Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest–Post-Test Explanatory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Endpoints
2.2. Study Design
2.3. Population
2.4. Dietary Supplement
2.5. Adverse Events
2.6. Biochemical Parameters
2.7. Anthropometric Measurements and Dietary Counseling
2.8. Body Composition
2.9. Assessment of Acne Status
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Xu, J.; Wang, Q.; Kuang, H. Systems pharmacology to investigate the interaction of berberine and other drugs in treating polycystic ovary syndrome. Sci. Rep. 2016, 6, 28089. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Faliva, M.; Monteferrario, F.; Repaci, E.; Allieri, F. Focus on metabolic and nutritional correlates of polycystic ovary syndrome and update on nutritional management of these critical phenomena. Arch. Gynecol. Obstet. 2014, 290, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Speca, S.; Napolitano, C.; Tagliaferri, G. The pathogenetic enigma of polycystic ovary syndrome. J. Ultrasound 2007, 10, 153–160. [Google Scholar] [CrossRef]
- Stein, I. Amenorrhea associated with bilateral polycystic ovaries. Am. J. Obstet. Gynecol. 1935, 29, 181–191. [Google Scholar] [CrossRef]
- Franks, S. Polycistic Ovary Syndrome: A changing perspective. Clin. Endocrinol. 1989, 31, 87–120. [Google Scholar] [CrossRef]
- Balen, A.; Conway, G.; Kaltsas, G.; Techatrasak, K.; Manning, P.; West, C.; Jacobs, H. Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum. Reprod. 1995, 10, 2107–2111. [Google Scholar] [CrossRef]
- Nestler, J.E.; Barlascini, C.O.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Clore, J.N.; Blackard, W.G. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1989, 68, 1027–1032. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Ciampelli, M.; Lanzone, A. Insulin and polycystic ovary syndrome: A new look at an old subject. Gynecol. Endocrinol. 1998, 12, 277–292. [Google Scholar] [CrossRef]
- Burghen, G.A.; Givens, J.R.; Kitabchi, A.E. Correlation of hyperandrogenism with hyperinsulinism in poly cystic ovarian disease. J. Clin. Endocrinol. Metab. 1980, 50, 113–116. [Google Scholar] [CrossRef]
- Stuart, C.A.; Peters, E.J.; Prince, M.J.; Richards, G.; Cavallo, A.; Meyer, W.J. Insulin resistance with acanthosis nigricans: The roles of obesity and androgen excess. Metabolism 1986, 35, 197–205. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Mitrakou, A.; Hennes, M.M.I.; Platanissiotis, D.; Kaklas, N.; Spina, J.; Georgiadou, E.; Hoffmann, R.G.; Kissebah, A.H.; Raptis, S. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism 1995, 44, 525–531. [Google Scholar] [CrossRef]
