Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment
Abstract
1. Introduction
2. Methods
3. Pediatric Obesity and Asthma
4. “Obese Asthma” Phenotype
5. Asthma Endotype
6. Adipose Tissue-Associated Inflammation and Asthma
7. Obesity and Pulmonary Function
8. Impact of Nutritional Status on Asthma Prevention and Treatment
8.1. Early-Life Nutrition and Asthma Prevention
8.1.1. Breastfeeding
8.1.2. Infant Formula
8.1.3. Cow’s Milk and Soy Milk
8.1.4. Complementary Feeding
8.2. Nutrition at Developmental Age: Prevention, Treatment, and Empowerment
8.2.1. Cow’s Milk
8.2.2. Animal Proteins
8.2.3. Dietary Patterns
Mediterranean Diet
Western Diet
8.3. Food Supplements
8.3.1. Polyunsaturated Fatty Acids
8.3.2. Antioxidants
8.3.3. Vitamin D
8.4. Probiotics, Prebiotics, and Synbiotics: Prevention and Treatment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Child Growth Standards. Available online: https://www.who.int/toolkits/child-growth-standards (accessed on 28 September 2021).
- Ferrante, G.; La Grutta, S. The Burden of Pediatric Asthma. Front. Pediatr. 2018, 6, 186. [Google Scholar] [CrossRef]
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma Costs and Social Impact. Asthma Res. Pract. 2017, 3, 1. [Google Scholar] [CrossRef]
- Forno, E.; Celedón, J.C. Health Disparities in Asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 1033–1035. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Asthma Data, Statistics, and Surveillance. 2016. Available online: http://www.cdc.gov/asthma/most_recent_data.htm (accessed on 28 September 2021).
- Vijayakanthi, N.; Greally, J.M.; Rastogi, D. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics 2016, 137, e20150812. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, X.; Xiao, L.; Xie, Z.; Bettini, M.; Deng, T. A Unique Population: Adipose-Resident Regulatory T Cells. Front. Immunol. 2018, 9, 2075. [Google Scholar] [CrossRef]
- Lang, J.E. Obesity and Childhood Asthma. Curr. Opin. Pulm. Med. 2019, 25, 34–43. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet Lond. Engl. 2017, 390, 2627–2642. [CrossRef]
- Asher, I.; Pearce, N. Global Burden of Asthma among Children. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2014, 18, 1269–1278. [Google Scholar] [CrossRef]
- Gold, D.R.; Damokosh, A.I.; Dockery, D.W.; Berkey, C.S. Body-Mass Index as a Predictor of Incident Asthma in a Prospective Cohort of Children. Pediatr. Pulmonol. 2003, 36, 514–521. [Google Scholar] [CrossRef]
- Rzehak, P.; Wijga, A.H.; Keil, T.; Eller, E.; Bindslev-Jensen, C.; Smit, H.A.; Weyler, J.; Dom, S.; Sunyer, J.; Mendez, M.; et al. Body Mass Index Trajectory Classes and Incident Asthma in Childhood: Results from 8 European Birth Cohorts--a Global Allergy and Asthma European Network Initiative. J. Allergy Clin. Immunol. 2013, 131, 1528–1536. [Google Scholar] [CrossRef]
- Lang, J.E.; Bunnell, H.T.; Hossain, M.J.; Wysocki, T.; Lima, J.J.; Finkel, T.H.; Bacharier, L.; Dempsey, A.; Sarzynski, L.; Test, M.; et al. Being Overweight or Obese and the Development of Asthma. Pediatrics 2018, 142, e20182119. [Google Scholar] [CrossRef]
- von Mutius, E.; Schwartz, J.; Neas, L.M.; Dockery, D.; Weiss, S.T. Relation of Body Mass Index to Asthma and Atopy in Children: The National Health and Nutrition Examination Study III. Thorax 2001, 56, 835–838. [Google Scholar] [CrossRef]
- Stoodley, I.; Williams, L.; Thompson, C.; Scott, H.; Wood, L. Evidence for Lifestyle Interventions in Asthma. Breathe Sheff. Engl. 2019, 15, e50–e61. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The Role of Nutrition in Asthma Prevention and Treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef]
- Di Genova, L.; Penta, L.; Biscarini, A.; Di Cara, G.; Esposito, S. Children with Obesity and Asthma: Which Are the Best Options for Their Management? Nutrients 2018, 10, 1634. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; de Courten, M.P.J.; Stojanovska, L.; Blatch, G.L.; Tangalakis, K.; de Courten, B. The Complex Immunological and Inflammatory Network of Adipose Tissue in Obesity. Mol. Nutr. Food Res. 2016, 60, 43–57. [Google Scholar] [CrossRef]
- Chen, Y.C.; Dong, G.H.; Lin, K.C.; Lee, Y.L. Gender Difference of Childhood Overweight and Obesity in Predicting the Risk of Incident Asthma: A Systematic Review and Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 222–231. [Google Scholar] [CrossRef]
- Egan, K.B.; Ettinger, A.S.; Bracken, M.B. Childhood Body Mass Index and Subsequent Physician-Diagnosed Asthma: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. BMC Pediatr. 2013, 13, 121. [Google Scholar] [CrossRef]
- De, A.; Rastogi, D. Association of Pediatric Obesity and Asthma, Pulmonary Physiology, Metabolic Dysregulation, and Atopy; and the Role of Weight Management. Expert Rev. Endocrinol. Metab. 2019, 14, 335–349. [Google Scholar] [CrossRef]
- Camargo, C.A.; Weiss, S.T.; Zhang, S.; Willett, W.C.; Speizer, F.E. Prospective Study of Body Mass Index, Weight Change, and Risk of Adult-Onset Asthma in Women. Arch. Intern. Med. 1999, 159, 2582–2588. [Google Scholar] [CrossRef]
- Chastang, J.; Baiz, N.; Parnet, L.; Cadwallader, J.S.; De Blay, F.; Caillaud, D.; Charpin, D.A.; Dwyer, J.; Lavaud, F.; Raherison, C.; et al. Changes in Body Mass Index during Childhood and Risk of Various Asthma Phenotypes: A Retrospective Analysis. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2017, 28, 273–279. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Gao, X.; Paul, T.K.; Cai, J.; Wang, Y. Sex Difference in the Association between Obesity and Asthma in U.S. Adults: Findings from a National Study. Respir. Med. 2015, 109, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Gennuso, J.; Epstein, L.H.; Paluch, R.A.; Cerny, F. The Relationship between Asthma and Obesity in Urban Minority Children and Adolescents. Arch. Pediatr. Adolesc. Med. 1998, 152, 1197–1200. [Google Scholar] [CrossRef]
- Koenig, K. Pilot Study of Low-Income Parents’ Perspectives of Managing Asthma in High-Risk Infants and Toddlers. Pediatr. Nurs. 2007, 33, 223–242. [Google Scholar]
- van den Bemt, L.; Kooijman, S.; Linssen, V.; Lucassen, P.; Muris, J.; Slabbers, G.; Schermer, T. How Does Asthma Influence the Daily Life of Children? Results of Focus Group Interviews. Health Qual. Life Outcomes 2010, 8, 5. [Google Scholar] [CrossRef]
- Meng, A.; McConnell, S. Symptom Perception and Respiratory Sensation: Clinical Applications. Nurs. Clin. N. Am. 2003, 38, 737–748. [Google Scholar] [CrossRef]
- Gilliland, F.D.; Berhane, K.; Islam, T.; McConnell, R.; Gauderman, W.J.; Gilliland, S.S.; Avol, E.; Peters, J.M. Obesity and the Risk of Newly Diagnosed Asthma in School-Age Children. Am. J. Epidemiol. 2003, 158, 406–415. [Google Scholar] [CrossRef]
- Mannino, D.M.; Mott, J.; Ferdinands, J.M.; Camargo, C.A.; Friedman, M.; Greves, H.M.; Redd, S.C. Boys with High Body Masses Have an Increased Risk of Developing Asthma: Findings from the National Longitudinal Survey of Youth (NLSY). Int. J. Obes. 2006, 30, 6–13. [Google Scholar] [CrossRef]
- Mamun, A.A.; Lawlor, D.A.; Alati, R.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M. Increasing Body Mass Index from Age 5 to 14 Years Predicts Asthma among Adolescents: Evidence from a Birth Cohort Study. Int. J. Obes. 2005 2007, 31, 578–583. [Google Scholar] [CrossRef]
- Weinmayr, G.; Forastiere, F.; Büchele, G.; Jaensch, A.; Strachan, D.P.; Nagel, G.; ISAAC Phase Two Study Group. Overweight/Obesity and Respiratory and Allergic Disease in Children: International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. PLoS ONE 2014, 9, e113996. [Google Scholar] [CrossRef] [PubMed]
- Mebrahtu, T.F.; Feltbower, R.G.; Greenwood, D.C.; Parslow, R.C. Childhood Body Mass Index and Wheezing Disorders: A Systematic Review and Meta-Analysis. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2015, 26, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Kerr, S.; Dunican, E.M.; Woodruff, P.G.; Fajt, M.L.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; et al. Refractory Airway Type 2 Inflammation in a Large Subgroup of Asthmatic Patients Treated with Inhaled Corticosteroids. J. Allergy Clin. Immunol. 2019, 143, 104–113.e14. [Google Scholar] [CrossRef]
- Ho, W.-C.; Lin, Y.-S.; Caffrey, J.L.; Lin, M.-H.; Hsu, H.-T.; Myers, L.; Chen, P.-C.; Lin, R.-S. Higher Body Mass Index May Induce Asthma among Adolescents with Pre-Asthmatic Symptoms: A Prospective Cohort Study. BMC Public Health 2011, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lai, H.J.; Roberg, K.A.; Gangnon, R.E.; Evans, M.D.; Anderson, E.L.; Pappas, T.E.; Dasilva, D.F.; Tisler, C.J.; Salazar, L.P.; et al. Early Childhood Weight Status in Relation to Asthma Development in High-Risk Children. J. Allergy Clin. Immunol. 2010, 126, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Dumas, O.; Varraso, R.; Gillman, M.W.; Field, A.E.; Camargo, C.A. Longitudinal Study of Maternal Body Mass Index, Gestational Weight Gain, and Offspring Asthma. Allergy 2016, 71, 1295–1304. [Google Scholar] [CrossRef]
- Mohanan, S.; Tapp, H.; McWilliams, A.; Dulin, M. Obesity and Asthma: Pathophysiology and Implications for Diagnosis and Management in Primary Care. Exp. Biol. Med. 2014, 239, 1531–1540. [Google Scholar] [CrossRef]
- Yao, T.-C.; Tsai, H.-J.; Chang, S.-W.; Chung, R.-H.; Hsu, J.-Y.; Tsai, M.-H.; Liao, S.-L.; Hua, M.-C.; Lai, S.-H.; Chen, L.-C.; et al. Obesity Disproportionately Impacts Lung Volumes, Airflow and Exhaled Nitric Oxide in Children. PLoS ONE 2017, 12, e0174691. [Google Scholar] [CrossRef]
- Tollefsen, E.; Langhammer, A.; Romundstad, P.; Bjermer, L.; Johnsen, R.; Holmen, T.L. Female Gender Is Associated with Higher Incidence and More Stable Respiratory Symptoms during Adolescence. Respir. Med. 2007, 101, 896–902. [Google Scholar] [CrossRef][Green Version]
- Fletcher, J.M.; Green, J.C.; Neidell, M.J. Long Term Effects of Childhood Asthma on Adult Health. J. Health Econ. 2010, 29, 377–387. [Google Scholar] [CrossRef]
- Hossain, M.J.; Xie, L.; Lang, J.E.; Wysocki, T.T.; Shaffer, T.H.; Bunnell, H.T. Piecewise Mixed Effects Model to Compare the Weight-Gain Patter Ns Before and After Diagnosis of Asthma in Children Younger than 5 Years. J. Biom. Biostat. 2015, 6, 248. [Google Scholar] [CrossRef]
- Chen, Z.; Salam, M.T.; Alderete, T.L.; Habre, R.; Bastain, T.M.; Berhane, K.; Gilliland, F.D. Effects of Childhood Asthma on the Development of Obesity among School-Aged Children. Am. J. Respir. Crit. Care Med. 2017, 195, 1181–1188. [Google Scholar] [CrossRef]
- Consilvio, N.P.; Di Pillo, S.; Verini, M.; de Giorgis, T.; Cingolani, A.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. The Reciprocal Influences of Asthma and Obesity on Lung Function Testing, AHR, and Airway Inflammation in Prepubertal Children. Pediatr. Pulmonol. 2010, 45, 1103–1110. [Google Scholar] [CrossRef][Green Version]
