280 |
| Hypertension | 92 (76.7) | 48 (76.2) | 44 (77.2) | 1.000 |
| Dyslipidemia | 65 (54.2) | 32 (50.8) | 33 (57.9) | 0.468 |
| Diabetes mellitus | 101 (84.2) | 53 (84.1) | 48 (84.2) | 1.000 |
| Chronic kidney disease | 86 (71.2) | 47 (74.6) | 39 (68.4) | 0.544 |
| Hemodialysis | 62 (51.2) | 42 (66.7) | 20 (35.1) | <0.001 |
| Prior PCI | 69 (57.5) | 39 (61.9) | 30 (52.6) | 0.357 |
| Prior CVD | 21 (17.5) | 6 (9.5) | 15 (26.3) | 0.018 |
| Smoking history | 79 (65.8) | 44 (69.8) | 35 (61.4) | 0.343 |
| Chronic hepatitis | 1 (0.8) | 0 (0) | 1 (1.8) | 0.475 |
| Ratherford 4/5/6 | 4 (3.3)/87 (72.5) /29 (24.1) | 2 (3.2)/46 (73.0) /16 (25.4) | 2 (3.5)/42 (73.7) /13 (22.8) | |
| Isolated BK lesion | 65 (54.1) | 35 (55.6) | 30 (52.6) | 0.871 |
| CONUT score | 4.9 ± 2.5 | 6.8 ± 1.7 | 2.9 ± 1.1 | <0.001 |
| WIfI High-risk | 73 (60.8) | 43 (68.3) | 30 (52.6) | 0.086 |
| CFS | 5.4 ± 3.7 | 5.9 ± 1.4 | 4.9 ± 1.9 | 0.005 |
| Aspirin | 70 (58.3) | 39 (61.9) | 31 (54.4) | 0.355 |
| Thienopyridine | 78 (65.0) | 42 (66.7) | 36 (63.2) | 0.706 |
| Cilostazol | 13 (10.8) | 2 (3.2) | 11 (19.3) | 0.006 |
| DOAC | 9 (7.5) | 6 (9.5) | 3 (5.2) | 0.496 |
| ARB/ACEI | 75 (62.5) | 32 (50.8) | 43 (75.4) | 0.007 |
| Statin | 73 (60.8) | 32 (50.8) | 41 (71.9) | 0.024 |
| Insulin | 19 (15.8) | 10 (15.9) | 9 (15.8) | 0.812 |
| Variables | Overall (n = 120) | Higher CONUT (n = 63) | Lower CONUT (n = 57) | p |
|---|---|---|---|---|
| Serum albumin (mg/dL) | 3.3 ± 0.6 | 3.0 ± 0.4 | 3.7 ± 0.4 | <0.001 |
| Total cholesterol (mg/dL) | 145.1 ± 33.3 | 135.6 ± 30.8 | 155.8 ± 33.1 | <0.001 |
| Lymphocyte count (103/mL) | 1169.4 ± 557.6 | 968.9 ± 405.1 | 1394.9 ± 619.8 | <0.001 |
| Hemoglobin (g/dL) | 11.2 ± 1.8 | 10.5 ± 1.7 | 11.9 ± 1.7 | <0.001 |
| CRP (mg/dL) | 2.5 ± 4.2 | 3.6 ± 5.2 | 1.4 ± 1.8 | 0.002 |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.26 | 0.6 ± 0.4 | 0.193 |
| HbA1c (%) | 6.4 ± 1.1 | 6.3 ± 1.1 | 6.5 ± 1.1 | 0.299 |
| HDL-C (mg/dL) | 40.9 ± 13.4 | 39.4 ± 12.0 | 42.7 ± 14.7 | 0.174 |
| LDL-C (mg/dL) | 80.7 ± 28.4 | 72.6 ± 25.5 | 89.9 ± 29.0 | <0.001 |
| TG (mg/dL) | 103.2 ± 55.0 | 98.4 ± 45.3 | 108.7 ± 64.3 | 0.311 |
| Variables | Overall (n = 120) | Higher CONUT (n = 63) | Lower CONUT (n = 57) | p |
|---|---|---|---|---|
| Death (%) | 16 (13.3) | 15 (23.8) | 1 (1.8) | <0.001 |
| TLR (%) | 25 (20.8) | 16 (25.4) | 9 (15.8) | 0.261 |
| MI (%) | 1 (0.8) | 1 (1.6) | 0 (0) | 1.000 |
| CVA (%) | 4 (3.3) | 3 (4.8) | 1 (1.8) | 0.621 |
| Major amputation (%) | 10 (8.3) | 4 (6.3) | 6 (10.5) | 0.515 |
| Minor amputation (%) | 47 (39.2) | 27 (42.9) | 20 (35.1) | 0.455 |
| MALE (%) | 42 (35.0) | 29 (46.0) | 13 (22.8) | 0.012 |
| Predictors of AFS | ||||
|---|---|---|---|---|