- Toprak, S.; Yönem, A.; Çakir, B.; Güler, S.; Azal, Ö.; Özata, M.; Çorakçi, A. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm. Res. Paediatr. 2001, 55, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Castello, R.; Negri, C.; Tosi, F.; Spiazzi, G.G.; Brun, E.; Balducci, R.; Toscano, V.; Muggeo, M. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: Apparent relative impairment of 17,20-lyase activity. J. Clin. Endocrinol. Metab. 1996, 81, 881–886. [Google Scholar] [PubMed]
- Rocca, M.L.; Venturella, R.; Mocciaro, R.; Di Cello, A.; Sacchinelli, A.; Russo, V.; Trapasso, S.; Zullo, F.; Morelli, M. Polycystic ovary syndrome: Chemical pharmacotherapy. Expert Opin. Pharmacother. 2015, 16, 1369–1393. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z. Adverse reactions of metformin. Chin. J. Misdiagn. 2006, 6, 285. [Google Scholar]
- Zhang, H.; Tian, X. Analysis of Renal Damage in Rats with Metformin Treated with Polycystic Ovary Syndrome. Shanxi Med. J. 2008, 37, 431. [Google Scholar]
- Ong, M.; Peng, J.; Jin, X.; Qu, X. Chinese Herbal Medicine for the Optimal Management of Polycystic Ovary Syndrome. Am. J. Chin. Med. 2017, 45, 405–422. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef]
- Saleem, F.; Rizvi, S.W. New Therapeutic Approaches in Obesity and Metabolic Syndrome Associated with Polycystic Ovary Syndrome. Cureus 2017, 9, e1844. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Al-thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bellone, I.; Rapacioli, G.; Putignano, P. Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 89–96. [Google Scholar] [CrossRef]
- di Pierro, F.; Villanova, N.; Agostini, F.; Marzocchi, R.; Soverini, V.; Marchesini, G. Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Orio, F.; Muscogiuri, G.; Palomba, S.; Savastano, S.; Volpe, A.; Orio, M.; Colarieti, G.; La Sala, G.B.; Colao, A.; Marciano, F.; et al. Berberine improves reproductive features in obese Caucasian women with polycystic ovary syndrome independently of changes of insulin sensitivity. ESPEN J. 2013, 8, e200–e204. [Google Scholar] [CrossRef]