- van Veen, W.J.; Driessen, J.M.M.; Kersten, E.T.G.; van Leeuwen, J.C.; Brusse-Keizer, M.G.J.; van Aalderen, W.M.C.; Thio, B.J. BMI Predicts Exercise Induced Bronchoconstriction in Asthmatic Boys. Pediatr. Pulmonol. 2017, 52, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.-S.; Kim, Y.-D.; Shin, J.-H.; Kim, J.-H.; Oh, J.-W.; Lee, H.-B. Serum Leptin and Adiponectin Levels Correlate with Exercise-Induced Bronchoconstriction in Children with Asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2011, 107, 14–21. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R.; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, F.; Montella, S.; De Stefano, S.; Sperlì, F.; Barbarano, F.; Valerio, G. Relationship between Exhaled Nitric Oxide and Body Mass Index in Children and Adolescents. J. Allergy Clin. Immunol. 2005, 116, 1163–1164, author reply 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Erkoçoğlu, M.; Kaya, A.; Ozcan, C.; Akan, A.; Vezir, E.; Azkur, D.; Kara, O.; Demirel, F.; Ginis, T.; Civelek, E.; et al. The Effect of Obesity on the Level of Fractional Exhaled Nitric Oxide in Children with Asthma. Int. Arch. Allergy Immunol. 2013, 162, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; den Dekker, H.T.; Kruithof, C.J.; Reiss, I.K.; Vrijheid, M.; de Jongste, J.C.; Jaddoe, V.W.V.; Duijts, L. Early Childhood Growth Patterns and School-Age Respiratory Resistance, Fractional Exhaled Nitric Oxide and Asthma. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2016, 27, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.F.; Li, C.Y.; Lam, C.W.K.; Au, C.S.S.; Yung, E.; Chan, I.H.S.; Wong, G.W.K.; Fok, T.F. The Relation between Obesity and Asthmatic Airway Inflammation. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2004, 15, 344–350. [Google Scholar] [CrossRef]
- Chow, J.S.W.; Leung, A.S.M.; Li, W.W.S.; Tse, T.P.K.; Sy, H.Y.; Leung, T.F. Airway Inflammatory and Spirometric Measurements in Obese Children. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2009, 15, 346–352. [Google Scholar] [PubMed]
- Linn, W.S.; Rappaport, E.B.; Berhane, K.T.; Bastain, T.M.; Avol, E.L.; Gilliland, F.D. Exhaled Nitric Oxide in a Population-Based Study of Southern California Schoolchildren. Respir. Res. 2009, 10, 28. [Google Scholar] [CrossRef]
- Forno, E.; Lescher, R.; Strunk, R.; Weiss, S.; Fuhlbrigge, A.; Celedón, J.C.; Childhood Asthma Management Program Research Group. Decreased Response to Inhaled Steroids in Overweight and Obese Asthmatic Children. J. Allergy Clin. Immunol. 2011, 127, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W. Biological Treatments for Severe Asthma: Where Do We Stand? Curr. Opin. Allergy Clin. Immunol. 2018, 18, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. Available online: www.ginasthma.org (accessed on 28 September 2021).
- Willeboordse, M.; van de Kant, K.D.G.; Tan, F.E.S.; Mulkens, S.; Schellings, J.; Crijns, Y.; van der Ploeg, L.; van Schayck, C.P.; Dompeling, E. A Multifactorial Weight Reduction Programme for Children with Overweight and Asthma: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0157158. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Physical Activity Basics. Available online: https://www.cdc.gov/physicalactivity/basics/index.htm (accessed on 28 September 2021).
- Black, M.H.; Zhou, H.; Takayanagi, M.; Jacobsen, S.J.; Koebnick, C. Increased Asthma Risk and Asthma-Related Health Care Complications Associated with Childhood Obesity. Am. J. Epidemiol. 2013, 178, 1120–1128. [Google Scholar] [CrossRef]
- di Palmo, E.; Filice, E.; Cavallo, A.; Caffarelli, C.; Maltoni, G.; Miniaci, A.; Ricci, G.; Pession, A. Childhood Obesity and Respiratory Diseases: Which Link? Children 2021, 8, 177. [Google Scholar] [CrossRef]
- Lang, J.E.; Hossain, J.; Dixon, A.E.; Shade, D.; Wise, R.A.; Peters, S.P.; Lima, J.J. American Lung Association-Asthma Clinical Research Centers Does Age Impact the Obese Asthma Phenotype? Longitudinal Asthma Control, Airway Function, and Airflow Perception among Mild Persistent Asthmatics. Chest 2011, 140, 1524–1533. [Google Scholar] [CrossRef]
- Wood, L.G. Metabolic Dysregulation. Driving the Obese Asthma Phenotype in Adolescents? Am. J. Respir. Crit. Care Med. 2015, 191, 121–122. [Google Scholar] [CrossRef]
- Okubo, Y.; Nochioka, K.; Hataya, H.; Sakakibara, H.; Terakawa, T.; Testa, M. Burden of Obesity on Pediatric Inpatients with Acute Asthma Exacerbation in the United States. J. Allergy Clin. Immunol. Pract. 2016, 4, 1227–1231. [Google Scholar] [CrossRef]
- Aragona, E.; El-Magbri, E.; Wang, J.; Scheckelhoff, T.; Scheckelhoff, T.; Hyacinthe, A.; Nair, S.; Khan, A.; Nino, G.; Pillai, D.K. Impact of Obesity on Clinical Outcomes in Urban Children Hospitalized for Status Asthmaticus. Hosp. Pediatr. 2016, 6, 211–218. [Google Scholar] [CrossRef]
- Diaz, J.; Farzan, S. Clinical Implications of the Obese-Asthma Phenotypes. Immunol. Allergy Clin. N. Am. 2014, 34, 739–751. [Google Scholar] [CrossRef]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The Immunology of Asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, Y.; Li, J.; Li, S.; Zhang, Y.; Shen, J.; Tan, W.; Wu, C. Detection of IL-9 Producing T Cells in the PBMCs of Allergic Asthmatic Patients. BMC Immunol. 2017, 18, 38. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Hwang, Y.Y.; Scanlon, S.T.; Zaghouani, H.; Garbi, N.; Fallon, P.G.; McKenzie, A.N.J. Group 2 Innate Lymphoid Cells License Dendritic Cells to Potentiate Memory TH2 Cell Responses. Nat. Immunol. 2016, 17, 57–64. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Fukunaga, K.; Suzuki, Y.; Miyata, J.; Masaki, K.; Betsuyaku, T.; Koyasu, S.; Asano, K. Thymic Stromal Lymphopoietin Induces Corticosteroid Resistance in Natural Helper Cells during Airway Inflammation. Nat. Commun. 2013, 4, 2675. [Google Scholar] [CrossRef] [PubMed]
- Shikotra, A.; Choy, D.F.; Ohri, C.M.; Doran, E.; Butler, C.; Hargadon, B.; Shelley, M.; Abbas, A.R.; Austin, C.D.; Jackman, J.; et al. Increased Expression of Immunoreactive Thymic Stromal Lymphopoietin in Patients with Severe Asthma. J. Allergy Clin. Immunol. 2012, 129, 104–111.e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wu, J.; Zhao, J.; Liu, F.; Chen, Y.; Bi, L.; Liu, S.; Dong, L. IL-33 Promotes Airway Remodeling and Is a Marker of Asthma Disease Severity. J. Asthma Off. J. Assoc. Care Asthma 2014, 51, 863–869. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Östling, J.; Ax, E.; Calvén, J.; Thörn, K.; Israelsson, E.; Öberg, L.; Singhania, A.; Lau, L.C.K.; Wilson, S.J.; et al. Epithelial IL-6 Trans-Signaling Defines a New Asthma Phenotype with Increased Airway Inflammation. J. Allergy Clin. Immunol. 2019, 143, 577–590. [Google Scholar] [CrossRef]
- Jackson, D.J.; Bacharier, L.B.; Calatroni, A.; Gill, M.A.; Hu, J.; Liu, A.H.; Wheatley, L.M.; Gern, J.E.; Gruchalla, R.S.; Khurana Hershey, G.K.; et al. Serum IL-6: A Biomarker in Childhood Asthma? J. Allergy Clin. Immunol. 2020, 145, 1701–1704.e3. [Google Scholar] [CrossRef]
- Morjaria, J.B.; Babu, K.S.; Vijayanand, P.; Chauhan, A.J.; Davies, D.E.; Holgate, S.T. Sputum IL-6 Concentrations in Severe Asthma and Its Relationship with FEV1. Thorax 2011, 66, 537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Wills-Karp, M. The Potential Role of Interleukin-17 in Severe Asthma. Curr. Allergy Asthma Rep. 2011, 11, 388–394. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Croce, S.; Avanzini, M.A.; Regalbuto, C.; Cordaro, E.; Vinci, F.; Zuccotti, G.; Calcaterra, V. Adipose Tissue Immunomodulation and Treg/Th17 Imbalance in the Impaired Glucose Metabolism of Children with Obesity. Children 2021, 8, 554. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid-Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. TEM 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Arslan, N.; Erdur, B.; Aydin, A. Hormones and Cytokines in Childhood Obesity. Indian Pediatr. 2010, 47, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Neves Miranda, V.P.; Gouveia Peluzio, M.d.C.; Rodrigues de Faria, E.; Castro Franceschini, S.d.C.; Eloiza Priore, S. Inflammatory Markers in Relation to Body Composition, Physical Activity and Assessment of Nutritional Status of the Adolescents. Nutr. Hosp. 2015, 31, 1920–1927. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage Infiltration into Omental versus Subcutaneous Fat across Different Populations: Effect of Regional Adiposity and the Comorbidities of Obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Krist, J.; Wieder, K.; Klöting, N.; Oberbach, A.; Kralisch, S.; Wiesner, T.; Schön, M.R.; Gärtner, D.; Dietrich, A.; Shang, E.; et al. Effects of Weight Loss and Exercise on Apelin Serum Concentrations and Adipose Tissue Expression in Human Obesity. Obes. Facts 2013, 6, 57–69. [Google Scholar] [CrossRef]
- Flaherman, V.; Rutherford, G.W. A Meta-Analysis of the Effect of High Weight on Asthma. Arch. Dis. Child. 2006, 91, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. Adv. Exp. Med. Biol. 2017, 960, 221–245. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Paul, W.E. Early Signaling Events That Underlie Fate Decisions of Naive CD4(+) T Cells toward Distinct T-Helper Cell Subsets. Immunol. Rev. 2013, 252, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Childhood Obesity: Immune Response and Nutritional Approaches. Front. Immunol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Ryba-Stanisławowska, M.; Skrzypkowska, M.; Myśliwiec, M.; Myśliwska, J. Loss of the Balance between CD4(+)Foxp3(+) Regulatory T Cells and CD4(+)IL17A(+) Th17 Cells in Patients with Type 1 Diabetes. Hum. Immunol. 2013, 74, 701–707. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef]
- Qiu, H.; He, Y.; Ouyang, F.; Jiang, P.; Guo, S.; Guo, Y. The Role of Regulatory T Cells in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2019, 8, e014201. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Cui, B.; Li, P.; Hua, F.; Lv, X.; Zhou, J.; Hu, Z.; Zhang, X. 1,25-Dihydroxyvitamin D3 Protects Obese Rats from Metabolic Syndrome via Promoting Regulatory T Cell-Mediated Resolution of Inflammation. Acta Pharm. Sin. B 2018, 8, 178–187. [Google Scholar] [CrossRef]
- Martinez-Sanchez, M.E.; Hiriart, M.; Alvarez-Buylla, E.R. The CD4+ T Cell Regulatory Network Mediates Inflammatory Responses during Acute Hyperinsulinemia: A Simulation Study. BMC Syst. Biol. 2017, 11, 64. [Google Scholar] [CrossRef]
- Mackey-Lawrence, N.M.; Petri, W.A. Leptin and Mucosal Immunity. Mucosal Immunol. 2012, 5, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Beck, M.A. The Impact of Obesity on the Immune Response to Infection. Proc. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of Leptin With Obesity and Insulin Resistance. Cureus 2020, 12, e12178. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, Fat Mass and Immune System: Role for Leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef]
- La Cava, A. Leptin in Inflammation and Autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef]
- Filková, M.; Haluzík, M.; Gay, S.; Senolt, L. The Role of Resistin as a Regulator of Inflammation: Implications for Various Human Pathologies. Clin. Immunol. Orlando Fla 2009, 133, 157–170. [Google Scholar] [CrossRef]
- Kelishadi, R.; Roufarshbaf, M.; Soheili, S.; Payghambarzadeh, F.; Masjedi, M. Association of Childhood Obesity and the Immune System: A Systematic Review of Reviews. Child. Obes. Print 2017, 13, 332–346. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor Necrosis Factor Alpha: A Key Component of the Obesity-Diabetes Link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, but Not Tumor Necrosis Factor-Alpha, in Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Mangova, M.; Lipek, T.; Vom Hove, M.; Körner, A.; Kiess, W.; Treudler, R.; Prenzel, F. Obesity-Associated Asthma in Childhood. Allergol. Sel. 2020, 4, 76–85. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and Asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Clément, K.; Baur, L.A.; Tordjman, J. Obesity and Low-Grade Inflammation: A Paediatric Perspective. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2010, 11, 118–126. [Google Scholar] [CrossRef]
