Cross-Sectional Associations between Dietary Daily Nicotinamide Intake and Patient-Reported Outcomes in Colorectal Cancer Survivors, 2 to 10 Years Post-Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Calculation of Average Daily Nicotinamide Intake
2.3. Measurement of Serum Protein Carbonyl Contents and NAD+ Levels
2.4. Outcome Measurement
2.5. Other Factors
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
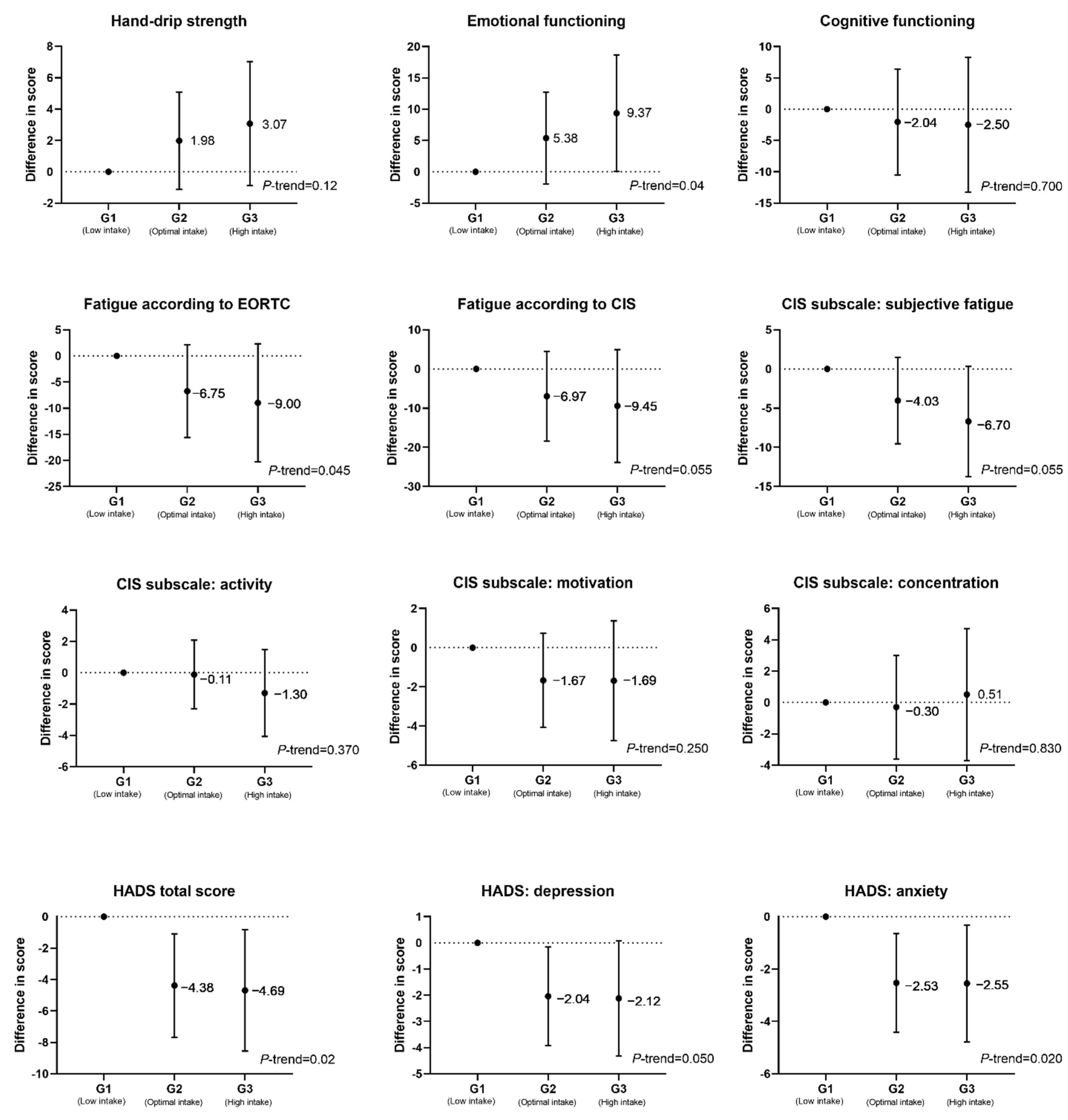
3.2. Associations between Dietary Nicotinamide Intake and Patient-Reported Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primer 2015, 1, 15065. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of Colorectal Cancer in Patients with Ulcerative Colitis: A Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J. Clin. 2015, 65, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Rametta Giuliano, S.; Tralongo, P. Early Colorectal Cancer: Diagnosis, Treatment and Survivorship Care. Crit. Rev. Oncol. Hematol. 2019, 136, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chini, C.C.S.; Tarragó, M.G.; Chini, E.N. Nad and the aging process: Role in life, death and everything in between. Mol. Cell. Endocrinol. 2017, 455, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD+ in Aging, Metabolism, and Neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Berg, J.; Clement, J.; Khorshidi, F.; Poljak, A.; Jayasena, T.; Grant, R.; Sachdev, P. Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes. Antioxid. Redox Signal. 2019, 30, 251–294. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Wong, M.; Poljak, A.; Sachdev, P.; Braidy, N. The Plasma NAD+ Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Res. 2019, 22, 121–130. [Google Scholar] [CrossRef]
