Children with Intestinal Failure Maintain Their Renal Function on Long-Term Parenteral Nutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data and Study Variables
2.3. Statistical Analysis
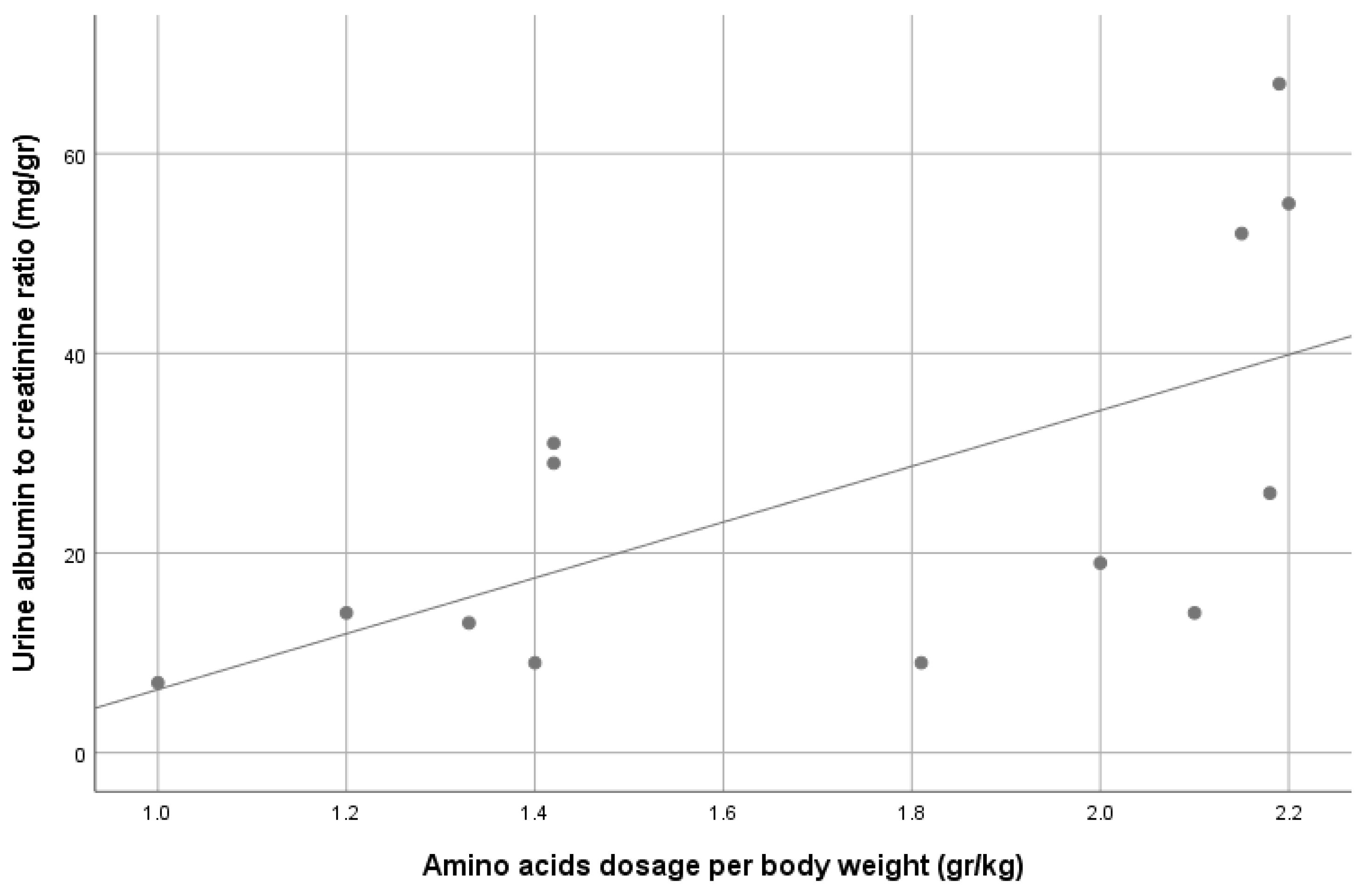
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.J.; Cohran, V.; Raphael, B.P.; Sentongo, T.; Volpert, D.; Warner, B.W.; Goday, P.S. Intestinal Rehabilitation Programs in the Management of Pediatric Intestinal Failure and Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 588–596. [Google Scholar] [CrossRef]
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Shamir, R.; Simchowitz, V.; Lohner, S.; Cai, W.; Decsi, T. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Complications. Clin. Nutr. 2018, 37, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Ksiazyk, J.; Prell, C.; Tabbers, M. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Home parenteral nutrition. Clin. Nutr. 2018, 37, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Boncompain-Gérard, M.; Robert, D.; Fouque, D.; Hadj-Aïssa, A. Renal function and urinary excretion of electrolytes in patients receiving cyclic parenteral nutrition. JPEN 2000, 24, 234–239. [Google Scholar] [CrossRef]
- Pironi, L.; Lauro, A.; Soverini, V.; Zanfi, C.; Agostini, F.; Guidetti, M.; Pazzeschi, C.; Pinna, A.D. Renal function in patients on long-term home parenteral nutrition and in intestinal transplant recipients. Nutrition 2014, 30, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, A.A.; Ament, M.E.; Buchman, A.; Dahlstrom, K.A.; Vargas, J. Renal function of children receiving long-term parenteral nutrition. J. Pediatr. 1991, 119, 864–868. [Google Scholar] [CrossRef]
- Kosar, C.; De Silva, N.; Avitzur, Y.; Steinberg, K.; Courtney-Martin, G.; Chambers, K.; Fitzgerald, K.; Harvey, E.; Wales, P.W. Prevalence of renal abnormality in pediatric intestinal failure. J. Pediatr. Surg. 2016, 51, 794–797. [Google Scholar] [CrossRef]
- Billing, H.; Traunspurger, A.; Sturm, E.; Busch, A. High Incidence of Proteinuria in Children with Chronic Intestinal Failure Under Long-term Parenteral Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 751–754. [Google Scholar] [CrossRef]
- Roberts, A.J.; Belza, C.; Wales, P.W.; Courtney-Martin, G.; Harvey, E.; Avitzur, Y. Nephrocalcinosis and Renal Dysfunction in Pediatric Intestinal Failure. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 789–793. [Google Scholar] [CrossRef]
- Fullerton, B.S.; Hong, C.R.; Jaksic, T. Long-term outcomes of pediatric intestinal failure. Semin. Pediatr. Surg. 2017, 26, 328–335. [Google Scholar] [CrossRef]