- Silva, L.R.; Stefanello, J.M.F.; Pizzi, J.; Timossi, L.S.; Leite, N. Atherosclerosis Subclinical and Inflammatory Markers in Obese and Nonobese Children and Adolescents. Rev. Bras. Epidemiol. Braz. J. Epidemiol. 2012, 15, 804–816. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, W.J.; Funahashi, T.; Tanaka, S.; Matsuzawa, Y.; Chao, C.L.; Chen, C.L.; Tai, T.Y.; Chuang, L.M. Weight Reduction Increases Plasma Levels of an Adipose-Derived Anti-Inflammatory Protein, Adiponectin. J. Clin. Endocrinol. Metab. 2001, 86, 3815–3819. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Naismith, E.; Miggitsch, C.; Carmona Arana, J.A.; Keller, M.; Grubeck-Loebenstein, B.; Weinberger, B. The Impact of Body Mass Index on Adaptive Immune Cells in the Human Bone Marrow. Immun. Ageing A 2020, 17, 15. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the Past Two Decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of Anti-Inflammatory Adipokines in Obesity-Related Diseases. Trends Endocrinol. Metab. TEM 2014, 25, 348–355. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in Health and Disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Cao, J.; Li, H.; Chen, L. Targeting Drugs to APJ Receptor: The Prospect of Treatment of Hypertension and Other Cardiovascular Diseases. Curr. Drug Targets 2015, 16, 148–155. [Google Scholar] [CrossRef]
- Itoh, N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, H.; Windsor, A.; Bessos, M.; Wolfe, L. Intra-Abdominal Pressure, Sagittal Abdominal Diameter and Obesity Comorbidity. J. Intern. Med. 1997, 241, 71–79. [Google Scholar] [CrossRef]
- Lazarus, R.; Colditz, G.; Berkey, C.S.; Speizer, F.E. Effects of Body Fat on Ventilatory Function in Children and Adolescents: Cross-Sectional Findings from a Random Population Sample of School Children. Pediatr. Pulmonol. 1997, 24, 187–194. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.-Y.; Mullen, J.; Celedón, J.C. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 570–581.e10. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Weiner, D.J.; Mullen, J.; Sawicki, G.; Kurland, G.; Han, Y.Y.; Cloutier, M.M.; Canino, G.; Weiss, S.T.; Litonjua, A.A.; et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am. J. Respir. Crit. Care Med. 2017, 195, 314–323. [Google Scholar] [CrossRef]
- Peters, J.I.; McKinney, J.M.; Smith, B.; Wood, P.; Forkner, E.; Galbreath, A.D. Impact of Obesity in Asthma: Evidence from a Large Prospective Disease Management Study. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2011, 106, 30–35. [Google Scholar] [CrossRef]
- Salome, C.M.; Munoz, P.A.; Berend, N.; Thorpe, C.W.; Schachter, L.M.; King, G.G. Effect of Obesity on Breathlessness and Airway Responsiveness to Methacholine in Non-Asthmatic Subjects. Int. J. Obes. 2005 2008, 32, 502–509. [Google Scholar] [CrossRef]
- Dixon, A. The Treatment of Asthma in Obesity. Expert Rev. Respir. Med. 2012, 6, 331–340. [Google Scholar] [CrossRef]
- in’t Veen, J.C.; Beekman, A.J.; Bel, E.H.; Sterk, P.J. Recurrent Exacerbations in Severe Asthma Are Associated with Enhanced Airway Closure during Stable Episodes. Am. J. Respir. Crit. Care Med. 2000, 161, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Dooley, A.A.; Pillai, D.K. Paediatric Obesity-Related Asthma: Disease Burden and Effects on Pulmonary Physiology. Paediatr. Respir. Rev. 2021, 37, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Canfield, S.M.; Andrade, A.; Isasi, C.R.; Hall, C.B.; Rubinstein, A.; Arens, R. Obesity-Associated Asthma in Children: A Distinct Entity. Chest 2012, 141, 895–905. [Google Scholar] [CrossRef]
- Rastogi, D.; Bhalani, K.; Hall, C.B.; Isasi, C.R. Association of Pulmonary Function with Adiposity and Metabolic Abnormalities in Urban Minority Adolescents. Ann. Am. Thorac. Soc. 2014, 11, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.J.; Mackenzie-Rife, K.A.; Witmans, M.B.; Montgomery, M.D.; Ball, G.D.C.; Egbogah, S.; Eves, N.D. Obesity Negatively Impacts Lung Function in Children and Adolescents. Pediatr. Pulmonol. 2014, 49, 1003–1010. [Google Scholar] [CrossRef]
- Salome, C.M.; King, G.G.; Berend, N. Physiology of Obesity and Effects on Lung Function. J. Appl. Physiol. Bethesda Md 1985 2010, 108, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.J.; Martin, J.G.; Macklem, P.T. Effects of Lung Volume on Maximal Methacholine-Induced Bronchoconstriction in Normal Humans. J. Appl. Physiol. Bethesda Md 1985 1987, 62, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- King, G.G.; Brown, N.J.; Diba, C.; Thorpe, C.W.; Muñoz, P.; Marks, G.B.; Toelle, B.; Ng, K.; Berend, N.; Salome, C.M. The Effects of Body Weight on Airway Calibre. Eur. Respir. J. 2005, 25, 896–901. [Google Scholar] [CrossRef]
- Mancini, M.C. Metabolic Syndrome in Children and Adolescents—Criteria for Diagnosis. Diabetol. Metab. Syndr. 2009, 1, 20. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H.; Khan, Z.S.; Tesfa, L.; Hall, C.B.; Macian, F. Inflammation, Metabolic Dysregulation, and Pulmonary Function among Obese Urban Adolescents with Asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, L.; Neal, W.A.; Ice, C.; Perez, M.K.; Piedimonte, G. Metabolic Abnormalities in Children with Asthma. Am. J. Respir. Crit. Care Med. 2011, 183, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bodas, M.; Bhatraju, N.K.; Pattnaik, B.; Gheware, A.; Parameswaran, P.K.; Thompson, M.; Freeman, M.; Mabalirajan, U.; Gosens, R.; et al. Hyperinsulinemia Adversely Affects Lung Structure and Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L837–L845. [Google Scholar] [CrossRef] [PubMed]
- Perdue, A.D.; Cottrell, L.A.; Lilly, C.L.; Gower, W.A.; Ely, B.A.; Foringer, B.; Wright, M.L.; Neal, W.A. Pediatric Metabolic Outcome Comparisons Based on a Spectrum of Obesity and Asthmatic Symptoms. J. Asthma Off. J. Assoc. Care Asthma 2019, 56, 388–394. [Google Scholar] [CrossRef]
- Schaafsma, D.; McNeill, K.D.; Stelmack, G.L.; Gosens, R.; Baarsma, H.A.; Dekkers, B.G.J.; Frohwerk, E.; Penninks, J.-M.; Sharma, P.; Ens, K.M.; et al. Insulin Increases the Expression of Contractile Phenotypic Markers in Airway Smooth Muscle. Am. J. Physiol. Cell Physiol. 2007, 293, C429–C439. [Google Scholar] [CrossRef]
- Al-Shawwa, B.A.; Al-Huniti, N.H.; DeMattia, L.; Gershan, W. Asthma and Insulin Resistance in Morbidly Obese Children and Adolescents. J. Asthma Off. J. Assoc. Care Asthma 2007, 44, 469–473. [Google Scholar] [CrossRef]
- Arshi, M.; Cardinal, J.; Hill, R.J.; Davies, P.S.W.; Wainwright, C. Asthma and Insulin Resistance in Children. Respirol. Carlton Vic. 2010, 15, 779–784. [Google Scholar] [CrossRef]
- Grasemann, H.; Holguin, F. Oxidative Stress and Obesity-Related Asthma. Paediatr. Respir. Rev. 2021, 37, 18–21. [Google Scholar] [CrossRef]
- North, M.L.; Khanna, N.; Marsden, P.A.; Grasemann, H.; Scott, J.A. Functionally Important Role for Arginase 1 in the Airway Hyperresponsiveness of Asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L911–L920. [Google Scholar] [CrossRef]
- Brown, S.D.; Baxter, K.M.; Stephenson, S.T.; Esper, A.M.; Brown, L.A.S.; Fitzpatrick, A.M. Airway TGF-Β1 and Oxidant Stress in Children with Severe Asthma: Association with Airflow Limitation. J. Allergy Clin. Immunol. 2012, 129, 388–396.e1–e8. [Google Scholar] [CrossRef]
- Munblit, D.; Treneva, M.; Peroni, D.; Colicino, S.; Chow, L.; Dissanayeke, S.; Pampura, A.; Boner, A.; Geddes, D.; Boyle, R.; et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef]
- Munblit, D.; Treneva, M.; Peroni, D.; Colicino, S.; Chow, L.; Dissanayeke, S.; Abrol, P.; Sheth, S.; Pampura, A.; Boner, A.; et al. Colostrum and Mature Human Milk of Women from London, Moscow, and Verona: Determinants of Immune Composition. Nutrients 2016, 8, 695. [Google Scholar] [CrossRef] [PubMed]
- Minniti, F.; Comberiati, P.; Munblit, D.; Piacentini, G.; Antoniazzi, E.; Zanoni, L.; Boner, A.; Peroni, D. Breast-Milk Characteristics Protecting Against Allergy. Endocrine‚ Metab. Immune Disord.-Drug Targets 2014, 14, 9–15. [Google Scholar] [CrossRef]
- Verhasselt, V.; Genuneit, J.; Metcalfe, J.R.; Tulic, M.K.; Rekima, A.; Palmer, D.J.; Prescott, S.L. Ovalbumin in Breastmilk Is Associated with a Decreased Risk of IgE-Mediated Egg Allergy in Children. Allergy 2020, 75, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Guibas, G.V.; Xepapadaki, P.; Moschonis, G.; Douladiris, N.; Filippou, A.; Tsirigoti, L.; Manios, Y.; Papadopoulos, N.G. Breastfeeding and Wheeze Prevalence in Pre-Schoolers and Pre-Adolescents: The Genesis and Healthy Growth Studies. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2013, 24, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet Lond. Engl. 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Lodge, C.; Tan, D.; Lau, M.; Dai, X.; Tham, R.; Lowe, A.; Bowatte, G.; Allen, K.; Dharmage, S. Breastfeeding and Asthma and Allergies: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Timing of Introduction of Complementary Foods, and Hydrolyzed Formulas. Pediatrics 2008, 121, 183–191. [Google Scholar] [CrossRef]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and Childhood Asthma: Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef]
- Trambusti, I.; Nuzzi, G.; Costagliola, G.; Verduci, E.; D’Auria, E.; Peroni, D.G.; Comberiati, P. Dietary Interventions and Nutritional Factors in the Prevention of Pediatric Asthma. Front. Pediatr. 2020, 8, 480. [Google Scholar] [CrossRef]
- Sozańska, B.; Sikorska-Szaflik, H. Diet Modifications in Primary Prevention of Asthma. Where Do We Stand? Nutrients 2021, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- El-Heneidy, A.; Abdel-Rahman, M.E.; Mihala, G.; Ross, L.J.; Comans, T.A. Milk Other Than Breast Milk and the Development of Asthma in Children 3 Years of Age. A Birth Cohort Study (2006–2011). Nutrients 2018, 10, 1798. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.C.; Allen, K.; Edwards, C.A.; Beyer, K.; Boulay, A.; van der Aa, L.B.; Sprikkelman, A.; Belohlavkova, S.; Clausen, M.; Dubakiene, R.; et al. Infant Feeding and Allergy Prevention: A Review of Current Knowledge and Recommendations. A EuroPrevall State of the Art Paper. Allergy 2009, 64, 1407–1416. [Google Scholar] [CrossRef]
- von Berg, A.; Filipiak-Pittroff, B.; Krämer, U.; Link, E.; Heinrich, J.; Koletzko, S.; Grübl, A.; Hoffmann, U.; Beckmann, C.; Reinhardt, D.; et al. The German Infant Nutritional Intervention Study (GINI) for the Preventive Effect of Hydrolysed Infant Formulas in Infants at High Risk for Allergic Diseases. Design and Selected Results. Allergol. Sel. 2017, 1, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Hansen, K.S.; Jacobsen, H.P.; Estmann, A.; Christensen, A.E.F.; Hansen, L.G.; Kier, S.R.; Lassen, K.; Lintrup, M.; Mortensen, S.; et al. Comparison of a Partially Hydrolyzed Infant Formula with Two Extensively Hydrolyzed Formulas for Allergy Prevention: A Prospective, Randomized Study: Comparison of Hydrolyzed Infant Formulas for Allergy Prevention. Pediatr. Allergy Immunol. 2000, 11, 149–161. [Google Scholar] [CrossRef]
- Browne, P.D.; Claassen, E.; Cabana, M.D. Microbiota in Health and Disease: From Pregnancy to Childhood; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; ISBN 978-90-8686-294-8. Available online: https://www.wageningenacademic.com/ (accessed on 28 September 2021).
