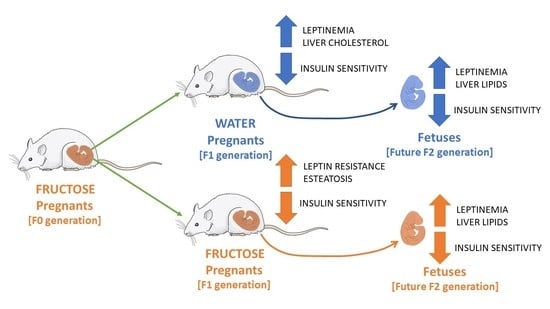
Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Plasma Determinations
2.3. Liver and Placenta Determinations
2.4. RNA Extraction and Gene Expression Determination by qPCR
2.5. Statistical Analysis
3. Results
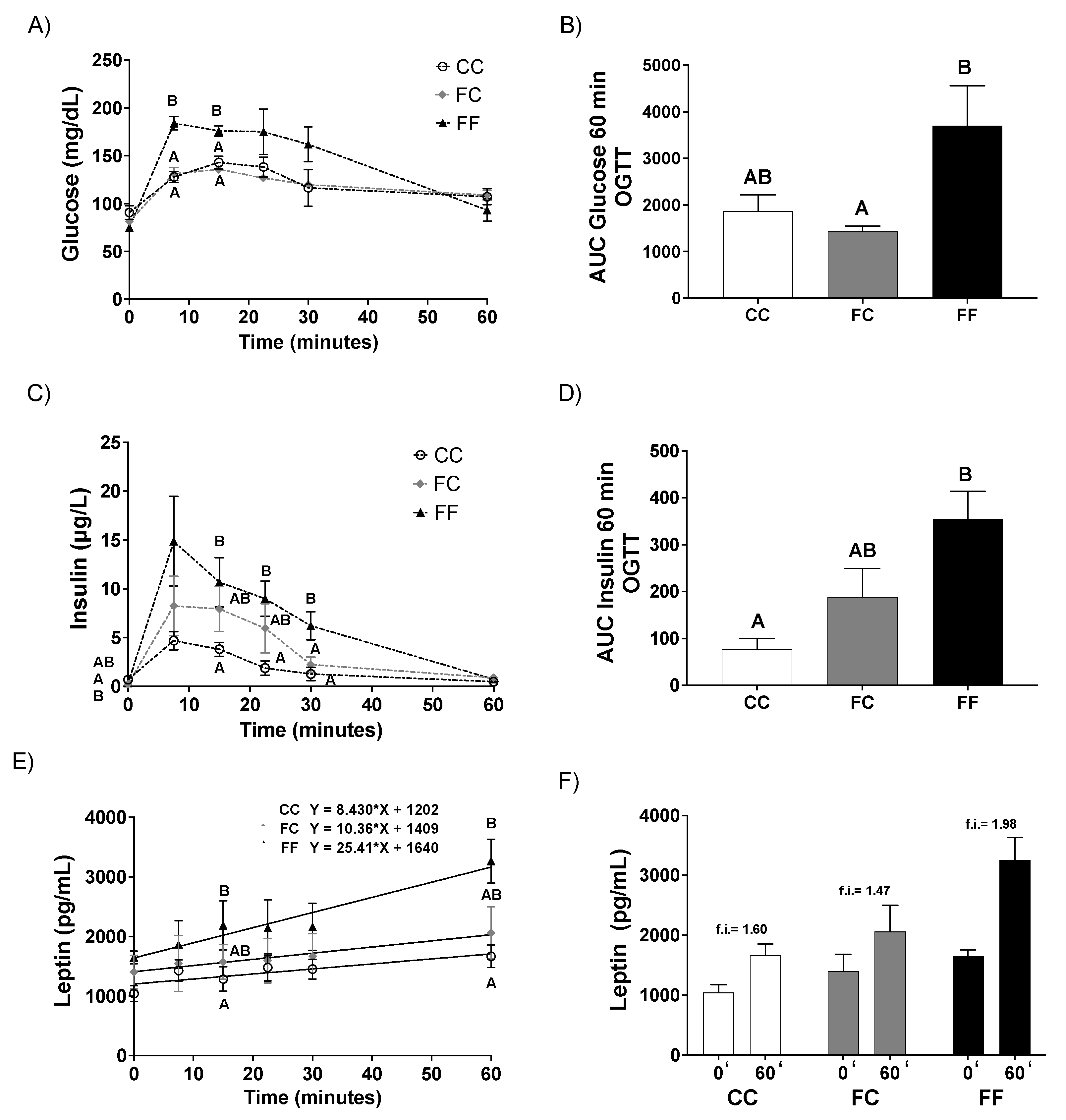
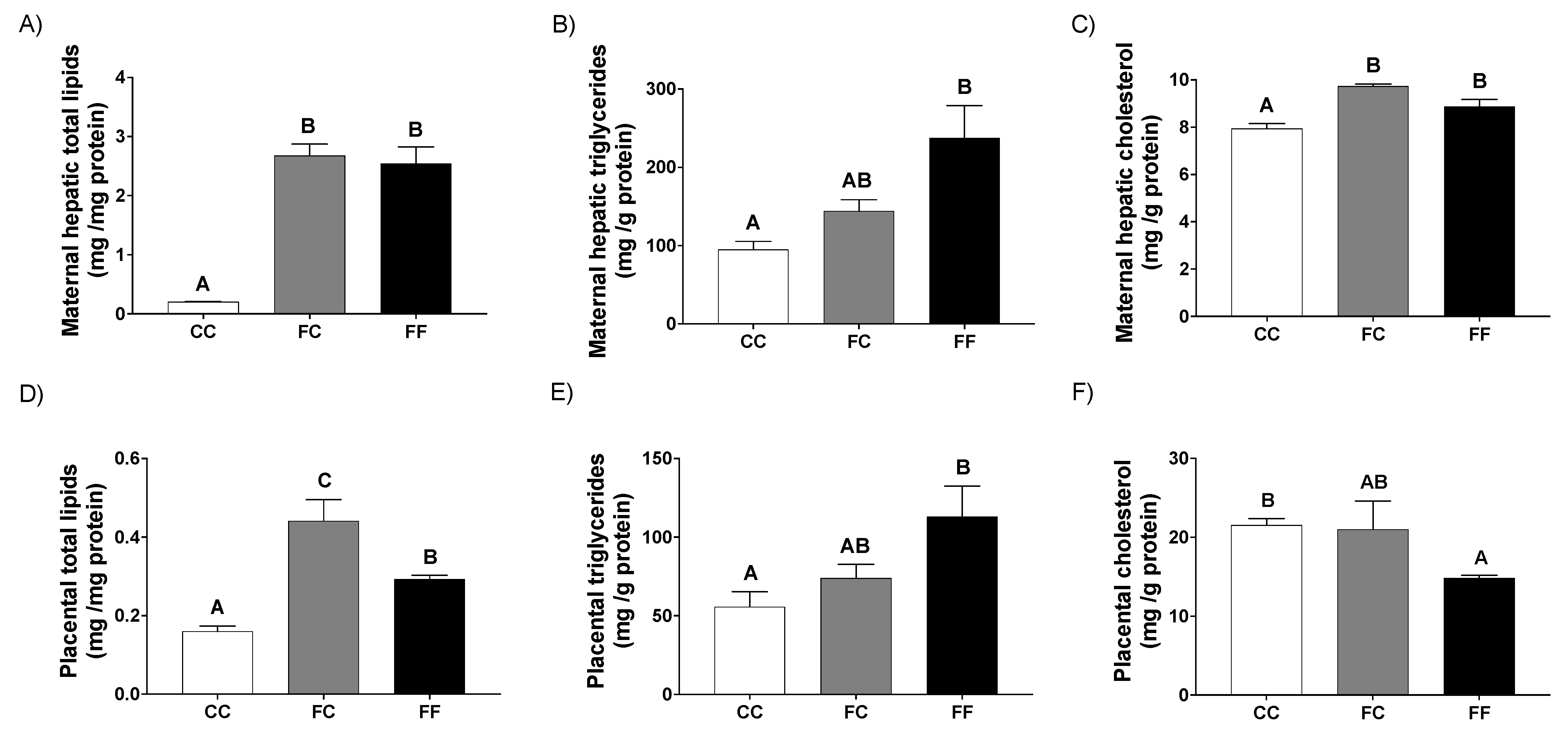
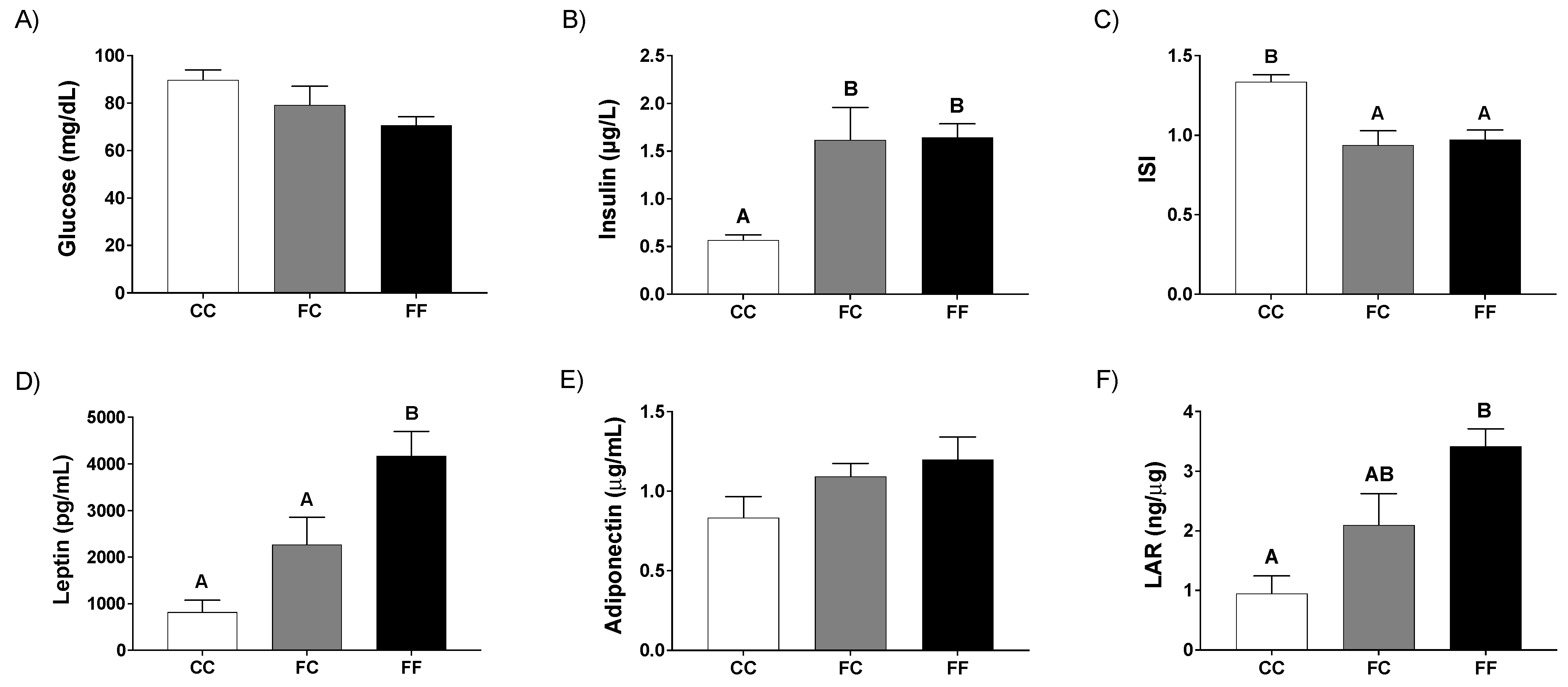
3.1. Pregnancy in Progeny from Fructose-Fed Mothers with or without Fructose Intake throughout Gestation Alters Insulin Sensitivity, Leptin Response, and Liver and Placenta Lipid Contents
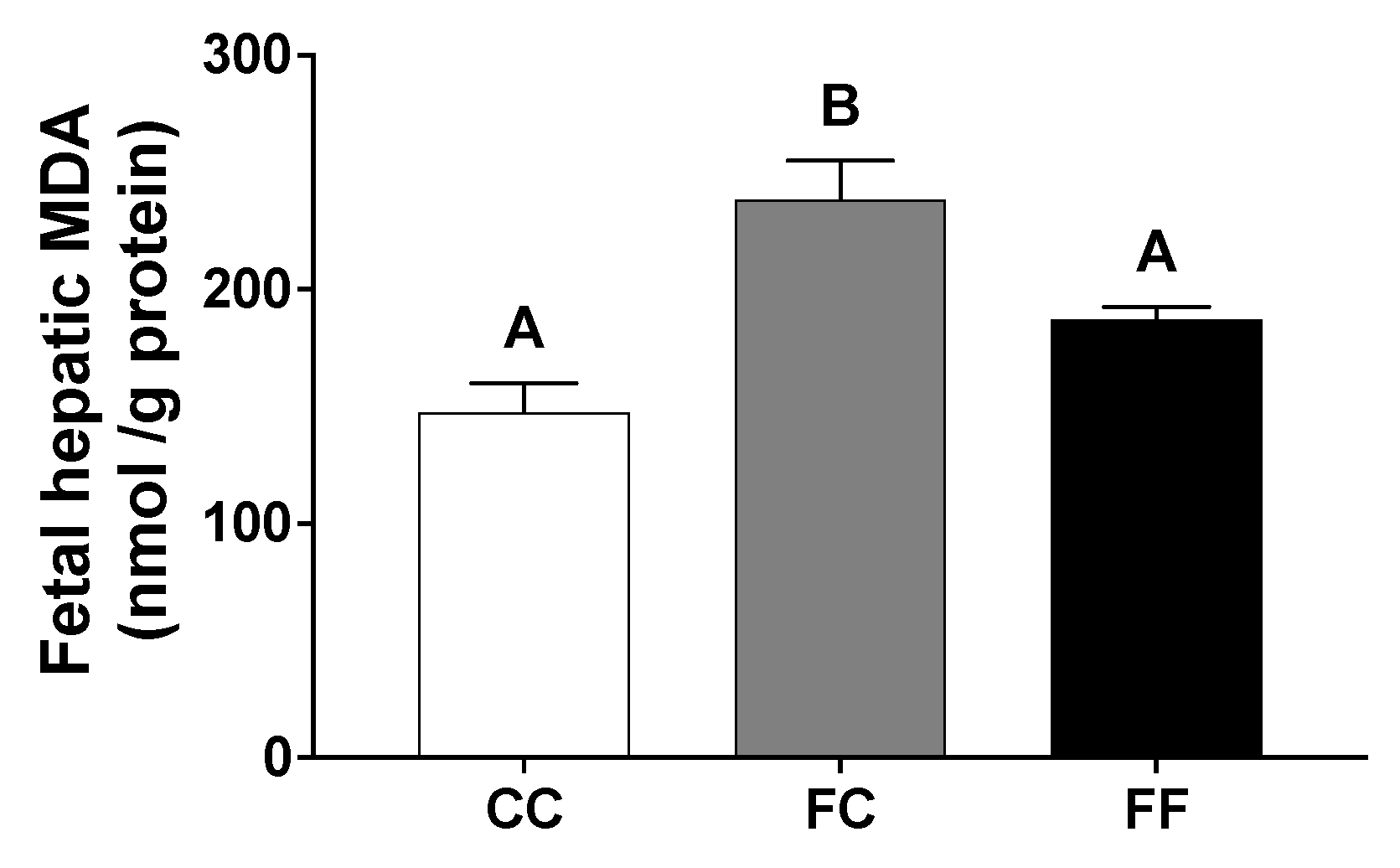
3.2. Pregnancy in Progeny from Fructose-Fed Mothers with or without Fructose Intake throughout Gestation Modifies the Insulin and Leptin Responses, Lipid Contents, and Oxidative Stress of Their Fetuses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, S.; Santacroce, A.; Picardi, A.; Buonocore, G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J. Clin. Pediatr. 2016, 5, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Gauster, M.; Wadsack, C. Placental transport in pregnancy pathologies. Am. J. Clin. Nutr. 2011, 94, 1896S–1902S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.E.; Sebert, S.; Hyatt, M.A.; Budge, H. Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef]
- Neri, C.; Edlow, A.G. Effects of Maternal Obesity on Fetal Programming: Molecular Approaches. Cold Spring Harb. Perspect. Med. 2016, 6, a026591. [Google Scholar] [CrossRef] [Green Version]
- Kendig, M.; Ekayanti, W.; Stewart, H.; Boakes, R.A.; Rooney, K. Metabolic Effects of Access to Sucrose Drink in Female Rats and Transmission of Some Effects to Their Offspring. PLOS ONE 2015, 10, e0131107. [Google Scholar] [CrossRef] [PubMed]