- Rondanelli, M.; Infantino, V.; Riva, A.; Petrangolini, G.; Faliva, M.A.; Peroni, G.; Naso, M.; Nichetti, M.; Spadaccini, D.; Gasparri, C.; et al. Polycystic ovary syndrome management: A review of the possible amazing role of berberine. Arch. Gynecol. Obstet. 2020, 301, 53–60. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Reggi, A.; Parini, A.; Morbini, M.; Rosticci, M.; Grandi, E.; Borghi, C. Berberine and monacolin effects on the cardiovascular risk profile of women with oestroprogestin-induced hypercholesterolemia. High Blood Press. Cardiovasc. Prev. 2014, 21, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kuang, H.; Shen, W.; Ma, H.; Zhang, Y.; Stener-Victorin, E.; Hung, E.; Ng, Y.; Liu, J.; Kuang, H.; et al. Letrozole, berberine, or their combination for anovulatory infertility in women with polycystic ovary syndrome: Study design of a double-blind randomised controlled trial. BMJ Open 2013, 3, e003934. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-K.; Wang, Y.-Y.; Liu, J.-P.; Liang, R.-N.; Xue, H.-Y.; Ma, H.-X.; Shao, X.-G.; Ng, E.H.Y.; Reproductive and Developmental Network in Chinese Medicine. Randomized controlled trial of letrozole, berberine, or a combination for infertility in the polycystic ovary syndrome. Fertil. Steril. 2016, 106, 757–765.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-w.; Zhou, J.; Gober, H.-J.; Leung, W.T.; Wang, L. Effect and mechanism of berberine against polycystic ovary syndrome. Biomed. Pharmacother. 2021, 138, 111468. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Kennedy, E.; Gonzalez, C.; Stern, M.P.; Miettinen, H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996, 19, 1138–1141. [Google Scholar] [CrossRef]
- Vermeulen, A.; Stoïca, T.; Verdonck, L. The apparent free testosterone concentration, an index of androgenicity. J. Clin. Endocrinol. Metab. 1971, 33, 759–767. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Wang, X.; Liao, X.; Li, L.; Yang, G.; Gao, L. Free androgen index and Irisin in polycystic ovary syndrome. J. Endocrinol. Investig. 2016, 39, 549–556. [Google Scholar] [CrossRef]
- Frisancho, A.R. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef]
- Mohammad, A.; De Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef]
- Thappa, D.M.; Adityan, B.; Kumari, R. Scoring systems in acne vulgaris. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 323–326. [Google Scholar] [CrossRef]
- Motley, R.J.; Finlay, A.Y. Practical use of a disability index in the routine management of acne. Clin. Exp. Dermatol. 1992, 17, 1–3. [Google Scholar] [CrossRef]