- Kewalramani, A.; Bollinger, M.E. The Impact of Food Allergy on Asthma. J. Asthma Allergy 2010, 3, 65–74. [Google Scholar] [CrossRef][Green Version]
- von Berg, A.; Filipiak-Pittroff, B.; Krämer, U.; Hoffmann, B.; Link, E.; Beckmann, C.; Hoffmann, U.; Reinhardt, D.; Grübl, A.; Heinrich, J.; et al. Allergies in High-Risk Schoolchildren after Early Intervention with Cow’s Milk Protein Hydrolysates: 10-Year Results from the German Infant Nutritional Intervention (GINI) Study. J. Allergy Clin. Immunol. 2013, 131, 1565–1573.e5. [Google Scholar] [CrossRef]
- Berg, A.; Filipiak-Pittroff, B.; Schulz, H.; Hoffmann, U.; Link, E.; Sußmann, M.; Schnappinger, M.; Brüske, I.; Standl, M.; Krämer, U.; et al. Allergic Manifestation 15 Years after Early Intervention with Hydrolyzed Formulas—The GINI Study. Allergy 2016, 71, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Gibson, P.G. Dietary Factors Lead to Innate Immune Activation in Asthma. Pharmacol. Ther. 2009, 123, 37–53. [Google Scholar] [CrossRef]
- Davisse-Paturet, C.; Raherison, C.; Adel-Patient, K.; Divaret-Chauveau, A.; Bois, C.; Dufourg, M.; Lioret, S.; Charles, M.; Lauzon-Guillain, B. Use of Partially Hydrolysed Formula in Infancy and Incidence of Eczema, Respiratory Symptoms or Food Allergies in Toddlers from the ELFE Cohort. Pediatr. Allergy Immunol. 2019, 30, 614–623. [Google Scholar] [CrossRef]
- Osborn, D.A.; Sinn, J.K.; Jones, L.J. Infant Formulas Containing Hydrolysed Protein for Prevention of Allergic Disease. Cochrane Database Syst. Rev. 2018, 10, CD003664. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Woods, R.K.; Walters, E.H.; Raven, J.M.; Wolfe, R.; Ireland, P.D.; Thien, F.C.K.; Abramson, M.J. Food and Nutrient Intakes and Asthma Risk in Young Adults. Am. J. Clin. Nutr. 2003, 78, 414–421. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Forno, E.; Brehm, J.M.; Acosta-Pérez, E.; Alvarez, M.; Colón-Semidey, A.; Rivera-Soto, W.; Campos, H.; Litonjua, A.A.; Alcorn, J.F.; et al. Diet, Interleukin-17, and Childhood Asthma in Puerto Ricans. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2015, 115, 288–293.e1. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, D.; Salameh, P.; Baldi, I.; Raherison, C. Diet and Allergic Diseases among Population Aged 0 to 18 Years: Myth or Reality? Nutrients 2013, 5, 3399–3423. [Google Scholar] [CrossRef] [PubMed]
- Dougkas, A.; Barr, S.; Reddy, S.; Summerbell, C.D. A Critical Review of the Role of Milk and Other Dairy Products in the Development of Obesity in Children and Adolescents. Nutr. Res. Rev. 2019, 32, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Comberiati, P.; Costagliola, G.; D’Elios, S.; Peroni, D. Prevention of Food Allergy: The Significance of Early Introduction. Medicina 2019, 55, 323. [Google Scholar] [CrossRef]
- Zutavern, A.; Brockow, I.; Schaaf, B.; von Berg, A.; Diez, U.; Borte, M.; Kraemer, U.; Herbarth, O.; Behrendt, H.; Wichmann, H.-E.; et al. Timing of Solid Food Introduction in Relation to Eczema, Asthma, Allergic Rhinitis, and Food and Inhalant Sensitization at the Age of 6 Years: Results from the Prospective Birth Cohort Study LISA. Pediatrics 2008, 121, e44–e52. [Google Scholar] [CrossRef]
- Lau, S. What Is New in the Prevention of Atopy and Asthma? Curr. Opin. Allergy Clin. Immunol. 2013, 13, 181–186. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Takkinen, H.-M.; Niemelä, O.; Kaila, M.; Erkkola, M.; Ahonen, S.; Haapala, A.-M.; Kenward, M.G.; Pekkanen, J.; Lahesmaa, R.; et al. Timing of Infant Feeding in Relation to Childhood Asthma and Allergic Diseases. J. Allergy Clin. Immunol. 2013, 131, 78–86. [Google Scholar] [CrossRef]
- Njå, F.; Nystad, W.; Lødrup Carlsen, K.; Hetlevik, Ø.; Carlsen, K.-H. Effects of Early Intake of Fruit or Vegetables in Relation to Later Asthma and Allergic Sensitization in School-Age Children. Acta Paediatr. 2005, 94, 147–154. [Google Scholar] [CrossRef]
- Virtanen, S.M.; Kaila, M.; Pekkanen, J.; Kenward, M.G.; Uusitalo, U.; Pietinen, P.; Kronberg-Kippilä, C.; Hakulinen, T.; Simell, O.; Ilonen, J.; et al. Early Introduction of Oats Associated with Decreased Risk of Persistent Asthma and Early Introduction of Fish with Decreased Risk of Allergic Rhinitis. Br. J. Nutr. 2010, 103, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Craig, L.C.A.; Allan, K.; Prabhu, N.; Turner, S.W.; McNeill, G.; Erkkola, M.; Seaton, A.; Devereux, G. Breastfeeding and Introduction of Complementary Foods during Infancy in Relation to the Risk of Asthma and Atopic Diseases up to 10 Years. Clin. Exp. Allergy 2013, 43, 1263–1273. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The Role of Fish Intake on Asthma in Children: A Meta-Analysis of Observational Studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Hesselmar, B.; Saalman, R.; Rudin, A.; Adlerberth, I.; Wold, A. Early Fish Introduction Is Associated with Less Eczema, but Not Sensitization, in Infants: Fish and Eczema. Acta Paediatr. 2010, 99, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Q.; Liu, B.; Li, J.; Luo, C.-Q.; Zhang, Q.; Chen, J.-L.; Sinha, A.; Li, Z.-Y. Fish Intake during Pregnancy or Infancy and Allergic Outcomes in Children: A Systematic Review and Meta-Analysis. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2017, 28, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sozańska, B. Raw Cow’s Milk and Its Protective Effect on Allergies and Asthma. Nutrients 2019, 11, 469. [Google Scholar] [CrossRef]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The Protective Effect of Farm Milk Consumption on Childhood Asthma and Atopy: The GABRIELA Study. J. Allergy Clin. Immunol. 2011, 128, 766–773.e4. [Google Scholar] [CrossRef]
- Brick, T.; Schober, Y.; Böcking, C.; Pekkanen, J.; Genuneit, J.; Loss, G.; Dalphin, J.-C.; Riedler, J.; Lauener, R.; Nockher, W.A.; et al. ω-3 Fatty Acids Contribute to the Asthma-Protective Effect of Unprocessed Cow’s Milk. J. Allergy Clin. Immunol. 2016, 137, 1699–1706.e13. [Google Scholar] [CrossRef]
- Abbring, S.; Verheijden, K.A.T.; Diks, M.A.P.; Leusink-Muis, A.; Hols, G.; Baars, T.; Garssen, J.; van Esch, B.C.A.M. Raw Cow’s Milk Prevents the Development of Airway Inflammation in a Murine House Dust Mite-Induced Asthma Model. Front. Immunol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, R.J.J.; Knol, E.F.; Heck, J.M.L.; Savelkoul, H.F.J. Which Factors in Raw Cow’s Milk Contribute to Protection against Allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, B.; Pfaffl, M.W.; Dumpler, J.; von Mutius, E.; Ege, M.J. MicroRNA in Native and Processed Cow’s Milk and Its Implication for the Farm Milk Effect on Asthma. J. Allergy Clin. Immunol. 2016, 137, 1893–1895.e13. [Google Scholar] [CrossRef]
- Wang, J.; Liu, A.H. Food Allergies and Asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.V.; Erler, N.S.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; van den Hooven, E.H.; Franco, O.H.; Voortman, T. Dietary Intake of Protein in Early Childhood Is Associated with Growth Trajectories between 1 and 9 Years of Age. J. Nutr. 2016, 146, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA) Dietary Reference Values for the European Union. Available online: https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values (accessed on 28 September 2021).