- Zemrani, B.; Bines, J.E. Monitoring of long-term parenteral nutrition in children with intestinal failure. JGH Open 2019, 3, 163–172. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. JASN 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Kruse, K.; Kracht, U.; Kruse, U. Reference values for urinary calcium excretion and screening for hypercalciuria in children and adolescents. Eur. J. Pediatr. 1984, 143, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Houser, M. Assessment of proteinuria using random urine samples. J. Pediatr. 1984, 104, 845–848. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, F.A.; Chen, C.F.; Liu, W.S.; Shih, C.J.; Ou, S.M.; Yang, W.C.; Lin, C.C.; Yang, A.H. Diagnostic Accuracy of Urine Protein/Creatinine Ratio Is Influenced by Urine Concentration. PLoS ONE 2015, 10, e0137460. [Google Scholar] [CrossRef]
- De Santo, N.G.; Di Iorio, B.; Capasso, G.; Paduano, C.; Stamler, R.; Langman, C.B.; Stamler, J. Population based data on urinary excretion of calcium, magnesium, oxalate, phosphate and uric acid in children from Cimitile (southern Italy). Pediatr. Nephrol. 1992, 6, 149–157. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Zhang, Y.; Yue, S.; Mei, X.; Bi, L.; Zhai, W.; Ren, X.; Ding, Y.; Zhang, S.; et al. Correlation of urine protein/creatinine ratios to 24-h urinary protein for quantitating proteinuria in children. Pediatr. Nephrol. 2020, 35, 463–468. [Google Scholar] [CrossRef]
- Justesen, T.I.; Petersen, J.L.; Ekbom, P.; Damm, P.; Mathiesen, E.R. Albumin-to-creatinine ratio in random urine samples might replace 24-h urine collections in screening for micro- and macroalbuminuria in pregnant woman with type 1 diabetes. Diabetes Care 2006, 29, 924–925. [Google Scholar] [CrossRef][Green Version]
- Eknoyan, G.; Lameire, N. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Ylinen, E.; Merras-Salmio, L.; Gunnar, R.; Jahnukainen, T.; Pakarinen, M.P. Intestinal failure as a significant risk factor for renal impairment in children. Nutrition 2018, 45, 90–93. [Google Scholar] [CrossRef]
- Messova, A.; Dziubak, R.; Köglmeier, J. Renal Function in Children on Long Term Home Parenteral Nutrition. Front. Pediatr. 2019, 7, 137. [Google Scholar] [CrossRef]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. CJASN 2008, 3, 348–354. [Google Scholar] [CrossRef]
- Agostini, F.; Sasdelli, A.S.; Guidetti, M.; Comai, G.; La Manna, G.; Pironi, L. Outcome of kidney function in adults on long-term home parenteral nutrition for chronic intestinal failure. Nutrition 2019, 60, 212–216. [Google Scholar] [CrossRef]
- Lauverjat, M.; Hadj Aissa, A.; Vanhems, P.; Boulétreau, P.; Fouque, D.; Chambrier, C. Chronic dehydration may impair renal function in patients with chronic intestinal failure on long-term parenteral nutrition. Clin. Nutr. 2006, 25, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Tomaschitz, A. Aldosterone and the kidney: A rapidly moving frontier (an update). Nephrol. Dial. Transplant. 2014, 29, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary protein intake and renal function. Nutr. Metab. 2005, 2, 25. [Google Scholar] [CrossRef]