- Green, P.; MacLeod, C.J. SIMR: An R package for power analysis of generalized linear mixed models by simulation. Methods Ecol. Evol. 2016, 7, 493–498. [Google Scholar] [CrossRef]
- Package “simr”. Power Analysis for Generalised Linear Mixed Models by Simulation. 2019. Available online: https://github.com/pitakakariki/simr (accessed on 29 January 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. R Dev. Core Team 2007, 3, 1–89. [Google Scholar]
- Benjamin, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Xie, L.; Zhang, D.; Ma, H.; He, H.; Xia, Q.; Shen, W.; Chang, H.; Deng, Y.; Wu, Q.; Cong, J.; et al. The Effect of Berberine on Reproduction and Metabolism in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Control Trials. Evid.-Based Complement. Altern. Med. 2019, 2019, 7918631. [Google Scholar] [CrossRef]
- An, Y.; Sun, Z.; Zhang, Y.; Liu, B.; Guan, Y.; Lu, M. The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment. Clin. Endocrinol. 2014, 80, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kuang, H.; Luo, Y.; Chen, Q. Clinical observation of berberine in intervening insulin resistance of polycystic ovary syndrome. J. Guangzhou Univ. Tradit. Chin. Med. 2017, 34, 172–177. [Google Scholar]
- Wei, W.; Zhao, H.; Wang, A.; Sui, M.; Liang, K.; Deng, H.; Ma, Y.; Zhang, Y.; Zhang, H.; Guan, Y. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 2012, 166, 99–105. [Google Scholar] [CrossRef]
- Lingxiao, W.; Yuanzhen, K.; Yanwei, R. Therapeutic Effect of Berberine Combined with Diformin for Women with Polycystic Ovary Syndrome and Insulin Resistance. J. Zhejiang Chin. Med. Univ. 2011, 35, 713–715. [Google Scholar]
- Shen, H.R.; Xu, X.; Li, X.L. Berberine exerts a protective effect on rats with polycystic ovary syndrome by inhibiting the inflammatory response and cell apoptosis. Reprod. Biol. Endocrinol. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Yu, J.; Ding, C.; Hua, Z.; Jiang, X.; Wang, C. Protective effects of berberine in a rat model of polycystic ovary syndrome mediated via the PI3K/AKT pathway. J. Obstet. Gynaecol. Res. 2021, 47, 1789–1803. [Google Scholar] [CrossRef]
- Benson, S.; Janssen, O.E.; Hahn, S.; Tan, S.; Dietz, T.; Mann, K.; Pleger, K.; Schedlowski, M.; Arck, P.C.; Elsenbruch, S. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain. Behav. Immun. 2008, 22, 177–184. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil. Steril. 2011, 95, 1048–1058. [Google Scholar] [CrossRef]