- Hörnell, A.; Lagström, H.; Lande, B.; Thorsdottir, I. Protein Intake from 0 to 18 Years of Age and Its Relation to Health: A Systematic Literature Review for the 5th Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 57, 21083. [Google Scholar] [CrossRef] [PubMed]
- Luque, V.; Closa-Monasterolo, R.; Escribano, J.; Ferré, N. Early Programming by Protein Intake: The Effect of Protein on Adiposity Development and the Growth and Functionality of Vital Organs. Nutr. Metab. Insights 2015, 8, 49–56. [Google Scholar] [CrossRef]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2017, 32, 318–325. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Haines, J.; Haycraft, E.; Lytle, L.; Nicklaus, S.; Kok, F.J.; Merdji, M.; Fisberg, M.; Moreno, L.A.; Goulet, O.; Hughes, S.O. Nurturing Children’s Healthy Eating: Position Statement. Appetite 2019, 137, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Barr, S.I.; McNulty, H.; Li, D.; Blumberg, J.B. Health Effects of Vitamin and Mineral Supplements. BMJ 2020, 369, m2511. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean Diet in Pregnancy Is Protective for Wheeze and Atopy in Childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Calatayud-Sáez, F.M.; Calatayud Moscoso Del Prado, B.; Gallego Fernández-Pacheco, J.G.; González-Martín, C.; Alguacil Merino, L.F. Mediterranean Diet and Childhood Asthma. Allergol. Immunopathol. (Madr.) 2016, 44, 99–105. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive Oil, the Mediterranean Diet, and Arterial Blood Pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Am. J. Clin. Nutr. 2004, 80, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Zallo, N.; Aguinaga-Ontoso, I.; Alvarez-Alvarez, I.; Marin-Fernandez, B.; Guillén-Grima, F.; Azcona-San Julián, C. Influence of the Mediterranean Diet during Pregnancy in the Development of Wheezing and Eczema in Infants in Pamplona, Spain. Allergol. Immunopathol. (Madr.) 2018, 46, 9–14. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Ramirez-Hernandez, M.; Padilla, O.; Pacheco-Gonzalez, R.M.; Pérez-Fernández, V.; Garcia-Marcos, L. Effect of Foods and Mediterranean Diet during Pregnancy and First Years of Life on Wheezing, Rhinitis and Dermatitis in Preschoolers. Allergol. Immunopathol. (Madr.) 2016, 44, 400–409. [Google Scholar] [CrossRef]
- Lange, N.E.; Rifas-Shiman, S.L.; Camargo, C.A.; Gold, D.R.; Gillman, M.W.; Litonjua, A.A. Maternal Dietary Pattern during Pregnancy Is Not Associated with Recurrent Wheeze in Children. J. Allergy Clin. Immunol. 2010, 126, 250–255.e1–e4. [Google Scholar] [CrossRef]
- Biagi, C.; Nunzio, M.D.; Bordoni, A.; Gori, D.; Lanari, M. Effect of Adherence to Mediterranean Diet during Pregnancy on Children’s Health: A Systematic Review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Kogevinas, M. Prenatal and Childhood Mediterranean Diet and the Development of Asthma and Allergies in Children. Public Health Nutr. 2009, 12, 1629–1634. [Google Scholar] [CrossRef]
- Baines, K.J.; Wood, L.G.; Gibson, P.G. The Nutrigenomics of Asthma: Molecular Mechanisms of Airway Neutrophilia Following Dietary Antioxidant Withdrawal. Omics J. Integr. Biol. 2009, 13, 355–365. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-Rich Treatments Modify Noneosinophilic Airway Inflammation in Asthma: Proof of Concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef]
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Vafa, M.R.; Sharma, S.; Kolahdooz, F. Fruit and Vegetable Intake and Risk of Wheezing and Asthma: A Systematic Review and Meta-Analysis. Nutr. Rev. 2014, 72, 411–428. [Google Scholar] [CrossRef]
- Lv, N.; Xiao, L.; Ma, J. Dietary Pattern and Asthma: A Systematic Review and Meta-Analysis. J. Asthma Allergy 2014, 7, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Sterne, J.A.; Thompson, R.L.; Songhurst, C.E.; Margetts, B.M.; Burney, P.G. Dietary Antioxidants and Asthma in Adults: Population-Based Case-Control Study. Am. J. Respir. Crit. Care Med. 2001, 164, 1823–1828. [Google Scholar] [CrossRef]
- Akcay, A.; Tamay, Z.; Hocaoglu, A.B.; Ergin, A.; Guler, N. Risk Factors Affecting Asthma Prevalence in Adolescents Living in Istanbul, Turkey. Allergol. Immunopathol. (Madr.) 2014, 42, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Yi, S.; Ichimura, Y.; Hori, A.; Izumi, S.; Sugiyama, H.; Kudo, K.; Mizoue, T.; Kobayashi, N. Effect of Lifestyle on Asthma Control in Japanese Patients: Importance of Periodical Exercise and Raw Vegetable Diet. PLoS ONE 2013, 8, e68290. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.-H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and Dietary Intake: An Overview of Systematic Reviews. Allergy 2016, 71, 433–442. [Google Scholar] [CrossRef]
- Thorsteinsdottir, F.; Maslova, E.; Jacobsen, R.; Frederiksen, P.; Keller, A.; Backer, V.; Heitmann, B.L. Exposure to Vitamin D Fortification Policy in Prenatal Life and the Risk of Childhood Asthma: Results From the D-Tect Study. Nutrients 2019, 11, 924. [Google Scholar] [CrossRef]
- Douros, K.; Thanopoulou, M.-I.; Boutopoulou, B.; Papadopoulou, A.; Papadimitriou, A.; Fretzayas, A.; Priftis, K.N. Adherence to the Mediterranean Diet and Inflammatory Markers in Children with Asthma. Allergol. Immunopathol. (Madr.) 2019, 47, 209–213. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What Are the Effects of a Mediterranean Diet on Allergies and Asthma in Children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Itsiopoulos, C.; Susanto, N.H.; Erbas, B. Does Adherence to the Mediterranean Dietary Pattern Reduce Asthma Symptoms in Children? A Systematic Review of Observational Studies. Public Health Nutr. 2017, 20, 2722–2734. [Google Scholar] [CrossRef]
- Tabak, C.; Wijga, A.H.; de Meer, G.; Janssen, N.A.H.; Brunekreef, B.; Smit, H.A. Diet and Asthma in Dutch School Children (ISAAC-2). Thorax 2006, 61, 1048–1053. [Google Scholar] [CrossRef]
- Kan, H.; Stevens, J.; Heiss, G.; Rose, K.M.; London, S.J. Dietary Fiber, Lung Function, and Chronic Obstructive Pulmonary Disease in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2008, 167, 570–578. [Google Scholar] [CrossRef]
- Wood, L.G. Diet, Obesity, and Asthma. Ann. Am. Thorac. Soc. 2017, 14, S332–S338. [Google Scholar] [CrossRef]
- McAleer, J.P.; Kolls, J.K. Contributions of the Intestinal Microbiome in Lung Immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary Fiber for the Treatment of Type 2 Diabetes Mellitus: A Meta-Analysis. J. Am. Board Fam. Med. JABFM 2012, 25, 16–23. [Google Scholar] [CrossRef]
- Qi, L.; Rimm, E.; Liu, S.; Rifai, N.; Hu, F.B. Dietary Glycemic Index, Glycemic Load, Cereal Fiber, and Plasma Adiponectin Concentration in Diabetic Men. Diabetes Care 2005, 28, 1022–1028. [Google Scholar] [CrossRef]
- Farshchi, M.K.; Azad, F.J.; Salari, R.; Mirsadraee, M.; Anushiravani, M. A Viewpoint on the Leaky Gut Syndrome to Treat Allergic Asthma: A Novel Opinion. J. Evid.-Based Complement. Altern. Med. 2017, 22, 378–380. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating Antioxidant Intake in Asthma: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Patel, S.; Custovic, A.; Smith, J.A.; Simpson, A.; Kerry, G.; Murray, C.S. Cross-Sectional Association of Dietary Patterns with Asthma and Atopic Sensitisation in Childhood—In a Cohort Study. Pediatr. Allergy Immunol. 2014, 25, 565–571. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI Position Paper: Influence of Dietary Fatty Acids on Asthma, Food Allergy, and Atopic Dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; El-Obeid, T.; Meftah, Z.; Alawi, A.; Said, S.; Ganji, V. Fast Food and Sweet Intake Pattern Is Directly Associated with the Prevalence of Asthma in a Qatari Population. Eur. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Wickens, K.; Barry, D.; Friezema, A.; Rhodius, R.; Bone, N.; Purdie, G.; Crane, J. Fast Foods—Are They a Risk Factor for Asthma? Allergy 2005, 60, 1537–1541. [Google Scholar] [CrossRef]