- Šedová, L.; Šeda, O.; Kazdová, L.; Chylikova, B.; Hamet, P.; Tremblay, J.; Křen, V.; Křenová, D. Sucrose feeding during pregnancy and lactation elicits distinct metabolic response in offspring of an inbred genetic model of metabolic syndrome. Am. J. Physiol. Metab. 2007, 292, E1318–E1324. [Google Scholar] [CrossRef]
- Ching, R.H.H.; Yeung, L.O.Y.; Tse, I.M.Y.; Sit, W.-H.; Li, E.T.S. Supplementation of Bitter Melon to Rats Fed a High-Fructose Diet During Gestation and Lactation Ameliorates Fructose-Induced Dyslipidemia and Hepatic Oxidative Stress in Male Offspring. J. Nutr. 2011, 141, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Portha, B.; Grandjean, V.; Movassat, J. Mother or Father: Who Is in the Front Line? Mechanisms Underlying the Non-Genomic Transmission of Obesity/Diabetes via the Maternal or the Paternal Line. Nutrients 2019, 11, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, H.Y.; Cho, H.M.; Kim, M.; Kim, I. Maternal High-Fructose Intake Induces Multigenerational Activation of the Renin-Angiotensin-Aldosterone System. Hypertension 2019, 74, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Astbury, S.; Hoedl, A.; Nielsen, B.; Symonds, M.E.; Bell, R.C. Lifetime Exposure to a Constant Environment Amplifies the Impact of a Fructose-Rich Diet on Glucose Homeostasis during Pregnancy. Nutrients 2017, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.S.; Nicklas, T.A. High-Fructose Corn Syrup Use in Beverages: Composition, Manufacturing, Properties, Consumption, and Health Effects. Beverage Impacts Health Nutr. 2016, 285–301. [Google Scholar] [CrossRef]
- A Bray, G.; Nielsen, S.J.; Popkin, B. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.-M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Alwahsh, S.M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2017, 91, 1545–1563. [Google Scholar] [CrossRef]
