Phospholipid Derivatives of Cinnamic Acid Restore Insulin Sensitivity in Insulin Resistance in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
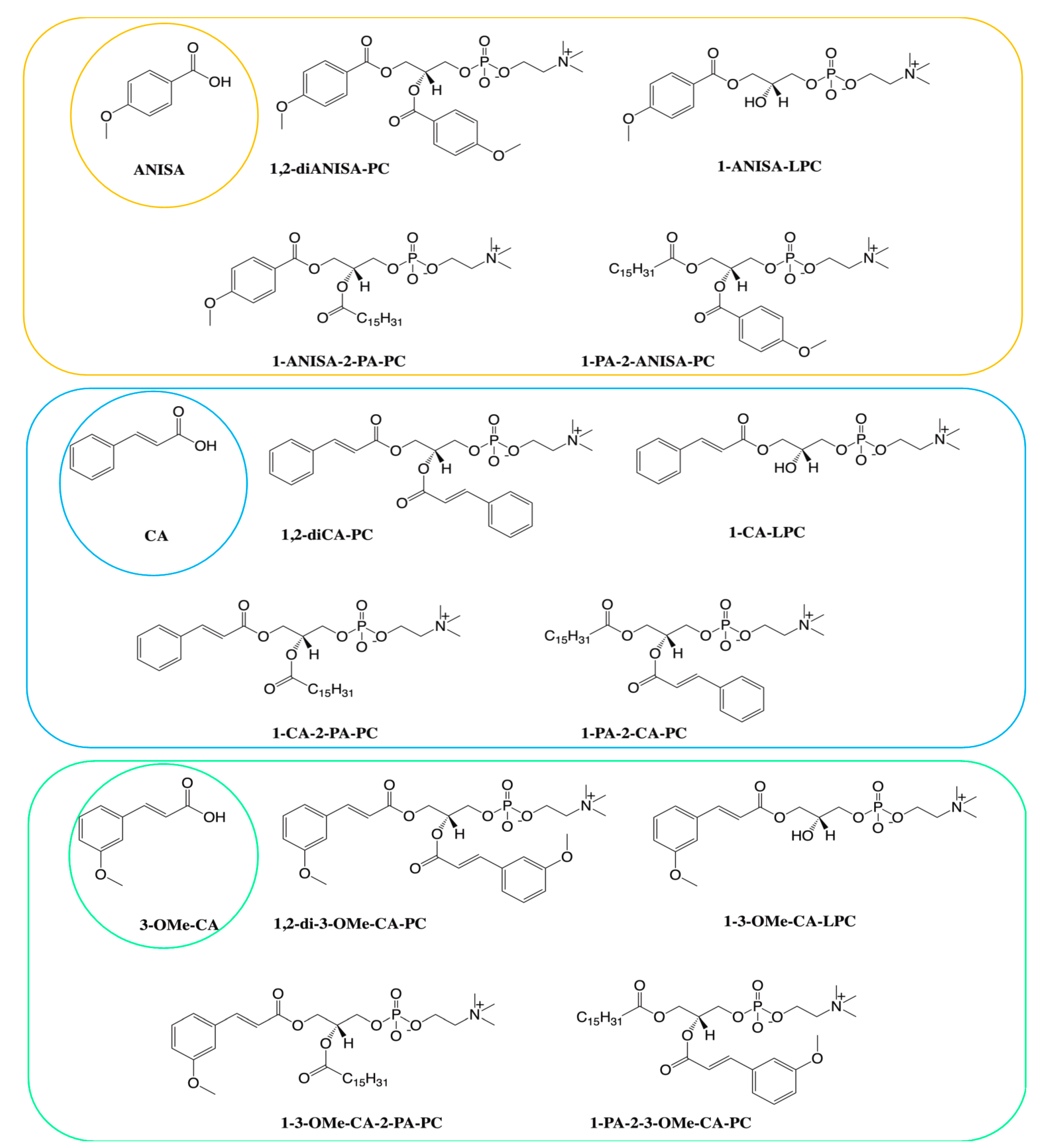
2.1. Chemicals and Reagents
2.2. 3L3-L1 Culturing and Differentiation
2.3. Viablitily Test (MTT)
2.4. Insulin Resistance Induction and the Effect of Phospholipid Derivatives
2.5. Glucose Uptake Test
2.6. Statistical Analysis
3. Results
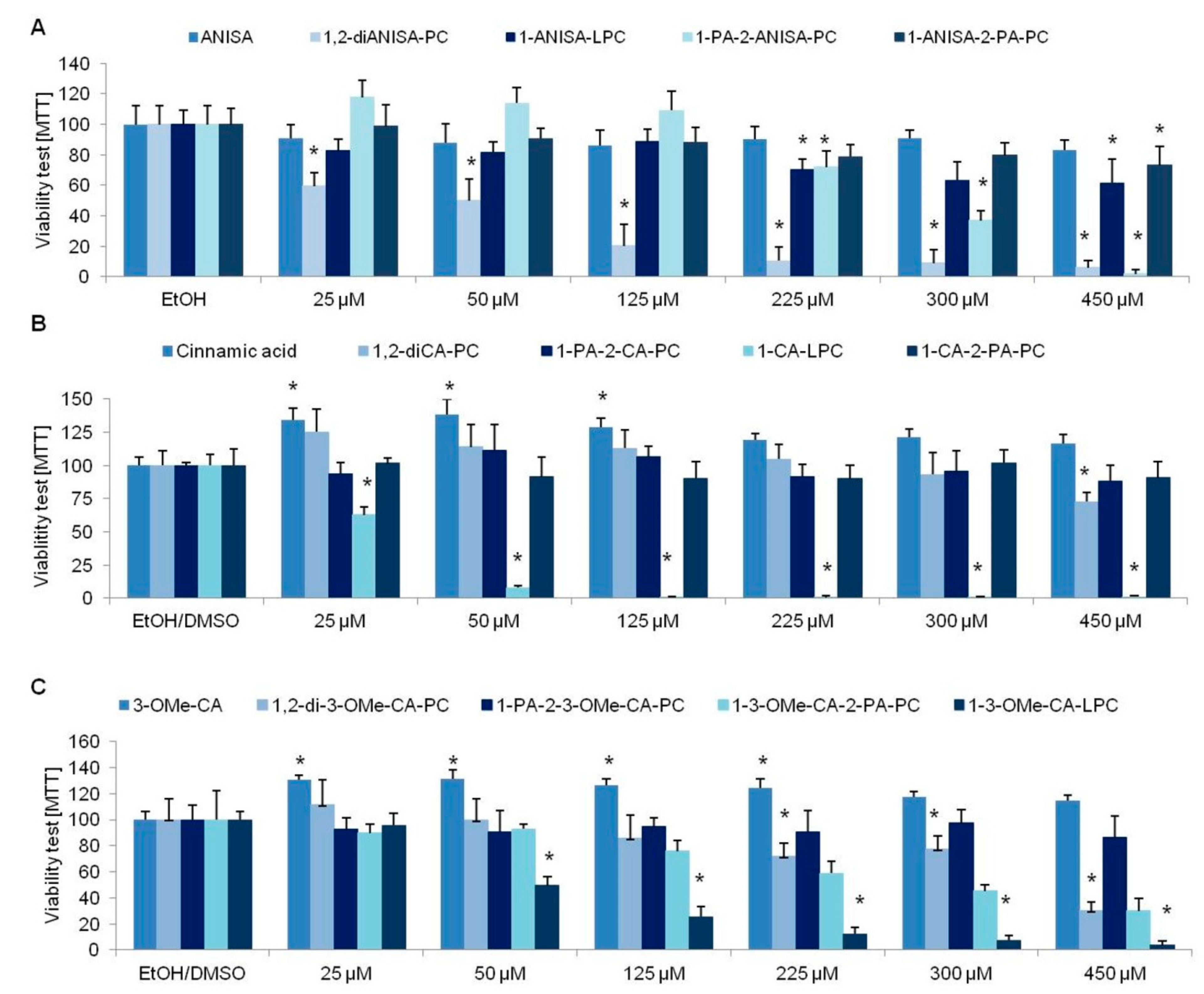
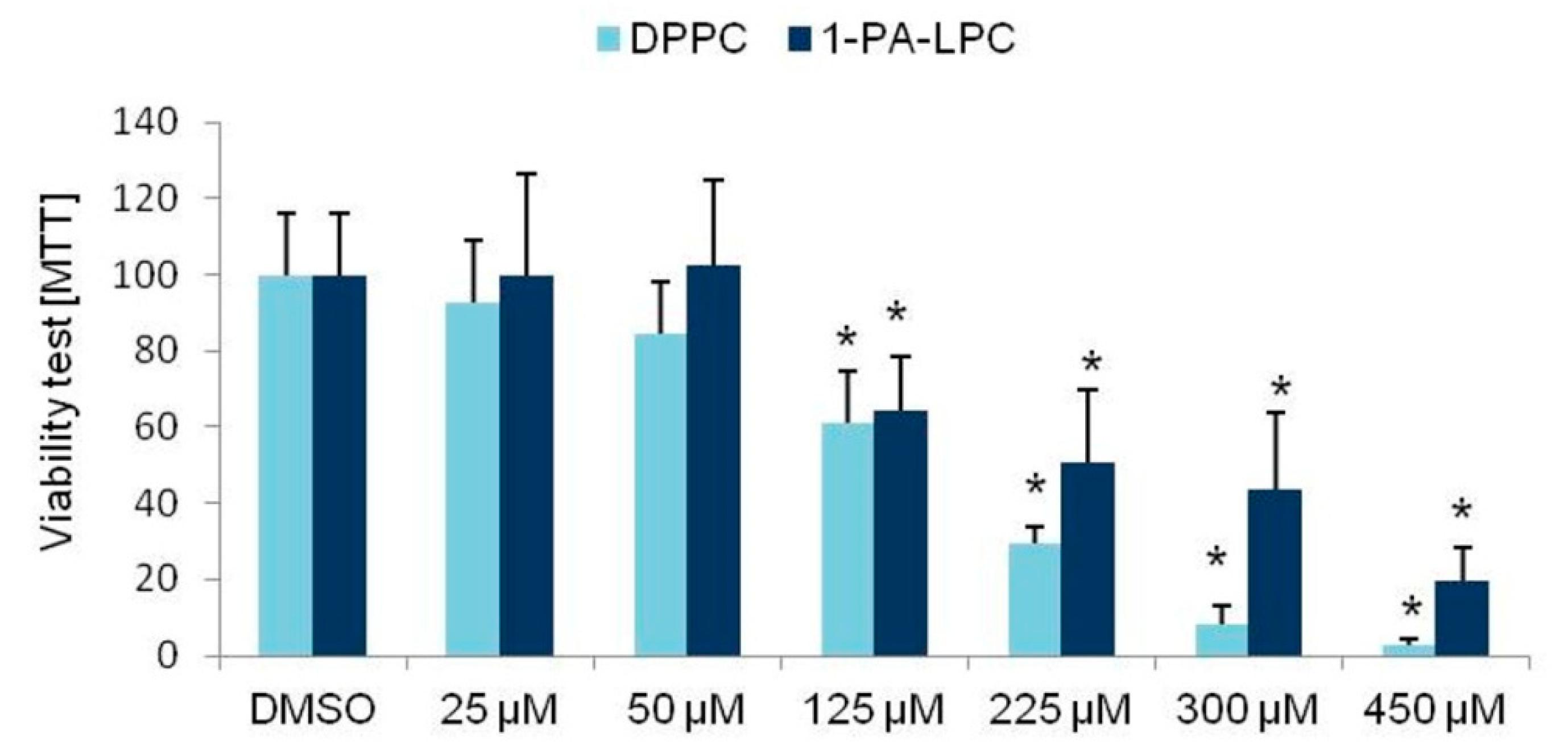
3.1. Viability Tests
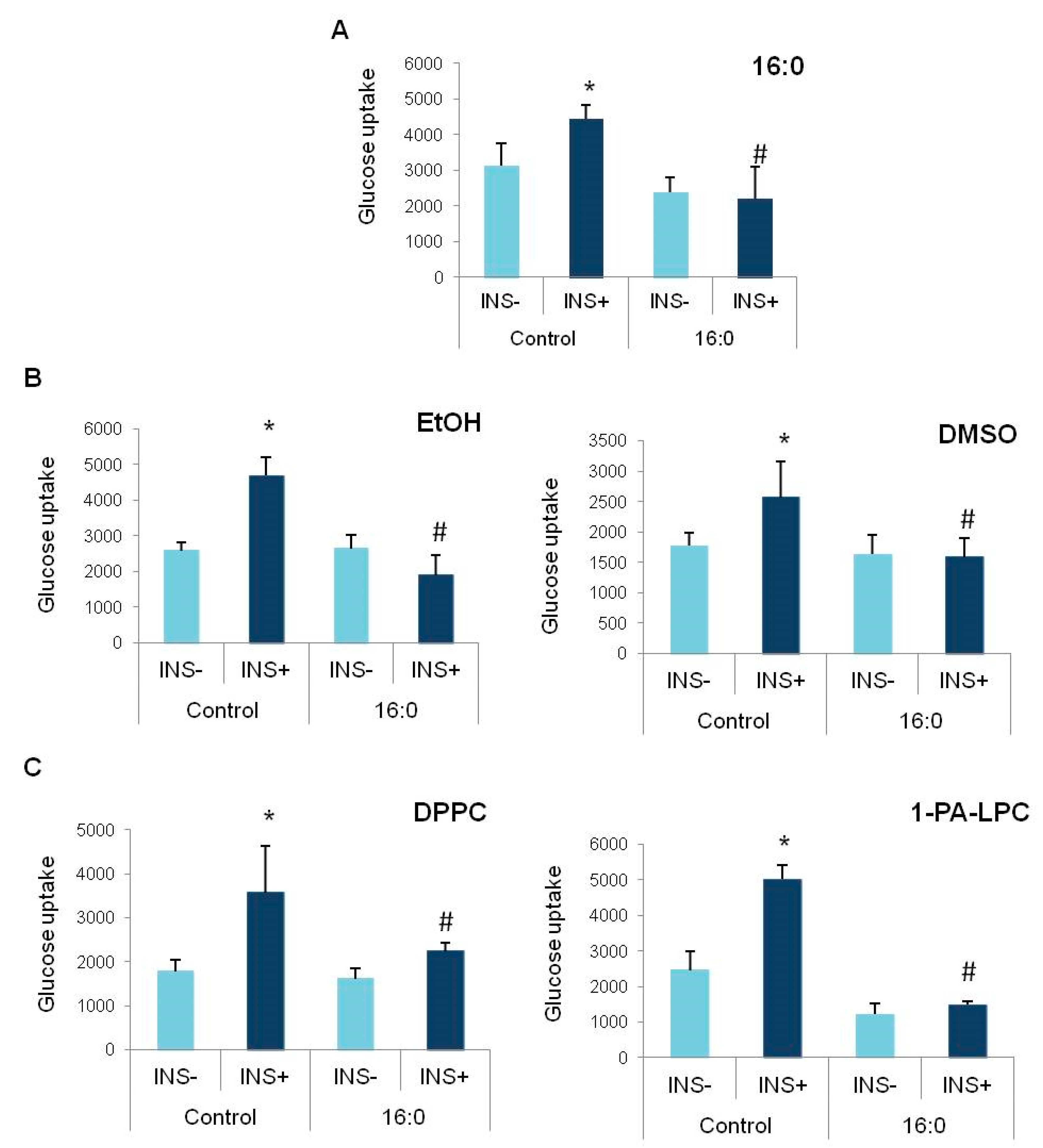
3.2. Insulin Resistance Induction and the Glucose Uptake
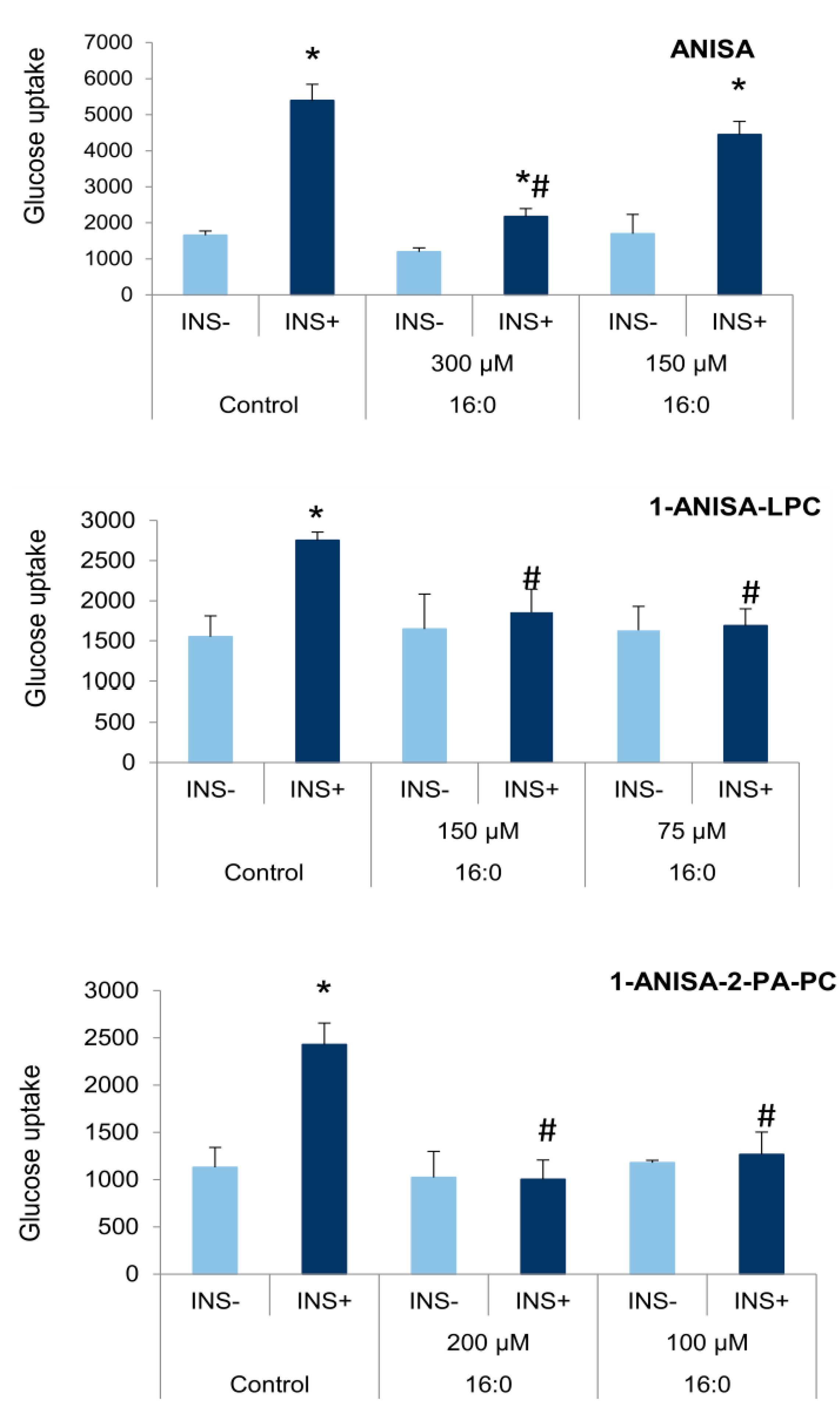