- Kuang, H.; Duan, Y.; Li, D.; Xu, Y.; Ai, W.; Li, W.; Wang, Y.; Liu, S.; Li, M.; Liu, X.; et al. The role of serum inflammatory cytokines and berberine in the insulin signaling pathway among women with polycystic ovary syndrome. PLoS ONE 2020, 15, e0235404. [Google Scholar] [CrossRef] [PubMed]
- Attallah, H.; Friedlander, A.L.; Hoffman, A.R. Visceral obesity, impaired glucose tolerance, metabolic syndrome, and growth hormone therapy. Growth Horm. IGF Res. 2006, 16, 62–67. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, Y.; Lu, H.; Li, L.; Sun, Z. Effect of berberine on clinical, metabolic and endocrine indexes and pregnancy ourcome in women with polycystic ovary syndrome undergoing IVF treatment. Mod. J. Integr. Tradit. Chin. West. Med. 2016, 25, 459–462. [Google Scholar]
- Li, L.; Li, C.; Pan, P.; Chen, X.; Wu, X.; Ng, E.H.Y.; Yang, D. A Single Arm Pilot Study of Effects of Berberine on the Menstrual Pattern, Ovulation Rate, Hormonal and Metabolic Profiles in Anovulatory Chinese Women with Polycystic Ovary Syndrome. PLoS ONE 2015, 10, e0144072. [Google Scholar]
- Kuang, H. Clinical Study on Berberine in the Treatment of Insulin Resistance in Women with Polycystic Ovary Syndrome; Guangzhou University of Chinese Medicine: Guangzhou, China, 2014. [Google Scholar]
- Ma, Y.; Yang, J.; Sui, M.; Liang, K.; Deng, H.; Wei, W. Study the therapeutic effect of berberine on PCOS patients with insulin resistance. Chin. J. Pract. Gynecol. Obstet. 2011, 27, 684–687. [Google Scholar]
- Shan, J.J.; Rodgers, K.; Lai, C.-T.; Sutherland, S.K. Challenges in natural health product research: The importance of standardization. Proc. West. Pharmacol. Soc. 2007, 50, 24–30. [Google Scholar] [PubMed]
| Parameters | t0 | t1 | t2 | ||
|---|---|---|---|---|---|
| Value Mean (SD) | Value Mean (SD) | Variation vs. t0 (%) | Value Mean (SD) | Variation vs. t0 (%) | |
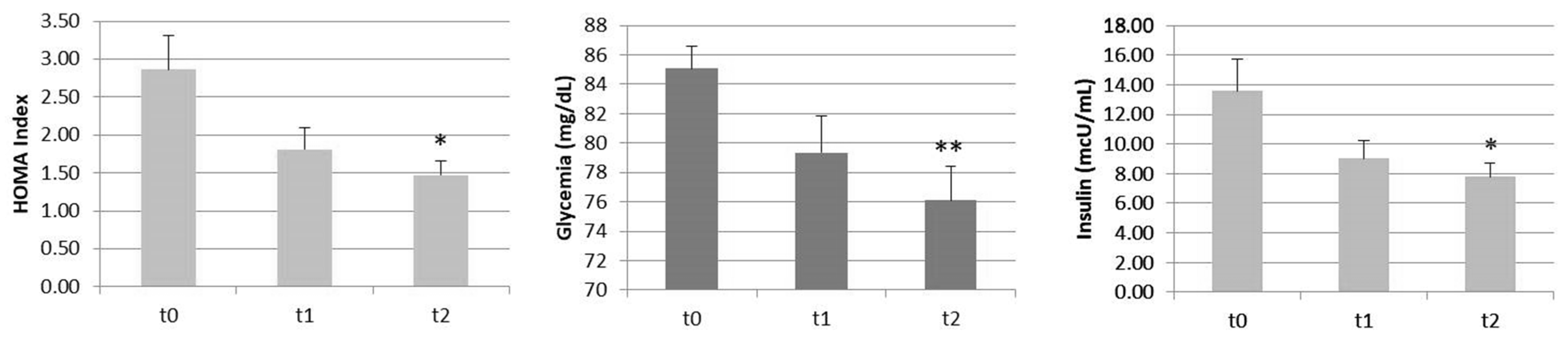
| HOMA (pt) | 2.86 (1.55) | 1.81 (0.98) | −36.7 | 1.47(0.63) | −48.6 * |
| Glycemia (mg/dL) | 85.08 (5.21) | 79.33 (8.79) | −6.8 | 76.08 (8.13) | −10.6 ** |
| Insulin (mcU/mL) | 13.46 (7.25) | 9.02 (4.31) | −33.0 | 7.79 (3.11) | −42.1 * |
| Parameters | t0 | t2 | |
|---|---|---|---|
| Value Mean (SD) | Value Mean (SD) | Variation vs. t0 (%) | |
| Total Cholesterol (mg/dL) | 160.08 (50.32) | 155.08 (44.74) | −3.1 |
| HDL (mg/dL) | 55.17 (10.60) | 57.75 (14.01) | 4.7 |
| LDL (mg/dL) | 87.13 (42.25) | 83.68 (34.31) | −4.0 |
| VLDL (mg/dL) | 17.78 (7.24) | 14.67 (5.23) | −17.5 * |