- Catena, C.; Giacchetti, G.; Novello, M.; Colussi, G.; Cavarape, A.; Sechi, L.A. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am. J. Hypertens. 2003, 16, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Reynolds, C.; Sloboda, D.; Gray, C.; Vickers, M. Maternal taurine supplementation attenuates maternal fructose-induced metabolic and inflammatory dysregulation and partially reverses adverse metabolic programming in offspring. J. Nutr. Biochem. 2015, 26, 267–276. [Google Scholar] [CrossRef]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, 378.e1–378.e6. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Leu, S.; Wu, K.L.H.; Lee, W.-C.; Chan, J.Y.H. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Otero, P.; Panadero, M.I.; Rodrigo, S.; Álvarez-Millán, J.J.; Bocos, C. Maternal Fructose Intake Induces Insulin Resistance and Oxidative Stress in Male, but Not Female, Offspring. J. Nutr. Metab. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Panadero, M.I.; Roglans, N.; Otero, P.; Álvarez-Millán, J.J.; Laguna, J.C.; Bocos, C. Fructose during pregnancy affects maternal and fetal leptin signaling. J. Nutr. Biochem. 2013, 24, 1709–1716. [Google Scholar] [CrossRef]
- Vickers, M.; Clayton, Z.; Yap, C.; Sloboda, D.M. Maternal Fructose Intake during Pregnancy and Lactation Alters Placental Growth and Leads to Sex-Specific Changes in Fetal and Neonatal Endocrine Function. Endocrinol. 2011, 152, 1378–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kereliuk, S.M.; Brawerman, G.M.; Dolinsky, V.W. Maternal Macronutrient Consumption and the Developmental Origins of Metabolic Disease in the Offspring. Int. J. Mol. Sci. 2017, 18, 1451. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, M.A.; Koski, K.G. Comparison of Effects of Dietary Glucose versus Fructose During Pregnancy on Fetal Growth and Development in Rats. J. Nutr. 1990, 120, 1312–1319. [Google Scholar] [CrossRef]
- Rodríguez, L.; Panadero, M.I.; Roglans, N.; Otero, P.; Rodrigo, S.; Álvarez-Millán, J.J.; Laguna, J.C.; Bocos, C. Fructose only in pregnancy provokes hyperinsulinemia, hypoadiponectinemia, and impaired insulin signaling in adult male, but not female, progeny. Eur. J. Nutr. 2016, 55, 665–674. [Google Scholar] [CrossRef]
- Rodríguez, L.; Panadero, M.I.; Rodrigo, S.; Roglans, N.; Otero, P.; Álvarez-Millán, J.J.; Laguna, J.C.; Bocos, C. Liquid fructose in pregnancy exacerbates fructose-induced dyslipidemia in adult female offspring. J. Nutr. Biochem. 2016, 32, 115–122. [Google Scholar] [CrossRef]
- Vilà, L.; Roglans, N.; Perna, V.; Sánchez, R.M.; Vázquez-Carrera, M.; Alegret, M.; Laguna, J.C. Liver AMP/ATP ratio and fructokinase expression are related to gender differences in AMPK activity and glucose intolerance in rats ingesting liquid fructose. J. Nutr. Biochem. 2011, 22, 741–751. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Carr, T.P.; Andresen, C.J.; Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993, 26, 39–42. [Google Scholar] [CrossRef]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N., Jr.; Sunderman, F.W., Jr. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1987, 33, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for General Users and for Biologist Programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45e. [Google Scholar] [CrossRef]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef] [Green Version]