3.3. The Influence of Anisic Acid and Its Phospholipid Derivatives on Glucose Uptake in Insulin-Resistant Adipocytes
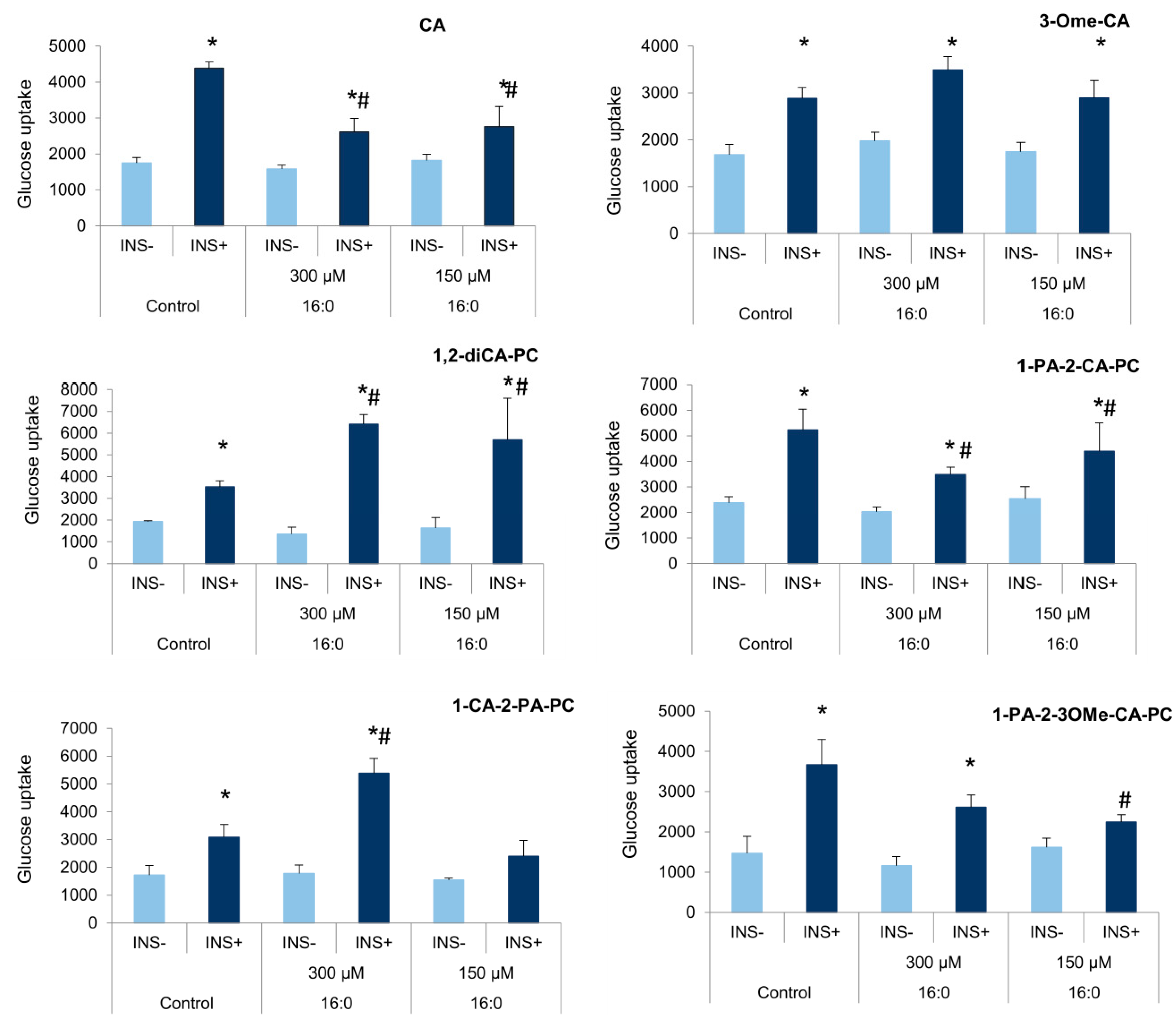
3.4. The Influence of Cinnamic Acid, 3-Methoxycinnamic Acid, and Their Phospholipid Derivatives on Glucose Uptake in Insulin-Resistant Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebovitz, H.E. Insulin Resistance: Definition and Consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. 2), S135–S148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ádány, R.; Pikó, P.; Fiatal, S.; Kósa, Z.; Sándor, J.; Bíró, É.; Kósa, K.; Paragh, G.; Bácsné Bába, É.; Veres-Balajti, I. Prevalence of Insulin Resistance in the Hungarian General and Roma