| Triglycerides (mg/dL) | 88.5 (36.23) | 73.08 (26.12) | −17.4 * |
| Parameters | t0 | t2 | |
|---|---|---|---|
| Value Mean (SD) | Value Mean (SD) | Variation vs. t0 (%) | |
| SHBG (nmol/l) | 62.73 (38.43) | 71.78 (39.25) | +14.4 * |
| Free Testosterone (ng/mL) | 0.46 (0.22) | 0.31 (0.31) | −32.6 ** |
| FAI (pt) | 3.18 (3.16) | 2.4 (1.70) | −24.5 |
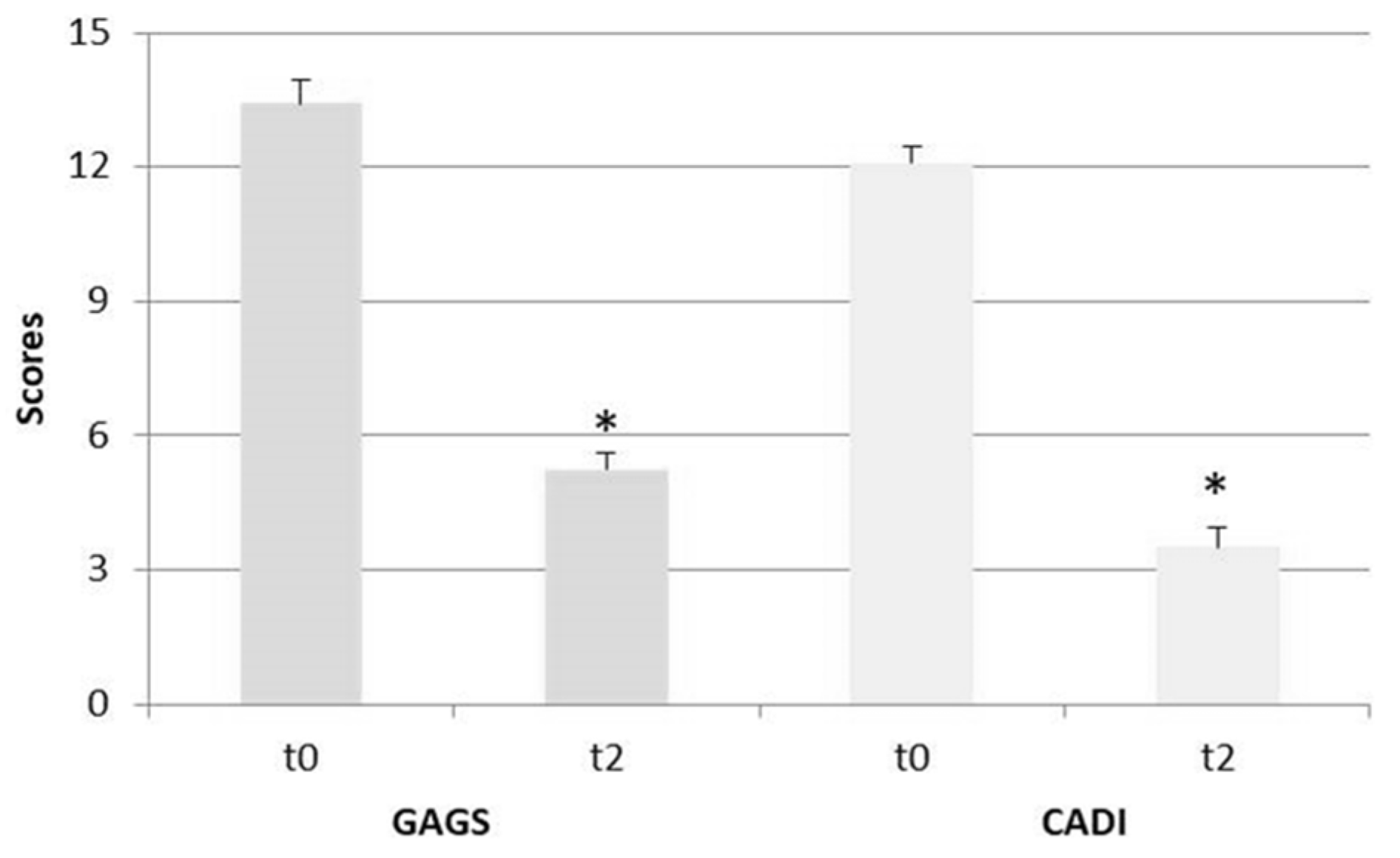
| GAGS | 13.42 (1.83) (Moderate) | 5.25 (1.29) (Mild) | −60.9 *** |
| CADI | 12.08 (1.38) (High) | 3.5 (1.62) (Low) | −71.0 *** |
| Parameters | t0 | t1 | t2 | ||
|---|---|---|---|---|---|
| Value Mean (SD) | Value Mean (SD) | Variation vs. t0 (%) | Value Mean (SD) | Variation vs. t0 (%) | |
| BMI (Kg/m2) | 25.39 (3.69) | 24.70 (3.76) | −2.7 | 24.57 (3.74) | −3.2 ** |
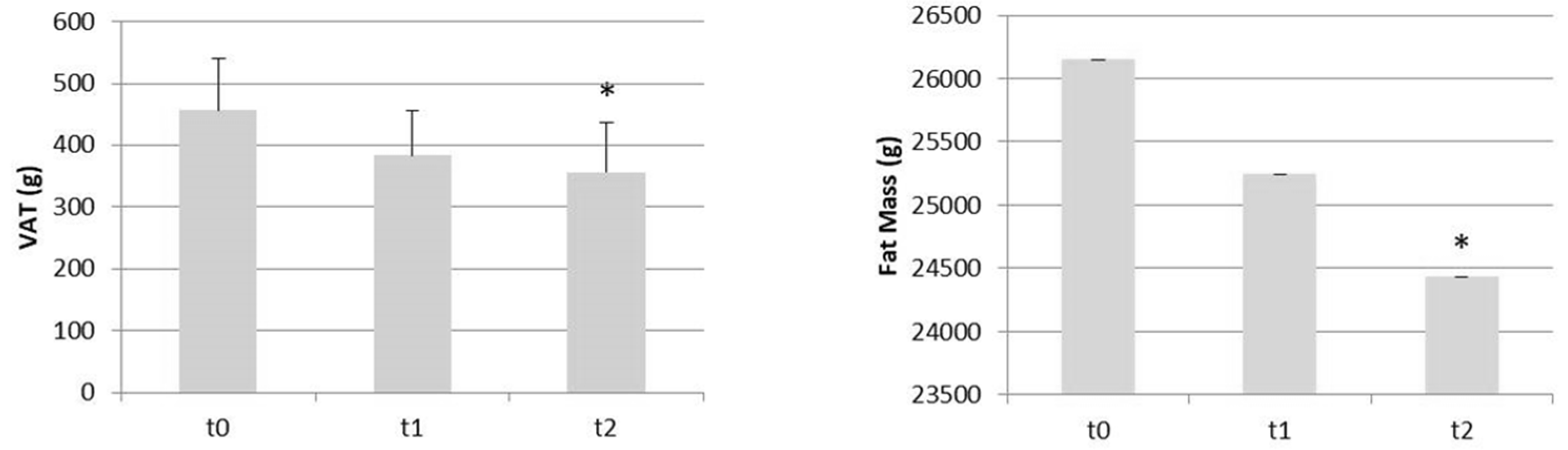
| VAT (g) | 456.92 (287.32) | 383,75 (254.10) | −16.0 | 357.00 (276.13) | −21.9 ** |
| Waist circumference (cm) | 87.46 (9.11) | 86 (8.88) | −1.7 | 84.62 (8.27) | −3.2 |
| Hip circumference (cm) | 106 (12.60) | 104,46 (12.65) | −1.5 | 104.08 (12.47) | −1.8 ** |
| W/H Ratio | 0.83 (0.06) | 0.83 (0.06) | 0.0 | 0.82 (0.06) | −1.2 * |
| Total mass (Kg) | 68.32 (10.87) | 66.71 (10.56) | −2.4 | 65.97 (65.97) | −3.4 ** |
| Fat mass (g) | 26147.58 (7230.92) | 25243.17 (6769.28) | −3.5 | 24433.08 (6565.32) | −6.6 ** |
| Lean mass (g) | 39863.17 (6011.00) | 39095.5 (5725.30) | −1.9 | 39249.67 (5571.37) | −1.5 |
| Parameters | t0 | t2 | |
|---|---|---|---|
| Value Mean (SD) | Value Mean (SD) | Variation vs. t0 (%) | |
| CRP (mg/dL) | 0.33 (0.39) | 0.19 (0.26) | −42.4 * |
| TNF α (pg/mL) | 12.24 (6.20) | 6.07 (2.70) | −50.4 ** |
| Parameters | t0 | t2 |
|---|---|---|
| Value Mean (SD) | Value Mean (SD) | |
| Total Bilirubin (mg/dL) | 0.43 (0.21) | 0.42 (0.17) |
| AST (IU/L) | 20.08 (9.59) | 18.08 (5.92) |
| ALT (IU/L) | 20.08 (13.15) | 17.42 (8.02) |
| GGT (U/L) | 13.58 (4.85) | 13.42 (4.23) |
| CPK (U/L) | 96.5 (42.84) | 67.5 (22.95) |
| Endpoints | Time × Group β [95%CI] | p-Value Unadjusted | p-Value Adjusted |
|---|---|---|---|
| Primary endpoint | |||
| HOMA | −0.69 [−1.06; −0.31] | 0.0009 | 0.003 |
| Secondary endpoints | |||
| Total Cholesterol (mg/dL) | −5.00 [−18.21; 8.21] | 0.42 | 0.49 |
| HDL (mg/dL) | 2.58 [−3.18; 8.35] | 0.34 | 0.45 |
| LDL (mg/dL) | −3.45 [−12.54; 5.64] | 0.42 | 0.49 |
| VLDL (mg/dL) | −3.11 [−5.55; −0.66] | 0.02 | 0.03 |
| CRP(mg/dL) | −0.14 [−0.23; −0.04] | 0.008 | 0.02 |