- Abele, F.; Höfer, K.; Bernhard, P.; Grawenhoff, J.; Seidel, M.; Krause, A.; Kopf, S.; Schröter, M.; Jäschke, A. A Novel NAD-RNA Decapping Pathway Discovered by Synthetic Light-Up NAD-RNAs. Biomolecules 2020, 10, 513. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Ratajczak, J.; Doig, C.L.; Oakey, L.A.; Callingham, R.; Da Silva Xavier, G.; Garten, A.; Elhassan, Y.S.; Redpath, P.; Migaud, M.E.; et al. Nicotinamide Riboside Kinases Display Redundancy in Mediating Nicotinamide Mononucleotide and Nicotinamide Riboside Metabolism in Skeletal Muscle Cells. Mol. Metab. 2017, 6, 819–832. [Google Scholar] [CrossRef]
- Alegre, J.; Rosés, J.M.; Javierre, C.; Ruiz-Baqués, A.; Segundo, M.J.; Fernández de Sevilla, T. Nicotinamida Adenina Dinucleótido (NADH) En Pacientes Con Síndrome de Fatiga Crónica. Rev. Clin. Esp. 2010, 210, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Bernier, M.; Aon, M.A.; Cortassa, S.; Kim, E.Y.; Fang, E.F.; Palacios, H.H.; Ali, A.; Navas-Enamorado, I.; Di Francesco, A.; et al. Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab. 2018, 27, 667–676.e4. [Google Scholar] [CrossRef]
- Pirinen, E.; Auranen, M.; Khan, N.A.; Brilhante, V.; Urho, N.; Pessia, A.; Hakkarainen, A.; Kuula, J.; Heinonen, U.; Schmidt, M.S.; et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020, 31, 1078–1090.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Prizment, A.; Thyagarajan, B.; Blaes, A. Cancer Treatment-Induced Accelerated Aging in Cancer Survivors: Biology and Assessment. Cancers 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and Aging: Causes, Consequences, and Therapeutic Avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- The Habitual Micronutrient Intake of Dutch Adults (VCP 2007–2010) Compared with Dietary Reference Values Set by the Health Council of The Netherlands (2014) and EFSA (2010–2017) (2018) RIVM. Available online: https://www.rivm.nl/documenten/habitual-micronutrient-intake-of-dutch-adults-vcp-2007-2010-compared-with-dietary (accessed on 8 July 2021).