Populations as Defined by Using Data Generated in a Complex Health (Interview and Examination) Survey. Int. J. Environ. Res. Public Health 2020, 17, 4833. [Google Scholar] [CrossRef] [PubMed]
- Rita, S.L.; Lubaki, F.J.-P.; Bompeka, L.F.; Ogunbanjo, G.A.; Ngwala, L.P. Prevalence and Determinants of Psychological Insulin Resistance among Type 2 Diabetic Patients in Kinshasa, Democratic Republic of Congo. Afr. J. Prim Health Care Fam. Med. 2019, 11, e1–e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapunar, J.; Aguilar-Farías, N.; Navarro, J.; Araneda, G.; Chandia-Poblete, D.; Manríquez, V.; Brito, R.; Cerda, A. High prevalence of overweight, obesity, insulin resistance and metabolic syndrome in rural children and adolescents. Rev. Med. Chil. 2018, 146, 978–986. [Google Scholar] [CrossRef] [Green Version]
- Van der Aa, M.P.; Knibbe, C.A.J.; de Boer, A.; van der Vorst, M.M.J. Definition of Insulin Resistance Affects Prevalence Rate in Pediatric Patients: A Systematic Review and Call for Consensus. J. Pediatr. Endocrinol. Metab. 2017, 30, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- You, D.; Nilsson, E.; Tenen, D.E.; Lyubetskaya, A.; Lo, J.C.; Jiang, R.; Deng, J.; Dawes, B.A.; Vaag, A.; Ling, C.; et al. Dnmt3a Is an Epigenetic Mediator of Adipose Insulin Resistance. Elife 2017, 6. [Google Scholar] [CrossRef]