- Hadj-Aïssa, A.; Bankir, L.; Fraysse, M.; Bichet, D.G.; Laville, M.; Zech, P.; Pozet, N. Influence of the level of hydration on the renal response to a protein meal. Kidney Int. 1992, 42, 1207–1216. [Google Scholar] [CrossRef]
- Dudley, J.; Rogers, R.; Sealy, L. Renal consequences of parenteral nutrition. Pediatr. Nephrol. 2014, 29, 375–385. [Google Scholar] [CrossRef]
- Johnson, E.; Vu, L.; Matarese, L.E. Bacteria, Bones, and Stones: Managing Complications of Short Bowel Syndrome. Nutr. Clin. Pract. 2018, 33, 454–466. [Google Scholar] [CrossRef]
- Swartz, R.D.; Wesley, J.R.; Somermeyer, M.G.; Lau, K. Hyperoxaluria and renal insufficiency due to ascorbic acid administration during total parenteral nutrition. Ann. Int. Med. 1984, 100, 530–531. [Google Scholar] [CrossRef]
- Buchman, A.L.; Moukarzel, A.A.; Ament, M.E. Excessive urinary oxalate excretion occurs in long-term TPN patients both with and without ileostomies. J. Am. Coll. Nutr. 1995, 14, 24–28. [Google Scholar] [CrossRef]
- Rudman, D.; Kutner, M.H.; Redd, S.C., 2nd; Waters, W.C.; Gerron, G.G.; Bleier, J. Hypocitraturia in calcium nephrolithiasis. J. Clin. Endocrinol. Metab. 1982, 55, 1052–1057. [Google Scholar] [CrossRef] [PubMed]

| Patient No. | Intestinal Failure Etiology | Underlying Disease | Small Bowel Length (cm) | Age (Years) | Weight (kg) | Weight Z-Score | Height (cm) | Height Z-Score | Duration of HPN (Years) | Other Diagnoses |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SBS | Midgut volvulus | 60 | 4 | 16 | 0.243 | 96 | −1 | 4 | |
| 2 | Dysmotilty | Pediatric intestinal pseudo-obstruction | full | 6 | 18 | −1.31 | 106 | −2.67 | 6 | |
| 3 | Enteropathy | Tricho-hepatoenteric syndrome | full | 2 | 10.5 | −2.43 | 83 | −1.98 | 2 | |
| 4 | SBS | Bowel resection post Hirschsprung disease | 80 | 6 | 19 | −0.92 | 107 | −1.89 | 6 | |
| 5 | SBS | Midgut volvulus | 20 | 4.5 | 17 | −0.34 | 103 | −0.99 | 4.5 | Prematurity |
| 6 | Dysmotilty | MMIHS | full | 11 | 35 | −0.8 | 133 | −2.17 | 11 | Dysplastic kidneys |
| 7 | SBS | Intestinal atresia | 10 | 1.5 | 11 | −0.39 | 79 | −0.11 | 1.5 | Prematurity |
| 8 | Dysmotilty | Hirschsprung disease (total colonic) | full | 8 | 19 | −1.95 | 112 | −2.81 | 8 | Mowat-Wilson Syndrome |
| 9 | Enteropathy | Microvillus inclusion disease | full | 15 | 45 | −1.82 | 150 | −2.65 | 15 | |
| 10 | SBS | Midgut volvulus | 20 | 8 | 22 | −1.09 | 122 | −0.68 | 4 | |
| 11 | Enteropathy | Microvillus inclusion disease | full | 12 | 22 | −3.2 | 110 | −5.14 | 1.5 | |
| 12 | Enteropathy | Congenital diarrhea | full | 9 | 25 | −1.11 | 135 | −0.16 | 1 | |
| 13 | SBS | Gastroschisis | 0 | 1.5 | 11 | −0.79 | 76 | −0.2 | 1.5 | Prematurity |
| 14 | Immune dysregulation | Autoimmune Enteropathy | full | 3 | 13 | −0.25 | 74 | −5.4 | 1.5 | |