- Fletcher, M.A.; Zeng, X.R.; Barnes, Z.; Levis, S.; Klimas, N.G. Plasma Cytokines in Women with Chronic Fatigue Syndrome. J. Transl. Med. 2009, 7, 96. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood Reflects Tissue Oxidative Stress: A Systematic Review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef]
- Van Roekel, E.H.; Bours, M.J.L.; Breedveld-Peters, J.J.L.; Meijer, K.; Kant, I.; Van Den Brandt, P.A.; Sanduleanu, S.; Beets, G.L.; Weijenberg, M.P. Light Physical Activity Is Associated with Quality of Life after Colorectal Cancer. Med. Sci. Sports Exerc. 2015, 47, 2493–2503. [Google Scholar] [CrossRef]
- Koole, J.L.; Bours, M.J.L.; Breedveld-Peters, J.J.L.; van Roekel, E.H.; van Dongen, M.C.J.M.; Eussen, S.J.P.M.; van Zutphen, M.; van Duijnhoven, F.J.B.; Boshuizen, H.C.; Weijenberg, M.P. Evaluating the Validity of a Food Frequency Questionnaire in Comparison with a 7-Day Dietary Record for Measuring Dietary Intake in a Population of Survivors of Colorectal Cancer. J. Acad. Nutr. Diet. 2020, 120, 245–257. [Google Scholar] [CrossRef]
- Koole, J.L.; Bours, M.J.L.; Breedveld-Peters, J.J.L.; van Roekel, E.H.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Vogelaar, F.J.; Aquarius, M.; Keulen, E.; Stoot, J.; et al. Is Dietary Supplement Use Longitudinally Associated with Fatigue in Stage I–III Colorectal Cancer Survivors? Clin. Nutr. 2020, 39, 234–241. [Google Scholar] [CrossRef]
- Breedveld-Peters, J.J.L.; Koole, J.L.; Müller-Schulte, E.; van der Linden, B.W.A.; Windhausen, C.; Bours, M.J.L.; van Roekel, E.H.; Weijenberg, M.P. Colorectal Cancers Survivors’ Adherence to Lifestyle Recommendations and Cross-Sectional Associations with Health-Related Quality of Life. Br. J. Nutr. 2018, 120, 188–197. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Bernofsky, C.; Swan, M. An Improved Cycling Assay for Nicotinamide Adenine Dinucleotide. Anal. Biochem. 1973, 53, 452–458. [Google Scholar] [CrossRef]
- Slocombe, L.L.; Colditz, I.G. A Rapid Colorimetric Assay for Measuring Low Concentrations of Haemoglobin in Large Numbers of Bovine Plasma Samples. Food Agric. Immunol. 2011, 22, 135–143. [Google Scholar] [CrossRef][Green Version]
- Peng, Z.; Xiang, W.; Zhou, J.; Cao, J.; Li, Z.; Gao, H.; Zhang, J.; Shen, H. Hemolytic Specimens in Complete Blood Cell Count: Red Cell Parameters Could Be Revised by Plasma Free Hemoglobin. J. Clin. Lab. Anal. 2020, 34, e23218. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, J. The Guide to Expression of Uncertainty in Measurement Approach for Estimating Uncertainty: An Appraisal. Clin. Chem. 2003, 49, 1822–1829. [Google Scholar] [CrossRef]
- Elands, R.J.J.; Simons, C.C.J.M.; van Dongen, M.; Schouten, L.J.; Verhage, B.A.J.; van den Brandt, P.A.; Weijenberg, M.P. A Systematic Literature Review and Meta-Regression Analysis on Early-Life Energy Restriction and Cancer Risk in Humans. PLoS ONE 2016, 11, e0158003. [Google Scholar] [CrossRef]
- Koole, J.L.; Bours, M.J.L.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; van Duijnhoven, F.J.B.; van den Ouweland, J.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Weijenberg, M.P. Higher Serum Vitamin D Concentrations Are Longitudinally Associated with Better Global Quality of Life and Less Fatigue in Colorectal Cancer Survivors up to 2 Years after Treatment. Cancer Epidemiol. Prev. Biomark. 2020, 29, 1135–1144. [Google Scholar] [CrossRef]
- Van Roekel, E.H.; Bours, M.J.L.; Te Molder, M.E.M.; Breedveld-Peters, J.J.L.; Olde Damink, S.W.M.; Schouten, L.J.; Sanduleanu, S.; Beets, G.L.; Weijenberg, M.P. Associations of Adipose and Muscle Tissue Parameters at Colorectal Cancer Diagnosis with Long-Term Health-Related Quality of Life. Qual. Life Res. 2017, 26, 1745–1759. [Google Scholar] [CrossRef]
- Van Roekel, E.H.; Winkler, E.A.H.; Bours, M.J.L.; Lynch, B.M.; Willems, P.J.B.; Meijer, K.; Kant, I.J.; Beets, G.L.; Sanduleanu, S.; Healy, G.N.; et al. Associations of Sedentary Time and Patterns of Sedentary Time Accumulation with Health-Related Quality of Life in Colorectal Cancer Survivors. Prev. Med. Rep. 2016, 4, 262–269. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of Physical Activities: Classification of Energy Costs of Human Physical Activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Niacin. Available online: https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/ (accessed on 21 October 2020).