- Mai, X.-M.; Becker, A.B.; Liem, J.J.; Kozyrskyj, A.L. Fast Food Consumption Counters the Protective Effect of Breastfeeding on Asthma in Children? Clin. Exp. Allergy 2009, 39, 556–561. [Google Scholar] [CrossRef]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the Association between Dietary Intake, Disease Severity and Airway Inflammation in Asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory Subtypes in Asthma: Assessment and Identification Using Induced Sputum. Respirology 2006, 11, 54–61. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.-J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-Producing Innate Lymphoid Cells and the NLRP3 Inflammasome Facilitate Obesity-Associated Airway Hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of Omega-3 Fatty Acids and Their Metabolites in Asthma and Allergic Diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef]
- Mizuta, K.; Matoba, A.; Shibata, S.; Masaki, E.; Emala, C.W. Obesity-Induced Asthma: Role of Free Fatty Acid Receptors. Jpn. Dent. Sci. Rev. 2019, 55, 103–107. [Google Scholar] [CrossRef]
- Matoba, A.; Matsuyama, N.; Shibata, S.; Masaki, E.; Emala, C.W.; Mizuta, K. The Free Fatty Acid Receptor 1 Promotes Airway Smooth Muscle Cell Proliferation through MEK/ERK and PI3K/Akt Signaling Pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L333–L348. [Google Scholar] [CrossRef]
- Losol, P.; Rezwan, F.I.; Patil, V.K.; Venter, C.; Ewart, S.; Zhang, H.; Arshad, S.H.; Karmaus, W.; Holloway, J.W. Effect of Gestational Oily Fish Intake on the Risk of Allergy in Children May Be Influenced by FADS1/2, ELOVL5 Expression and DNA Methylation. Genes Nutr. 2019, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Frumento, P.; Harb, H.; Alashkar Alhamwe, B.; Johansson, C.; Eick, L.; Alm, J.; Renz, H.; Scheynius, A.; Potaczek, D. Histone Acetylation of Immune Regulatory Genes in Human Placenta in Association with Maternal Intake of Olive Oil and Fish Consumption. Int. J. Mol. Sci. 2019, 20, 1060. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Irvine, J.; Amarasekera, M.; Hii, C.S.; Kesper, D.A.; Ma, Y.; D’Vaz, N.; Renz, H.; Potaczek, D.P.; Prescott, S.L.; et al. The Role of PKCζ in Cord Blood T-Cell Maturation towards Th1 Cytokine Profile and Its Epigenetic Regulation by Fish Oil. Biosci. Rep. 2017, 37, BSR20160485. [Google Scholar] [CrossRef]
- Blümer, N.; Renz, H. Consumption of Ω3-Fatty Acids during Perinatal Life: Role in Immuno-Modulation and Allergy Prevention. J. Perinat. Med. 2007, 35, S12–S18. [Google Scholar] [CrossRef]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil–Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- Hansen, S.; Strøm, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granström, C.; Halldorsson, T.I.; et al. Fish Oil Supplementation during Pregnancy and Allergic Respiratory Disease in the Adult Offspring. J. Allergy Clin. Immunol. 2017, 139, 104–111.e4. [Google Scholar] [CrossRef]
- Gunaratne, A.W.; Makrides, M.; Collins, C.T. Maternal Prenatal and/or Postnatal n-3 Long Chain Polyunsaturated Fatty Acids (LCPUFA) Supplementation for Preventing Allergies in Early Childhood. Cochrane Database Syst. Rev. 2015, CD010085. [Google Scholar] [CrossRef]
- Vahdaninia, M.; Mackenzie, H.; Dean, T.; Helps, S. ω-3 LCPUFA Supplementation during Pregnancy and Risk of Allergic Outcomes or Sensitization in Offspring. Ann. Allergy. Asthma. Immunol. 2019, 122, 302–313.e2. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Roggi, C.; Cena, H. N-3 LC-PUFA Supplementation: Effects on Infant and Maternal Outcomes. Eur. J. Nutr. 2014, 53, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Rago, D.; Rasmussen, M.A.; Lee-Sarwar, K.A.; Weiss, S.T.; Lasky-Su, J.; Stokholm, J.; Bønnelykke, K.; Chawes, B.L.; Bisgaard, H. Fish-Oil Supplementation in Pregnancy, Child Metabolomics and Asthma Risk. EBioMedicine 2019, 46, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Øien, T.; Schjelvaag, A.; Storrø, O.; Johnsen, R.; Simpson, M.R. Fish Consumption at One Year of Age Reduces the Risk of Eczema, Asthma and Wheeze at Six Years of Age. Nutrients 2019, 11, 1969. [Google Scholar] [CrossRef]
- Birch, E.E.; Khoury, J.C.; Berseth, C.L.; Castañeda, Y.S.; Couch, J.M.; Bean, J.; Tamer, R.; Harris, C.L.; Mitmesser, S.H.; Scalabrin, D.M. The Impact of Early Nutrition on Incidence of Allergic Manifestations and Common Respiratory Illnesses in Children. J. Pediatr. 2010, 156, 902–906.e1. [Google Scholar] [CrossRef] [PubMed]
- D’Vaz, N.; Meldrum, S.J.; Dunstan, J.A.; Martino, D.; McCarthy, S.; Metcalfe, J.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Postnatal Fish Oil Supplementation in High-Risk Infants to Prevent Allergy: Randomized Controlled Trial. Pediatrics 2012, 130, 674–682. [Google Scholar] [CrossRef]
- Marks, G.B.; Mihrshahi, S.; Kemp, A.S.; Tovey, E.R.; Webb, K.; Almqvist, C.; Ampon, R.D.; Crisafulli, D.; Belousova, E.G.; Mellis, C.M. Prevention of Asthma during the First 5 Years of Life: A Randomized Controlled Trial. J. Allergy Clin. Immunol. 2006, 118, 53–61. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, Y.; Wang, H.; Jiang, H. A Dose–Response Meta-Analysis Between Maternal Fish Oil Supplement And Risk of Asthma/Wheeze In Offspring 2021. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Lee, S.-C.; Yang, Y.-H.; Chuang, S.-Y.; Huang, S.-Y.; Pan, W.-H. Reduced Medication Use and Improved Pulmonary Function with Supplements Containing Vegetable and Fruit Concentrate, Fish Oil and Probiotics in Asthmatic School Children: A Randomised Controlled Trial. Br. J. Nutr. 2013, 110, 145–155. [Google Scholar] [CrossRef]
- Yokoyama, A.; Hamazaki, T.; Ohshita, A.; Kohno, N.; Sakai, K.; Zhao, G.-D.; Katayama, H.; Hiwada, K. Effect of Aerosolized Docosahexaenoic Acid in a Mouse Model of Atopic Asthma. Int. Arch. Allergy Immunol. 2000, 123, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Forno, E.; Holguin, F.; Celedón, J.C. Diet and Asthma: An Update. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 369–374. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Blatter, J.; Brehm, J.M.; Forno, E.; Litonjua, A.A.; Celedón, J.C. Diet and Asthma: Vitamins and Methyl Donors. Lancet Respir. Med. 2013, 1, 813–822. [Google Scholar] [CrossRef]
- Allen, S.; Britton, J.R.; Leonardi-Bee, J.A. Association between Antioxidant Vitamins and Asthma Outcome Measures: Systematic Review and Meta-Analysis. Thorax 2009, 64, 610–619. [Google Scholar] [CrossRef]
- Litonjua, A.A. Fat-Soluble Vitamins and Atopic Disease: What Is the Evidence? Proc. Nutr. Soc. 2012, 71, 67–74. [Google Scholar] [CrossRef]