| TNF α (pg/mL) | −6.17 [−9.91; −2.44] | 0.004 | 0.009 |
| Triglycerides (mg/dL) | −15.42 [−27.65; −3.18] | 0.02 | 0.03 |
| Glycemia (mg/dL) | −4.50 [−6.20; −2.80] | <0.0001 | 0.0001 |
| Insulin (mcU/mL) | −2.83 [−4.51; −1.15] | 0.002 | 0.005 |
| SHBG (nmol/L) | 9.04 [2.07; 16.01] | 0.02 | 0.03 |
| CPK (U/L) | −29.00 [−50.58; −7.42] | 0.01 | 0.02 |
| Free Testosterone (ng/mL) | −0.15 [−0.24; −0.06] | 0.003 | 0.007 |
| FAI | −0.78 [−2.04; 0.47] | 0.20 | 0.28 |
| Total Bilirubin (mg/dL) | −0.01 [−0.13; 0.12] | 0.91 | 0.91 |
| AST (IU/L) | −2.00 [−7.85; 3.85] | 0.47 | 0.52 |
| ALT (IU/L) | −2.67 [−8.86; 3.52] | 0.36 | 0.45 |
| GGT (U/L) | −0.17 [−1.62; 1.29] | 0.81 | 0.84 |
| BMI (kg/m2) | −0.41 [−0.64; −0.19] | 0.001 | 0.003 |
| Waist Circumference (cm) | −1.42 [−2.27; −0.57] | 0.002 | 0.005 |
| Hip Circumference (cm) | −0.96 [−1.49; −0.42] | 0.001 | 0.003 |
| W/H ratio (pt) | −0.006 [−0.01; −0.0004] | 0.03 | 0.04 |
| VAT (g) | −49.96 [−75.85; −24.06] | 0.0006 | 0.003 |
| Total Mass (kg) | −1.17 [−1.73; −0.61] | 0.0003 | 0.002 |
| Fat Mass (g) | −857.25 [−1334.43; −380.06] | 0.001 | 0.003 |
| Lean Mass (g) | −306.75 [−661.89; 48.39] | 0.09 | 0.13 |
| GAGS (pt) | −8.17 [−9.28; −7.05] | <0.0001 | <0.0001 |
| CADI (pt) | −8.58 [−9.65; −7.52] | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Giacosa, A.; Fazia, T.; Bernardinelli, L.; Gasparri, C.; Peroni, G.; Perna, S. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest–Post-Test Explanatory Study. Nutrients 2021, 13, 3665. https://doi.org/10.3390/nu13103665
Rondanelli M, Riva A, Petrangolini G, Allegrini P, Giacosa A, Fazia T, Bernardinelli L, Gasparri C, Peroni G, Perna S. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest–Post-Test Explanatory Study. Nutrients. 2021; 13(10):3665. https://doi.org/10.3390/nu13103665
Chicago/Turabian StyleRondanelli, Mariangela, Antonella Riva, Giovanna Petrangolini, Pietro Allegrini, Attilio Giacosa, Teresa Fazia, Luisa Bernardinelli, Clara Gasparri, Gabriella Peroni, and Simone Perna. 2021. "Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest–Post-Test Explanatory Study" Nutrients 13, no. 10: 3665. https://doi.org/10.3390/nu13103665
APA StyleRondanelli, M., Riva, A., Petrangolini, G., Allegrini, P., Giacosa, A., Fazia, T., Bernardinelli, L., Gasparri, C., Peroni, G., & Perna, S. (2021). Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest–Post-Test Explanatory Study. Nutrients, 13(10), 3665. https://doi.org/10.3390/nu13103665