- Thong, M.S.Y.; Mols, F.; Wang, X.S.; Lemmens, V.E.P.P.; Smilde, T.J.; van de Poll-Franse, L.V. Quantifying Fatigue in (Long-Term) Colorectal Cancer Survivors: A Study from the Population-Based Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry. Eur. J. Cancer Oxf. Engl. 2013, 49, 1957–1966. [Google Scholar] [CrossRef]
- Bower, J.E.; Bak, K.; Berger, A.; Breitbart, W.; Escalante, C.P.; Ganz, P.A.; Schnipper, H.H.; Lacchetti, C.; Ligibel, J.A.; Lyman, G.H.; et al. Screening, Assessment, and Management of Fatigue in Adult Survivors of Cancer: An American Society of Clinical Oncology Clinical Practice Guideline Adaptation. J. Clin. Oncol. 2014, 32, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Bours, M.J.; Beijer, S.; Winkels, R.M.; van Duijnhoven, F.J.; Mols, F.; Breedveld-Peters, J.J.; Kampman, E.; Weijenberg, M.P.; van de Poll-Franse, L.V. Dietary Changes and Dietary Supplement Use, and Underlying Motives for These Habits Reported by Colorectal Cancer Survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) Registry. Br. J. Nutr. 2015, 114, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Koole, J.L.; Bours, M.J.L.; Geijsen, A.J.M.R.; Gigic, B.; Ulvik, A.; Kok, D.E.; Brezina, S.; Ose, J.; Baierl, A.; Böhm, J.; et al. Circulating B-Vitamin Biomarkers and B-Vitamin Supplement Use in Relation to Quality of Life in Patients with Colorectal Cancer: Results from the FOCUS Consortium. Am. J. Clin. Nutr. 2021, 113, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, M.-F.; van der Linden, B.W.A.; Breedveld-Peters, J.J.L.; Koole, J.L.; van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Associations of the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Recommendations with Patient-Reported Outcomes in Colorectal Cancer Survivors 2–10 Years Post-Diagnosis: A Cross-Sectional Analysis. Br. J. Nutr. 2021, 125, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional Status as a Mediator of Fatigue and Its Underlying Mechanisms in Older People. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic Fatigue Syndrome (CFS): Suggestions for a Nutritional Treatment in the Therapeutic Approach. Biomed. Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef]
- Herbison, C.E.; Hickling, S.; Allen, K.L.; O’Sullivan, T.A.; Robinson, M.; Bremner, A.P.; Huang, R.-C.; Beilin, L.J.; Mori, T.A.; Oddy, W.H. Low Intake of B-Vitamins Is Associated with Poor Adolescent Mental Health and Behaviour. Prev. Med. 2012, 55, 634–638. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Veasey, R.; Watson, A.; Dodd, F.; Jones, E.; Maggini, S.; Haskell, C.F. Effects of High-Dose B Vitamin Complex with Vitamin C and Minerals on Subjective Mood and Performance in Healthy Males. Psychopharmacology 2010, 211, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Arakawa, M. Dietary Folate, Riboflavin, Vitamin B-6, and Vitamin B-12 and Depressive Symptoms in Early Adolescence: The Ryukyus Child Health Study. Psychosom. Med. 2010, 72, 763–768. [Google Scholar] [CrossRef]