| Univariate | p | Multivariate | p | |
| Male sex | 0.44 (0.18–1.13) | 0.090 | ||
| Diabetes mellitus | 0.96 (0.30–3.23) | 0.980 | ||
| Chronic kidney disease | 1.02 (0.38–2.72) | 0.967 | ||
| Hemodialysis | 1.53 (0.63–3.75) | 0.351 | 1.29 (0.45–3.71) | 0.640 |
| Anemia (<Hb 9 g/dL) | 0.56 (0.22–1.42) | 0.221 | ||
| CRP (>3 mg/dL) | 1.74 (0.70–4.37) | 0.236 | 1.08 (0.39–3.01) | 0.885 |
| Higher CONUT | 3.67 (1.35–10.0) | 0.011 | 2.24 (0.69–7.23) | 0.179 |
| WIfI High-risk | 2.02 (0.73–5.56) | 0.175 | ||
| Moderate-to-severe frailty | 23.50 (3.05–181.0) | 0.002 | 20.50 (2.61–161.0) | 0.005 |
| Predictors of delayed wound healing | ||||
| Univariate | p | Multivariate | p | |
| Male sex | 1.29 (0.32–5.10) | 0.340 | ||
| Diabetes mellitus | 0.37 (0.10–1.37 | 0.136 | ||
| Chronic kidney disease | 1.29 (0.33–5.10) | 0.716 | ||
| Hemodialysis | 2.47 (0.71–8.63) | 0.157 | 1.84 (0.45–7.48) | 0.396 |
| Anemia (<Hb 9 g/dL) | 2.08 (0.63–6.87) | 0.232 | ||
| CRP (>3 mg/dL) | 4.05 (1.22–13.5) | 0.023 | 3.05 (0.81–11.5) | 0.099 |
| Higher CONUT | 16.70 (2.07–124.0) | 0.008 | 11.20 (1.29–97.5) | 0.028 |
| WIfI High-risk | 0.57 (0.18–1.83) | 0.342 | ||
| Moderate-to-severe frailty | 1.60 (0.49–5.28) | 0.441 | 0.95 (0.25–3.58) | 0.941 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mine, K.; Sugihara, M.; Fujita, T.; Kato, Y.; Gondo, K.; Arimura, T.; Takamiya, Y.; Shiga, Y.; Kuwano, T.; Miura, S.-i. Impact of Controlling a Nutritional Status Score on Wound Healing in Patients with Chronic Limb-Threatening Ischemia after Endovascular Treatment. Nutrients 2021, 13, 3710. https://doi.org/10.3390/nu13113710
Mine K, Sugihara M, Fujita T, Kato Y, Gondo K, Arimura T, Takamiya Y, Shiga Y, Kuwano T, Miura S-i. Impact of Controlling a Nutritional Status Score on Wound Healing in Patients with Chronic Limb-Threatening Ischemia after Endovascular Treatment. Nutrients. 2021; 13(11):3710. https://doi.org/10.3390/nu13113710
Chicago/Turabian StyleMine, Kaori, Makoto Sugihara, Takafumi Fujita, Yuta Kato, Koki Gondo, Tadaaki Arimura, Yosuke Takamiya, Yuhei Shiga, Takashi Kuwano, and Shin-ichiro Miura. 2021. "Impact of Controlling a Nutritional Status Score on Wound Healing in Patients with Chronic Limb-Threatening Ischemia after Endovascular Treatment" Nutrients 13, no. 11: 3710. https://doi.org/10.3390/nu13113710
APA StyleMine, K., Sugihara, M., Fujita, T., Kato, Y., Gondo, K., Arimura, T., Takamiya, Y., Shiga, Y., Kuwano, T., & Miura, S.-i. (2021). Impact of Controlling a Nutritional Status Score on Wound Healing in Patients with Chronic Limb-Threatening Ischemia after Endovascular Treatment. Nutrients, 13(11), 3710. https://doi.org/10.3390/nu13113710