| 15 | SBS | Bowel resection post Hirschsprung disease | 160 | 8 | 21 | −1.64 | 125 | −0.7 | 1 |
| Patient No. | eGFR (mL/min/1.73 m2) | FENa (%) | Renin (mcIU/mL) | Aldosterone (pmol/L) | uCa/Cr (mg/mg) | PTH (pg/mL) | 25(OH) Vitamin D (nmol/L) | 1,25 (2OH) Vitamin D (pmol/L) | UrOxalate/Cr (mmol/mol) | UrCitrate/Cr (mg/gr) | uPr/Cr (mg/mg) | uAlb/Cr (mg/gr) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 142 | 0.25 | 132 | 359 | 0.57 | 54.2 | 36.7 | 99.2 | 76 | 635 | 0.29 | 7.00 |
| 2 | 175 | 0.18 | 656.5 | 387 | 0.12 | 83 | 73.3 | 200 | 52 | 362 | 0.30 | 19.00 |
| 3 | 127 | 2.40 | 58.4 | 874 | 0.23 | 17.9 | 69.4 | 187 | 73 | 556 | 0.36 | 67.00 |
| 4 | 158 | 0.07 | 739.8 | 449 | 0.34 | 20 | 64.4 | 56.5 | 43 | 489 | 0.10 | 9.00 |
| 5 | 203 | 0.18 | 183.8 | 798 | 1.11 | 25 | 56.5 | 184 | 132 | 48 | 0.51 | 29.00 |
| 6 | 82 | 0.24 | 978.3 | 2770 | 0.39 | 21 | 102 | 31.2 | 102 | 735 | 0.71 | 31.00 |
| 7 | 172 | 0.25 | 181.5 | 1100 | 0.31 | 33 | 123 | 88 | ||||
| 8 | 178 | 73.6 | 661 | 36 | 65.9 | 172 | ||||||
| 9 | 129 | 0.4 | 53.4 | 624 | 0.16 | 62 | 34.8 | 162 | 70 | 121 | 0.14 | 13.00 |
| 10 | 112 | 0.55 | 80.1 | 266 | 0.26 | 32 | 68.3 | 114 | 131 | 19 | 0.14 | 9.00 |
| 11 | 216 | 0.30 | 56.4 | 361 | 0.09 | 85 | 40.2 | 153 | 70 | 80 | 0.73 | 55.00 |
| 12 | 207 | 0.99 | 14.6 | 168 | 0.17 | 18 | 64.7 | 151 | 64 | 985 | 0.17 | 14.00 |
| 13 | 224 | 0.35 | 307.6 | 2660 | 0.04 | 30 | 63.3 | 173 | 22.5 | 1435 | 0.44 | 26.00 |
| 14 | 170 | 0.28 | 38.5 | 822 | 0.78 | 18 | 23.2 | 76.4 | 97.2 | 820 | 0.83 | 52.00 |
| 15 | 191 | 0.83 | 117.2 | 568 | 0.08 | 78 | 49.2 | 170 | 61 | 21 | 0.24 | 14.00 |
| Median (IQR) | 171.7 (135.3–196.9) | 0.29 (0.24–0.52) | 98.6 (56.9–183.2) | 624 (374–848) | 0.25 (0.13–0.38) | 32 (20.5–58.1 | 64.4 (44.7–68.8) | 153 (93.6–172.5) | 70 (61–97.2) | 489 (80–735) | 0.3 (0.17–0.51) | 19 (13–31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guz Mark, A.; Levi, S.; Davidovits, M.; Marderfeld, L.; Shamir, R. Children with Intestinal Failure Maintain Their Renal Function on Long-Term Parenteral Nutrition. Nutrients 2021, 13, 3647. https://doi.org/10.3390/nu13103647
Guz Mark A, Levi S, Davidovits M, Marderfeld L, Shamir R. Children with Intestinal Failure Maintain Their Renal Function on Long-Term Parenteral Nutrition. Nutrients. 2021; 13(10):3647. https://doi.org/10.3390/nu13103647
Chicago/Turabian StyleGuz Mark, Anat, Shelly Levi, Miriam Davidovits, Luba Marderfeld, and Raanan Shamir. 2021. "Children with Intestinal Failure Maintain Their Renal Function on Long-Term Parenteral Nutrition" Nutrients 13, no. 10: 3647. https://doi.org/10.3390/nu13103647
APA StyleGuz Mark, A., Levi, S., Davidovits, M., Marderfeld, L., & Shamir, R. (2021). Children with Intestinal Failure Maintain Their Renal Function on Long-Term Parenteral Nutrition. Nutrients, 13(10), 3647. https://doi.org/10.3390/nu13103647