- Sangüesa, G.; Montañés, J.C.; Baena, M.; Sánchez, R.M.; Roglans, N.; Alegret, M.; Laguna, J.C. Chronic fructose intake does not induce liver steatosis and inflammation in female Sprague-Dawley rats, but causes hypertriglyceridemia related to decreased VLDL receptor expression. Eur. J. Nutr. 2019, 58, 1283–1297. [Google Scholar] [CrossRef]
- Finucane, F.M.; Luan, J.; Wareham, N.J.; Sharp, S.J.; O’Rahilly, S.; Balkau, B.; Flyvbjerg, A.; Walker, M.; Højlund, K.; Nolan, J.J.; et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetol. 2009, 52, 2345–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norata, G.D.; Raselli, S.; Grigore, L.; Garlaschelli, K.; Dozio, E.; Magni, P.; Catapano, A.L. Leptin:Adiponectin Ratio Is an Independent Predictor of Intima Media Thickness of the Common Carotid Artery. Stroke 2007, 38, 2844–2846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forhead, A.J.; Fowden, A.L. The hungry fetus? Role of leptin as a nutritional signal before birth. J. Physiol. 2009, 587, 1145–1152. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Sabinari, I.W.; Olatunji, L.A. Oral L-glutamine rescues fructose-induced poor fetal outcome by preventing placental triglyceride and uric acid accumulation in Wistar rats. Heliyon 2020, 6, e05863. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.V.L.; Dyson, R.M.; Berry, M.J.; Gray, C. Fructose Consumption During Pregnancy Influences Milk Lipid Composition and Offspring Lipid Profiles in Guinea Pigs. Front. Endocrinol. 2020, 11, 550. [Google Scholar] [CrossRef]