- Checkley, W.; West, K.P.; Wise, R.A.; Wu, L.; LeClerq, S.C.; Khatry, S.; Katz, J.; Christian, P.; Tielsch, J.M.; Sommer, A. Supplementation with Vitamin A Early in Life and Subsequent Risk of Asthma. Eur. Respir. J. 2011, 38, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; West, K.P.; Wise, R.A.; Baldwin, M.R.; Wu, L.; LeClerq, S.C.; Christian, P.; Katz, J.; Tielsch, J.M.; Khatry, S.; et al. Maternal Vitamin A Supplementation and Lung Function in Offspring. N. Engl. J. Med. 2010, 362, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Bando, N.; Yamanishi, R.; Terao, J. Inhibition of Immunoglobulin E Production in Allergic Model Mice by Supplementation with Vitamin E and β-Carotene. Biosci. Biotechnol. Biochem. 2003, 67, 2176–2182. [Google Scholar] [CrossRef][Green Version]
- Devereux, G. Early Life Events in Asthma—Diet. Pediatr. Pulmonol. 2007, 42, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedón, J.C.; Castro-Rodriguez, J.A. Maternal Nutrition during Pregnancy and Risk of Asthma, Wheeze, and Atopic Diseases during Childhood: A Systematic Review and Meta-Analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Virtanen, S.M.; Alfthan, G.; Karvonen, A.M.; Genuneit, J.; Lauener, R.P.; Dalphin, J.-C.; Hyvärinen, A.; Pfefferle, P.; Riedler, J.; et al. Serum Vitamin E Concentrations at 1 Year and Risk of Atopy, Atopic Dermatitis, Wheezing, and Asthma in Childhood: The PASTURE Study. Allergy 2014, 69, 87–94. [Google Scholar] [CrossRef]
- Patel, B.D. Dietary Antioxidants and Asthma in Adults. Thorax 2006, 61, 388–393. [Google Scholar] [CrossRef]
- Romieu, I.; Varraso, R.; Avenel, V.; Leynaert, B.; Kauffmann, F.; Clavel-Chapelon, F. Fruit and Vegetable Intakes and Asthma in the E3N Study. Thorax 2006, 61, 209–215. [Google Scholar] [CrossRef]
- Pearson, P.J.K.; Lewis, S.A.; Britton, J.; Fogarty, A. Vitamin E Supplements in Asthma: A Parallel Group Randomised Placebo Controlled Trial. Thorax 2004, 59, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Newson, R.B.; Rayman, M.P.; Wong, A.P.-L.; Tumilty, M.K.; Phillips, J.M.; Potts, J.F.; Kelly, F.J.; White, P.T.; Burney, P.G.J. Randomised, Double Blind, Placebo-Controlled Trial of Selenium Supplementation in Adult Asthma. Thorax 2007, 62, 483–490. [Google Scholar] [CrossRef]
- Wilkinson, M.; Hart, A.; Milan, S.J.; Sugumar, K. Vitamins C and E for Asthma and Exercise-Induced Bronchoconstriction. Cochrane Database Syst. Rev. 2014, 6, CD010749. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L.; Tangpricha, V. Vitamin D and Molecular Actions on the Immune System: Modulation of Innate and Autoimmunity. J. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef]
- Bozzetto, S.; Carraro, S.; Giordano, G.; Boner, A.; Baraldi, E. Asthma, Allergy and Respiratory Infections: The Vitamin D Hypothesis. Allergy 2012, 67, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Sievert, L.L.; Muttukrishna, S.; Begum, K.; Murphy, L.; Sharmeen, T.; Gunu, R.; Chowdhury, O.; Bentley, G.R. Mismatch: A Comparative Study of Vitamin D Status in British-Bangladeshi Migrants. Evol. Med. Public Health 2021, 9, 164–173. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Szaflik, H.; Sozańska, B. The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients 2020, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Hollams, E.M.; Teo, S.M.; Kusel, M.; Holt, B.J.; Holt, K.E.; Inouye, M.; De Klerk, N.H.; Zhang, G.; Sly, P.D.; Hart, P.H.; et al. Vitamin D over the First Decade and Susceptibility to Childhood Allergy and Asthma. J. Allergy Clin. Immunol. 2017, 139, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In Utero Exposure to 25-Hydroxyvitamin D and Risk of Childhood Asthma, Wheeze, and Respiratory Tract Infections: A Meta-Analysis of Birth Cohort Studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef]
- Comberiati, P.; Di Cicco, M.E.; D’Elios, S.; Peroni, D.G. How Much Asthma Is Atopic in Children? Front. Pediatr. 2017, 5, 122. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of Prenatal Supplementation with Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef] [PubMed]
- von Mutius, E.; Martinez, F.D. Vitamin D Supplementation during Pregnancy and the Prevention of Childhood Asthma. N. Engl. J. Med. 2020, 382, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary Factors during Pregnancy and Atopic Outcomes in Childhood: A Systematic Review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Yepes-Nuñez, J.J.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Cuello-García, C.; Zhang, Y.; Morgano, G.P.; Agarwal, A.; Gandhi, S.; Terracciano, L.; et al. Vitamin D Supplementation in Primary Allergy Prevention: Systematic Review of Randomized and Non-Randomized Studies. Allergy 2018, 73, 37–49. [Google Scholar] [CrossRef]
- Gupta, A.; Sjoukes, A.; Richards, D.; Banya, W.; Hawrylowicz, C.; Bush, A.; Saglani, S. Relationship between Serum Vitamin D, Disease Severity, and Airway Remodeling in Children with Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 1342–1349. [Google Scholar] [CrossRef]
- Bose, S.; Diette, G.B.; Woo, H.; Koehler, K.; Romero, K.; Rule, A.M.; Detrick, B.; Brigham, E.; McCormack, M.C.; Hansel, N.N. Vitamin D Status Modifies the Response to Indoor Particulate Matter in Obese Urban Children with Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1815–1822.e2. [Google Scholar] [CrossRef] [PubMed]
- Chinellato, I.; Piazza, M.; Sandri, M.; Peroni, D.; Piacentini, G.; Boner, A.L. Vitamin D Serum Levels and Markers of Asthma Control in Italian Children. J. Pediatr. 2011, 158, 437–441. [Google Scholar] [CrossRef]
- Batmaz, S.B.; Arıkoğlu, T.; Tamer, L.; Eskandari, G.; Kuyucu, S. Seasonal Variation of Asthma Control, Lung Function Tests and Allergic Inflammation in Relation to Vitamin D Levels: A Prospective Annual Study. Postepy Dermatol. Alergol. 2018, 35, 99–105. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus Statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.P.; James, L.; Majure, J.M.; Bickel, S.; Phan, L.-T.; Serrano Gonzalez, M.; Staples, H.; Tam-Williams, J.; Lang, J.; Snowden, J.; et al. Obesity-Related Asthma in Children: A Role for Vitamin D. Pediatr. Pulmonol. 2021, 56, 354–361. [Google Scholar] [CrossRef]
- Brehm, J.M.; Acosta-Pérez, E.; Klei, L.; Roeder, K.; Barmada, M.; Boutaoui, N.; Forno, E.; Kelly, R.; Paul, K.; Sylvia, J.; et al. Vitamin D Insufficiency and Severe Asthma Exacerbations in Puerto Rican Children. Am. J. Respir. Crit. Care Med. 2012, 186, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Riverin, B.D.; Maguire, J.L.; Li, P. Vitamin D Supplementation for Childhood Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0136841. [Google Scholar] [CrossRef]
- Fiamenghi, V.I.; Mello, E.D.; de Vitamin, D. Deficiency in Children and Adolescents with Obesity: A Meta-Analysis. J. Pediatr. (Rio J.) 2021, 97, 273–279. [Google Scholar] [CrossRef]
- Nassar, M.F.; Emam, E.K.; Allam, M.F. Is There a Benefit of Vitamin D Supplementation in Deficient Children and Adolescents Suffering from Obesity? A Meta-Analysis. Glob. Pediatr. Health 2021, 8. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in Pediatric Age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, Jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, V.K.; Arrieta, M.-C. Host–Microbiome Intestinal Interactions during Early Life: Considerations for Atopy and Asthma Development. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 138–148. [Google Scholar] [CrossRef]
- Tan, J.-Y.; Tang, Y.-C.; Huang, J. Gut Microbiota and Lung Injury. Adv. Exp. Med. Biol. 2020, 1238, 55–72. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery Mode and Gut Microbial Changes Correlate with an Increased Risk of Childhood Asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef] [PubMed]