- Murakami, K.; Mizoue, T.; Sasaki, S.; Ohta, M.; Sato, M.; Matsushita, Y.; Mishima, N. Dietary Intake of Folate, Other B Vitamins, and ω-3 Polyunsaturated Fatty Acids in Relation to Depressive Symptoms in Japanese Adults. Nutrition 2008, 24, 140–147. [Google Scholar] [CrossRef]
- Mishra, G.D.; McNaughton, S.A.; O’Connell, M.A.; Prynne, C.J.; Kuh, D. Intake of B Vitamins in Childhood and Adult Life in Relation to Psychological Distress among Women in a British Birth Cohort. Public Health Nutr. 2009, 12, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Mahdavifar, B.; Hosseinzadeh, M.; Salehi-Abargouei, A.; Mirzaei, M.; Vafa, M. Dietary Intake of B Vitamins and Their Association with Depression, Anxiety, and Stress Symptoms: A Cross-Sectional, Population-Based Survey. J. Affect. Disord. 2021, 288, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, B.C.; Gudmundsrud, R.; Frank, J.; Hov, A.; Lautrup, S.; Aman, Y.; Røsjø, H.; Brenner, C.; Ziegler, M.; Tysnes, O.-B.; et al. Targeting NAD+ in Translational Research to Relieve Diseases and Conditions of Metabolic Stress and Ageing. Mech. Ageing Dev. 2020, 186, 111208. [Google Scholar] [CrossRef] [PubMed]
- De Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.; Seals, D.R. Nicotinamide Mononucleotide Supplementation Reverses Vascular Dysfunction and Oxidative Stress with Aging in Mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.-S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD+/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Sáez-Francàs, N.; Segundo, M.J.; Calvo, N.; Faro, M.; Aliste, L.; Fernández de Sevilla, T.; Alegre, J. Effect of Coenzyme Q10 plus Nicotinamide Adenine Dinucleotide Supplementation on Maximum Heart Rate after Exercise Testing in Chronic Fatigue Syndrome—A Randomized, Controlled, Double-Blind Trial. Clin. Nutr. 2016, 35, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; van Baar, H.; van Zutphen, M.; Tibosch, M.; Kouwenhoven, E.A.; Keulen, E.T.P.; Kok, D.E.; van Halteren, H.K.; Breukink, S.O.; de Wilt, J.H.W.; et al. Inflammation Is a Mediating Factor in the Association between Lifestyle and Fatigue in Colorectal Cancer Patients. Cancers 2020, 12, 3701. [Google Scholar] [CrossRef]
- Liu, L.; Su, X.; Quinn, W.J.; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Lim, C.K.; Grant, R.; Brew, B.J.; Guillemin, G.J. Serum Nicotinamide Adenine Dinucleotide Levels through Disease Course in Multiple Sclerosis. Brain Res. 2013, 1537, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Salech, F.; Ponce, D.P.; Paula-Lima, A.C.; SanMartin, C.D.; Behrens, M.I. Nicotinamide, a Poly ADP-Ribose Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Monk, B.J. PARP Inhibitor and Chemotherapy Combination Trials for the Treatment of Advanced Malignancies: Does a Development Pathway Forward Exist? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 443–447. [Google Scholar] [CrossRef]
- Sachdev, E.; Tabatabai, R.; Roy, V.; Rimel, B.J.; Mita, M.M. PARP Inhibition in Cancer: An Update on Clinical Development. Target. Oncol. 2019, 14, 657–679. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T.; Shibata, K. Nutritional Aspect of Tryptophan Metabolism. Int. J. Tryptophan Res. 2013, 6, 3–8. [Google Scholar] [CrossRef]