- Školníková, E.; Šedová, L.; Šeda, O. Grandmother’s Diet Matters: Early Life Programming with Sucrose Influences Metabolic and Lipid Parameters in Second Generation of Rats. Nutrients 2020, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.W.; Dumke, K.A.; Goran, M.I. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrients 2014, 30, 928–935. [Google Scholar] [CrossRef] [Green Version]
- White, J.S.; Hobbs, L.J.; Fernandez, S. Fructose content and composition of commercial HFCS-sweetened carbonated beverages. Int. J. Obes. 2014, 39, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.R.; Lesko, J.; Krieg, R.J., Jr.; Adler, R.A.; Stevens, W. Leptin responses to glucose infusions in obesity-prone rats. Am J Physiol Endocrinol Metab. 2000, 279, E1088–E1096. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Signalling mechanisms linking hepatic glucose and lipid metabolism. Diabetol. 2006, 49, 1732–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.-T.; Wang, Z.-W.; Higa, M.; Newgard, C.B.; Unger, R.H. Reversing adipocyte differentiation: Implications for treatment of obesity. Proc. Natl. Acad. Sci. 1999, 96, 2391–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H.; Zhou, Y.-T.; Orci, L. Regulation of fatty acid homeostasis in cells: Novel role of leptin. Proc. Natl. Acad. Sci. 1999, 96, 2327–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [Green Version]
- Paul, C.; Laganà, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef]
- Rodrigo, S.; Fauste, E.; de la Cuesta, M.; Rodríguez, L.; Álvarez-Millán, J.J.; Panadero, M.I.; Otero, P.; Bocos, C. Maternal fructose induces gender-dependent changes in both LXRα promoter methylation and cholesterol metabolism in progeny. J. Nutr. Biochem. 2018, 61, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.; Rodríguez, L.; Otero, P.; Panadero, M.I.; García, A.; Barbas, C.; Roglans, N.; Ramos, S.; Goya, L.; Laguna, J.C.; et al. Fructose during pregnancy provokes fetal oxidative stress: The key role of the placental heme oxygenase-1. Mol. Nutr. Food Res. 2016, 60, 2700–2711. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Herrera, E.; Bonet, B. Dual effect of glucose on LDL oxidation: Dependence on vitamin E. Free. Radic. Biol. Med. 2002, 33, 1133–1140. [Google Scholar] [CrossRef]


| CC | FC | FF | |
|---|---|---|---|
| Maternal body weight at day 0 (g) | 203.2 ± 0.2 B | 180.0 ± 5.7 A | 176.5 ± 1.5 A |
| Maternal body weight at day 21 (g) | 364.0 ± 6.1 | 339.4 ± 7.1 | 334.8 ± 8.3 |
| Maternal body weight increase (conceptus free) (g) | 161.0 ± 5.0 | 159.4 ± 5.6 | 156.1 ± 7.7 |
| Conceptus weight (g) | 87.6 ± 8.1 | 87.2 ± 4.7 | 79.8 ± 5.8 |
| Number fetus/litter | 11.8 ± 1.2 | 11.3 ± 1.0 | 10.8 ± 1.0 |
| Fetal body weight (g) | 5.6 ± 0.1 | 5.9 ± 0.4 | 5.6 ± 0.1 |
| AUC consumed diet (g/21 days per rat) | 449.6 ± 16.6 | 437.1 ± 12.5 | 371.1 ± 18.5 |
| AUC ingested liquid (mL/21 days per rat) | 635.0 ± 41.7 A | 699.4 ± 48.2 A | 1479.7 ± 139.7 B |
| Total amount of ingested energy (Kcal/21 days per rat) | 1304.0 ± 48.2 A | 1267.4 ± 36.3 A | 1668.2 ± 28.7 B |
| CC | FC | FF | |
|---|---|---|---|
| Maternal liver mRNA gene expression (a.u.) | |||
| SREBP1c | 0.94 ± 0.20 | 1.21 ± 0.36 | 1.52 ± 0.46 |
| FAS | 18.9 ± 2.1 | 25.7 ± 5.8 | 38.0 ± 11.8 |
| ATP citrate lyase | 0.230 ± 0.041 | 0.463 ± 0.106 | 0.536 ± 0.175 |
| SCD1 | 0.79 ± 0.08 | 1.34 ± 0.41 | 2.31 ± 0.75 |
| CPT1 | 0.760 ± 0.025 AB | 0.900 ± 0.239 B | 0.273 ± 0.063 A |
| HMG-CoA Reductase | 0.59 ± 0.10 AB | 1.03 ± 0.32 B | 0.32 ± 0.02 A |
| Placental mRNA gene expression (a.u.) | |||
| SREBP1c | 0.140 ± 0.016 | 0.175 ± 0.010 | 0.112 ± 0.020 |
| FAS | 0.568 ± 0.010 | 0.735 ± 0.120 | 0.526 ± 0.060 |