- Meirlaen, L.; Levy, E.I.; Vandenplas, Y. Prevention and Management with Pro-, Pre and Synbiotics in Children with Asthma and Allergic Rhinitis: A Narrative Review. Nutrients 2021, 13, 934. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-García, D.; Jiménez, M.; Rivas-Santiago, C.E.; Gallegos-Alcalá, P.; Hernández-Mercado, A.; Santoyo-Payán, L.S.; Loera-Arias, M.D.J.; Saucedo-Cardenas, O.; Montes de Oca-Luna, R.; Salinas, E. Lactococcus Lactis NZ9000 Prevents Asthmatic Airway Inflammation and Remodelling in Rats through the Improvement of Intestinal Barrier Function and Systemic TGF-β Production. Int. Arch. Allergy Immunol. 2021, 182, 277–291. [Google Scholar] [CrossRef]
- Spacova, I.; Van Beeck, W.; Seys, S.; Devos, F.; Vanoirbeek, J.; Vanderleyden, J.; Ceuppens, J.; Petrova, M.; Lebeer, S. Lactobacillus Rhamnosus Probiotic Prevents Airway Function Deterioration and Promotes Gut Microbiome Resilience in a Murine Asthma Model. Gut Microbes 2020, 11, 1729–1744. [Google Scholar] [CrossRef]
- Wang, H.T.; Anvari, S.; Anagnostou, K. The Role of Probiotics in Preventing Allergic Disease. Children 2019, 6, 24. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between Probiotic Supplementation and Asthma Incidence in Infants: A Meta-Analysis of Randomized Controlled Trials. J. Asthma 2020, 57, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Agarwal, A.; Gandhi, S.; Terracciano, L.; Schünemann, H.J.; et al. Prebiotics for the Prevention of Allergies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Exp. Allergy 2017, 47, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections during the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K. Prebiotics in Infants for Prevention of Allergy. Cochrane Database Syst. Rev. 2013, 3, CD006474. [Google Scholar] [CrossRef]
- van der Aa, L.B.; van Aalderen, W.M.C.; Heymans, H.S.A.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.J.; Ben Amor, K.; Sprikkelman, A.B.; Synbad Study Group. Synbiotics Prevent Asthma-like Symptoms in Infants with Atopic Dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef]
- Vliagoftis, H.; Kouranos, V.D.; Betsi, G.I.; Falagas, M.E. Probiotics for the Treatment of Allergic Rhinitis and Asthma: Systematic Review of Randomized Controlled Trials. Ann. Allergy. Asthma. Immunol. 2008, 101, 570–579. [Google Scholar] [CrossRef]
- Das, R.R.; Naik, S.S.; Singh, M. Probiotics as Additives on Therapy in Allergic Airway Diseases: A Systematic Review of Benefits and Risks. BioMed Res. Int. 2013, 2013, 231979. [Google Scholar] [CrossRef]
- Hassanzad, M.; Maleki Mostashari, K.; Ghaffaripour, H.; Emami, H.; Rahimi Limouei, S.; Velayati, A.A. Synbiotics and Treatment of Asthma: A Double-Blinded, Randomized, Placebo-Controlled Clinical Trial. Galen Med. J. 2019, 8, 1350. [Google Scholar] [CrossRef]
- McTernan, P.G.; McTernan, C.L.; Chetty, R.; Jenner, K.; Fisher, F.M.; Lauer, M.N.; Crocker, J.; Barnett, A.H.; Kumar, S. Increased Resistin Gene and Protein Expression in Human Abdominal Adipose Tissue. J. Clin. Endocrinol. Metab. 2002, 87, 2407. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.; Watson, R. The Mediterranean Diet (2nd Edition)—An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-818649-7. [Google Scholar]
- Wang, W.; Luo, X.; Zhang, Q.; He, X.; Zhang, Z.; Wang, X. Bifidobacterium Infantis Relieves Allergic Asthma in Mice by Regulating Th1/Th2. Med. Sci. Monit. 2020, 26, e920583. [Google Scholar] [CrossRef] [PubMed]
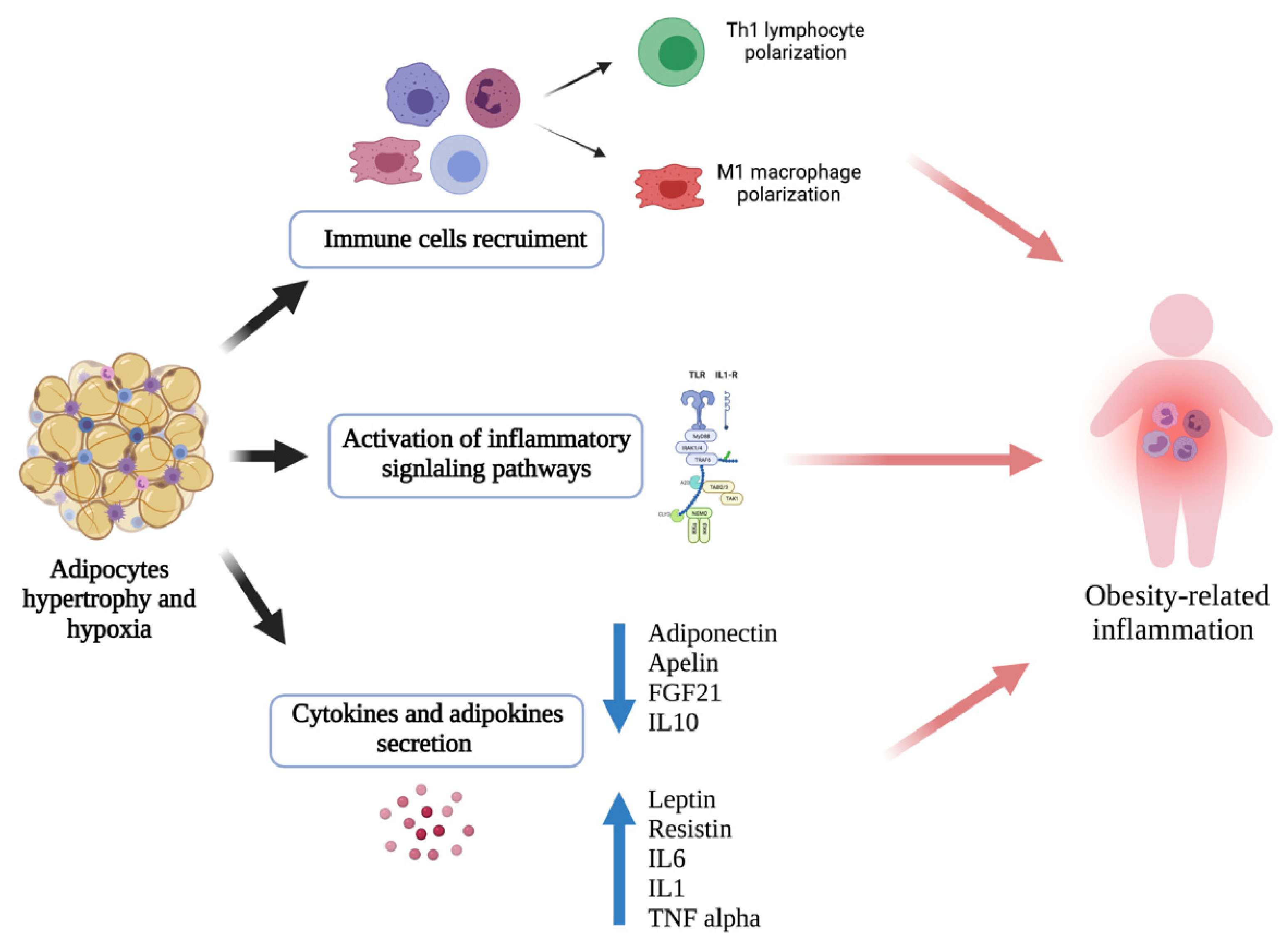
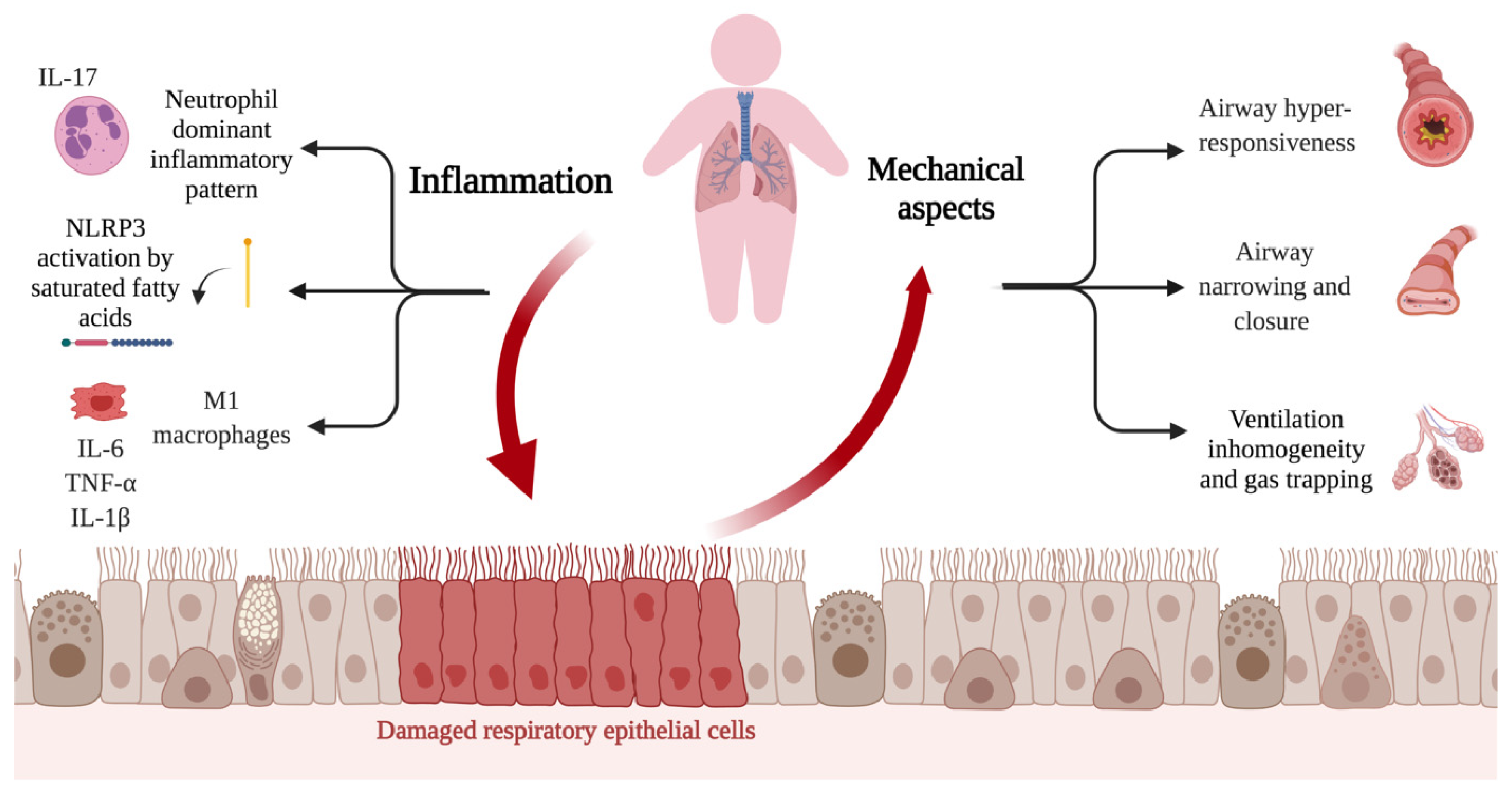
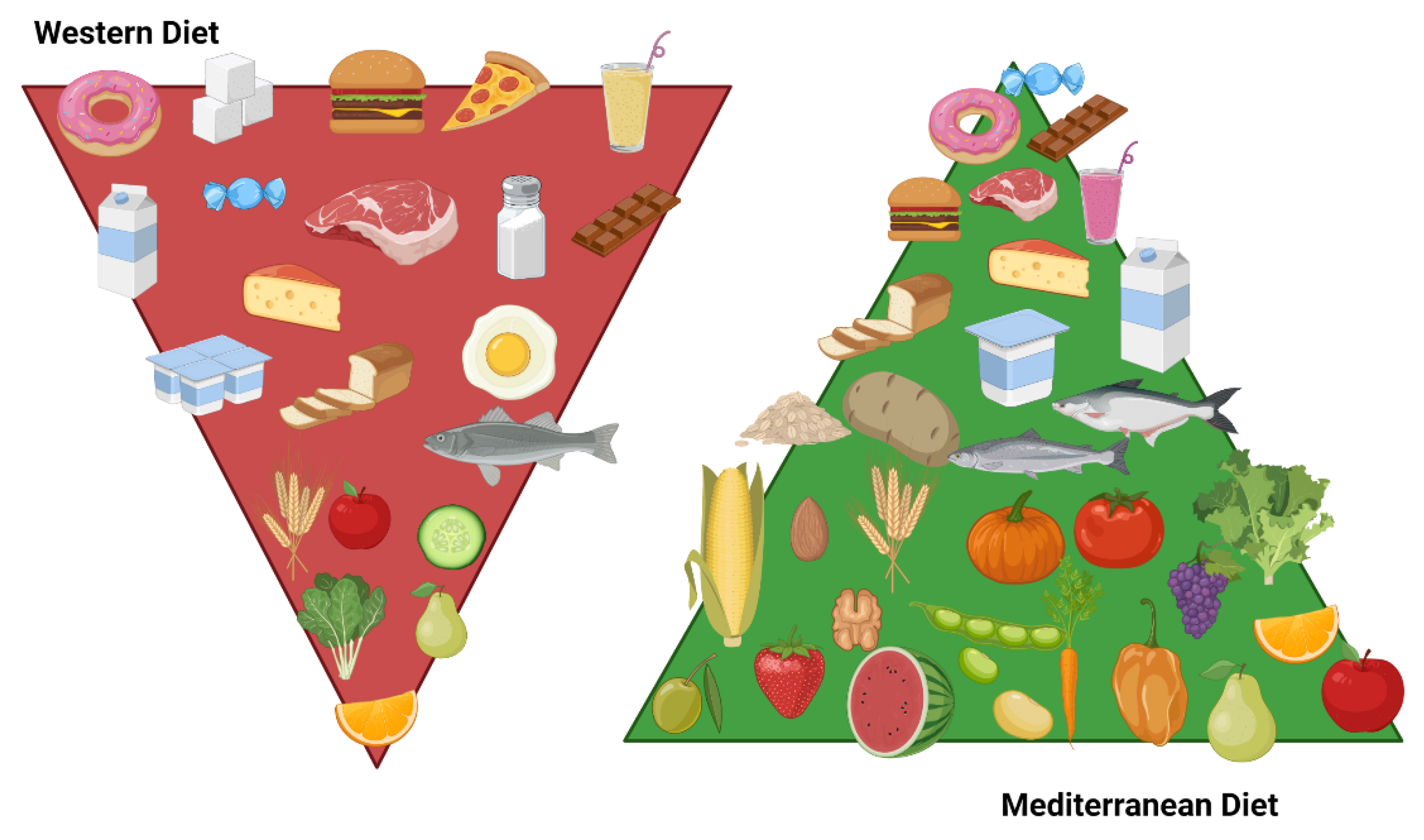
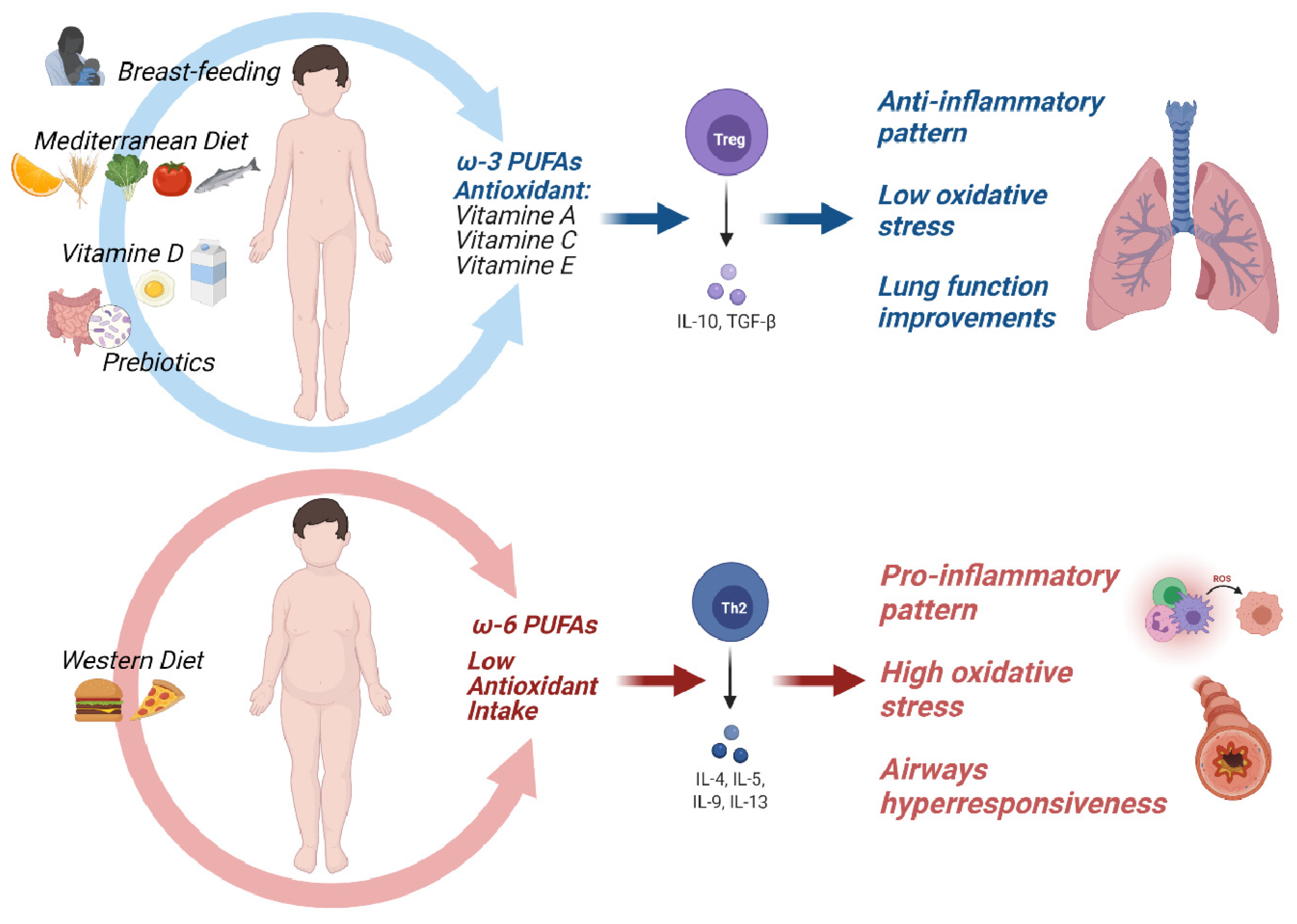
| Nutrient | Dietary Source | Mechanism of Action | Effect on Asthma | References | Future Prospective | References |
|---|---|---|---|---|---|---|
| Vitamin C | Lemon, orange, pepper, broccoli, spinach, radicchio, tomato |
| Antioxidant and anti-inflammatory effects | [241,242] |
| [205] |
| Vitamin A | Orange-yellow fruits and vegetables, milk, eggs |
| Antioxidant and anti-inflammatory effects | [244,245] |
| |
| Vitamin E | Green vegetables, nuts, seeds, vegetable oils |
| Antioxidant and anti-inflammatory effects | [247,248] |
| [254] |
| Flavonoids | Apples, grapes, eomatoes, salad, cabbage |
| Antioxidant and anti-inflammatory effects | [16] |
| [16,198] |
| Early-Life Nutrition | ||
|---|---|---|
| Type | Prevention | |
| Results | References | |
| Breastfeeding | Protection with a dose-dependent role on preschool wheezing and asthma. | [152,153,154,155,156] |
| Infant Formula | No evidence to show that the use of hydrolyzed formula versus conventional milk in the prevention of wheezing and asthma. | [161,162,165,166,168,169] |
| Soy Milk | No evidence to show it offers any protection against asthma. | [171,172,174,190] |
| Timing of Complementary Feeding | No evidence to show it offers any protection against asthma. | [182,183] |
| Developmental Age Nutrition | ||||
|---|---|---|---|---|
| Type | Prevention | Treatment | ||
| Results | References | Results | References | |
| Cow’s Milk | No effective role in preventing asthma. | [171,172,174,190] | No effective role in treating asthma. | [171,172,174,190] |
| Dietary Patterns | ||||
| Mediterranean Diet (MD) | Is shown to protect against preschool wheezing and asthma. | [16,156,212,216,217,219,220] | Is shown to protect against asthma exacerbations and lung function. | [16,212,217,219,220,222,223] |
| Western Diet (WD) | Higher risk of preschool wheezing and asthma. | [158,224,231,238] | Deteriorating effect on pulmonary function. | [231,236,237] |
| Food Supplements | ||||
| Polyunsaturated Fatty Acids | Inconclusive evidence on the protective role on preschool wheezing asthma prevention. | [250,251,252] | No effective role in treating asthma. | [239] |
| Antioxidants | Is shown to protect against preschool wheezing and asthma. | [224,239,275] | No effective role in treating asthma. | [277] |
| Vitamin D | Inconclusive evidence on the protective role on preschool wheezing asthma prevention. | [157,286,287,288,290] | Possible protective role on asthma exacerbations. | [298] |
| Probiotics, Prebiotics and Synbiotics | ||||
| No effective role in preventing asthma. | [308,310] | No effective role in preventing asthma. | [305,316] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Verduci, E.; Ghezzi, M.; Cena, H.; Pascuzzi, M.C.; Regalbuto, C.; Lamberti, R.; Rossi, V.; Manuelli, M.; Bosetti, A.; et al. Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients 2021, 13, 3708. https://doi.org/10.3390/nu13113708
Calcaterra V, Verduci E, Ghezzi M, Cena H, Pascuzzi MC, Regalbuto C, Lamberti R, Rossi V, Manuelli M, Bosetti A, et al. Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients. 2021; 13(11):3708. https://doi.org/10.3390/nu13113708
Chicago/Turabian StyleCalcaterra, Valeria, Elvira Verduci, Michele Ghezzi, Hellas Cena, Martina Chiara Pascuzzi, Corrado Regalbuto, Rossella Lamberti, Virginia Rossi, Matteo Manuelli, Alessandra Bosetti, and et al. 2021. "Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment" Nutrients 13, no. 11: 3708. https://doi.org/10.3390/nu13113708
APA StyleCalcaterra, V., Verduci, E., Ghezzi, M., Cena, H., Pascuzzi, M. C., Regalbuto, C., Lamberti, R., Rossi, V., Manuelli, M., Bosetti, A., & Zuccotti, G. V. (2021). Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients, 13(11), 3708. https://doi.org/10.3390/nu13113708