- Post, A.; Huberts, M.; Poppe, E.; van Faassen, M.; Kema, I.P.; Vogels, S.; Geleijnse, J.M.; Westerhuis, R.; Ipema, K.J.R.; Bakker, S.J.L.; et al. Tryptophan Intake and Tryptophan Losses in Hemodialysis Patients: A Balance Study. Nutrients 2019, 11, 2851. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Haroon, E.; Welle, J.R.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.; Patel, T.; Felger, J.C.; Miller, A.H. Associations among Peripheral and Central Kynurenine Pathway Metabolites and Inflammation in Depression. Neuropsychopharmacology 2020, 45, 998–1007. [Google Scholar] [CrossRef]

| Male (n = 91) | Female (n = 54) | Total Population (n = 145) | |
|---|---|---|---|
| Age, mean (SD) | 70.0 (8.0) | 70.0 (10.0) | 70.0 (9.0) |
| BMI, n (%) | |||
| <25 kg/m2 | 21 (23.1) | 17 (31.5) | 38 (26.2) |
| ≥25 kg/m2 | 70 (76.9) | 37 (68.5) | 107 (73.8) |
| Smoking status, n (%) | |||
| Former or never | 79 (86.8) | 50 (92.6) | 127 (87.5) |
| Current | 12 (13.2) | 4 (7.4) | 18 (12.5) |
| Number of comorbidities, n (%) | |||
| 0 | 25 (27.5) | 11 (20.4) | 35 (24.1) |
| 1 | 22 (24.2) | 12 (22.2) | 34 (23.4) |
| ≥2 | 44 (48.4) | 31 (57.4) | 76 (52.4) |
| Cancer stage, n(%) | |||
| I | 23 (23.5) | 16 (29.6) | 49 (33.8) |
| II | 31 (34.1) | 21 (38.9) | 52 (35.9) |
| III | 30 (33.0) | 17 (31.5) | 47 (32.4) |
| Received chemotherapy, n (%) | |||
| Yes | 48 (52.7) | 27 (50) | 75 (51.7) |
| Received radiotherapy, n (%) | |||
| Yes | 41 (45.1) | 13 (24.1) | 54 (37.2) |
| Received surgery, n (%) | |||
| Yes | 86 (94.5) | 53 (98.1) | 139 (95.9) |
| Years since diagnosis, median (IQR) | 6.0 (3.0) | 6.0 (3.0) | 6.0 (3.0) |
| Hours per week of MVPA, median (IQR) | 9.9 (12.9) | 7.0 (8.3) | 8.6 (10.3) |
| Dietary intake | |||
| Energy (kcal/d), median (IQR) | 2157.6 (494.0) | 1578.9 (382.2) | 1930.4 (654.0) |
| Nicotinamide (mg/d), median (IQR) | 19.1 (7.5) | 14.4 (6.3) | 17.3 (8.0) |
| Supplement use (yes/no), n (%) | |||
| Yes | 38 (41.8) | 26 (48.1) | 64 (44.1) |
| Serum protein carbonyl contents (nmol/mg protein), mean (SD) | 32.7 (19.6) | 38.5 (23.3) | 34.9 (21.2) |
| NAD+(nmol/L), mean (SD) | 1572.4 (731.5) | 1462.6 (566.4) | 1531.3 (674.4) |
| Univariate | Model I a | Model II b | ||||
|---|---|---|---|---|---|---|
| β c | 95% CI | β c | 95% CI | β c | 95% CI | |
| Hand-grip strength | 19.13 | 14.53, 23.74 | 4.81 | 0.87, 8.75 | 2.30 | −2.41, 7.02 |
| Emotional functioning | 10.90 | 3.37, 18.43 | 11.77 | 2.53, 21.01 | 10.31 | −0.65, 21.26 |
| Cognitive functioning | 6.63 | −1.99, 15.25 | 6.77 | −3.91, 17.45 | 8.04 | −4.50, 20.58 |
| Fatigue | ||||||
| According to EORTC | −17.25 | −26.43, −8.01 | −19.64 | −31.03, −8.25 | −14.85 | −28.14, −1.56 |
| According to CIS total | −16.45 | −28.44, −4.45 | −19.91 | −34.72, −5.11 | −17.52 | −34.54, −0.51 |
| CIS subjective fatigue | −9.47 | −15.23, −3.70 | −11.63 | −18.74, −4.53 | −10.47 | −18.70, −2.25 |
| CIS activity | −3.19 | −5.00, −0.24 | −3.88 | −6.79, −1.00 | −2.93 | −6.20, 0.33 |