| ATP citrate lyase | 0.056 ± 0.002 | 0.060 ± 0.004 | 0.060 ± 0.004 |
| SCD1 | 0.0015 ± 0.0003 | 0.0035 ± 0.0010 | 0.0011 ± 0.0001 |
| CPT1 | 0.044 ± 0.007 | 0.033 ± 0.005 | 0.026 ± 0.003 |
| HMG-CoA Reductase | 0.070 ± 0.007 | 0.095 ± 0.010 | 0.106 ± 0.014 |
| CC | FC | FF | |
|---|---|---|---|
| Fetal plasma levels | |||
| Triglycerides (mg/dL) | 16.3 ± 4.4 | 12.2 ± 1.2 | 10.2 ± 1.8 |
| Cholesterol (mg/dL) | 58.8 ± 2.1 | 61.5 ± 3.5 | 60.4 ± 3.5 |
| NEFA (mmol/L) | 0.084 ± 0.014 | 0.064 ± 0.010 | 0.053 ± 0.005 |
| Fetal liver mRNA gene expression (a.u.) | |||
| SREBP1c | 0.510 ± 0.062 | 0.545 ± 0.081 | 0.506 ± 0.043 |
| FAS | 4.20 ± 0.87 | 4.68 ± 1.70 | 4.85 ± 0.35 |
| ATP citrate lyase | 0.158 ± 0.022 | 0.170 ± 0.037 | 0.170 ± 0.009 |
| SCD1 | 0.088 ± 0.008 | 0.138 ± 0.058 | 0.143 ± 0.010 |
| CPT1 | 0.436 ± 0.111 | 0.333 ± 0.085 | 0.302 ± 0.024 |
| HMG-CoA Reductase | 0.628 ± 0.148 | 0.663 ± 0.221 | 0.614 ± 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauste, E.; Panadero, M.I.; Donis, C.; Otero, P.; Bocos, C. Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses. Nutrients 2021, 13, 3667. https://doi.org/10.3390/nu13103667
Fauste E, Panadero MI, Donis C, Otero P, Bocos C. Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses. Nutrients. 2021; 13(10):3667. https://doi.org/10.3390/nu13103667
Chicago/Turabian StyleFauste, Elena, María I. Panadero, Cristina Donis, Paola Otero, and Carlos Bocos. 2021. "Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses" Nutrients 13, no. 10: 3667. https://doi.org/10.3390/nu13103667
APA StyleFauste, E., Panadero, M. I., Donis, C., Otero, P., & Bocos, C. (2021). Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses. Nutrients, 13(10), 3667. https://doi.org/10.3390/nu13103667