| CIS motivation | −3.19 | −5.75, −0.63 | −3.36 | −6.54, −0.18 | −2.19 | −5.79, 1.42 |
| CIS concentration | −1.28 | −4.62, 2.07 | −0.83 | −5.00, 3.33 | −0.86 | −5.82, 4.11 |
| HADS total (distress) | −3.13 | −5.81, −0.45 | −4.38 | −7.67, −1.09 | −4.69 | −8.55, −0.83 |
| HADS depression | −1.17 | −2.69, 0.35 | −2.04 | −3.92, −0.16 | −2.12 | −4.32, 0.07 |
| HADS anxiety | −2.11 | −3.68, −0.55 | −2.53 | −4.42, −0.65 | −2.55 | −4.79, −0.32 |
| b G2 vs. G1 (Ref) | P-Interaction | ||||
|---|---|---|---|---|---|
| Male (n = 70) | Female (n = 29) | ||||
| β | 95% CI | β | 95% CI | ||
| Hand-grip strength a | 3.35 | −1.23, 7.93 | −0.08 | −3.40, 3.33 | <0.001 |
| Emotional functioning a | 11.21 | 1.64, 20.79 | −1.42 | −12.35, 9.50 | 0.002 |
| Cognitive functioning a | −0.71 | −11.19, 9.77 | −4.31 | −18.28, 9.67 | 0.924 |
| Fatigue | |||||
| According to EORTC a | −10.43 | −20.93, −0.17 | −2.60 | −18.14, 12.93 | 0.005 |
| According to CIS (total) a | −8.97 | −23.14, −0.20 | −0.85 | −20.41, 18.72 | 0.270 |
| CIS subjective fatigue a | −6.35 | −13.57, −0.18 | −0.73 | −9.65, 8.20 | 0.076 |
| CIS activity a | −0.91 | −3.67, 1.85 | 0.68 | −2.81, 4.18 | 0.789 |
| CIS motivation a | −1.08 | −4.18, 2.03 | −1.96 | −5.88, 1.96 | 0.207 |
| CIS concentration a | −1.60 | −6.00, 2.78 | 1.75 | −3.16, 6.67 | 0.614 |
| HADS total (distress) a | −3.50 | −6.94, −0.50 | −2.08 | −5.60, 1.45 | 0.050 |
| HADS depression a | −1.57 | −3.61, 0.48 | −0.82 | −2.60, 0.96 | 0.579 |
| HADS anxiety a | −2.31 | −4.31, −0.30 | −1.14 | −3.35, 1.07 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Bours, M.J.L.; Koole, A.; Kenkhuis, M.-F.; Eussen, S.J.P.M.; Breukink, S.O.; van Schooten, F.-J.; Weijenberg, M.P.; Hageman, G.J. Cross-Sectional Associations between Dietary Daily Nicotinamide Intake and Patient-Reported Outcomes in Colorectal Cancer Survivors, 2 to 10 Years Post-Diagnosis. Nutrients 2021, 13, 3707. https://doi.org/10.3390/nu13113707
Wu W, Bours MJL, Koole A, Kenkhuis M-F, Eussen SJPM, Breukink SO, van Schooten F-J, Weijenberg MP, Hageman GJ. Cross-Sectional Associations between Dietary Daily Nicotinamide Intake and Patient-Reported Outcomes in Colorectal Cancer Survivors, 2 to 10 Years Post-Diagnosis. Nutrients. 2021; 13(11):3707. https://doi.org/10.3390/nu13113707
Chicago/Turabian StyleWu, Wenbo, Martijn J. L. Bours, Annaleen Koole, Marlou-Floor Kenkhuis, Simone J. P. M. Eussen, Stephanie O. Breukink, Frederik-Jan van Schooten, Matty P. Weijenberg, and Geja J. Hageman. 2021. "Cross-Sectional Associations between Dietary Daily Nicotinamide Intake and Patient-Reported Outcomes in Colorectal Cancer Survivors, 2 to 10 Years Post-Diagnosis" Nutrients 13, no. 11: 3707. https://doi.org/10.3390/nu13113707
APA StyleWu, W., Bours, M. J. L., Koole, A., Kenkhuis, M.-F., Eussen, S. J. P. M., Breukink, S. O., van Schooten, F.-J., Weijenberg, M. P., & Hageman, G. J. (2021). Cross-Sectional Associations between Dietary Daily Nicotinamide Intake and Patient-Reported Outcomes in Colorectal Cancer Survivors, 2 to 10 Years Post-Diagnosis. Nutrients, 13(11), 3707. https://doi.org/10.3390/nu13113707