- Cierzniak, A.; Pawelka, D.; Kaliszewski, K.; Rudnicki, J.; Dobosz, T.; Malodobra-Mazur, M. DNA Methylation in Adipocytes from Visceral and Subcutaneous Adipose Tissue Influences Insulin-Signaling Gene Expression in Obese Individuals. Int. J. Obes. Lond. 2021, 45, 650–658. [Google Scholar] [CrossRef]
- Defronzo, R.A. Glucose Intolerance and Aging: Evidence for Tissue Insensitivity to Insulin. Diabetes 1979, 28, 1095–1101. [Google Scholar] [CrossRef]
- Lankarani, M.; Valizadeh, N.; Heshmat, R.; Peimani, M.; Sohrabvand, F. Evaluation of Insulin Resistance and Metabolic Syndrome in Patients with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2009, 25, 504–507. [Google Scholar] [CrossRef]
- Bailey, J.L.; Zheng, B.; Hu, Z.; Price, S.R.; Mitch, W.E. Chronic Kidney Disease Causes Defects in Signaling through the Insulin Receptor Substrate/Phosphatidylinositol 3-Kinase/Akt Pathway: Implications for Muscle Atrophy. J. Am. Soc. Nephrol. 2006, 17, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swan, J.W.; Anker, S.D.; Walton, C.; Godsland, I.F.; Clark, A.L.; Leyva, F.; Stevenson, J.C.; Coats, A.J. Insulin Resistance in Chronic Heart Failure: Relation to Severity and Etiology of Heart Failure. J. Am. Coll. Cardiol. 1997, 30, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Pagano, G.; Cavallo-Perin, P.; Cassader, M.; Bruno, A.; Ozzello, A.; Masciola, P.; Dall’omo, A.M.; Imbimbo, B. An in Vivo and in Vitro Study of the Mechanism of Prednisone-Induced Insulin Resistance in Healthy Subjects. J. Clin. Investig. 1983, 72, 1814–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruz, P.W. HIV Protease Inhibitors and Insulin Resistance: Lessons from in-Vitro, Rodent and Healthy Human Volunteer Models. Curr. Opin. HIV AIDS 2008, 3, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Banach, M.; Malodobra-Mazur, M.; Gluba, A.; Katsiki, N.; Rysz, J.; Dobrzyn, A. Statin Therapy and New-Onset Diabetes: Molecular Mechanisms and Clinical Relevance. Curr. Pharm. Des. 2013, 19, 4904–4912. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K. Hyperinsulinemic-Euglycemic Clamp to Assess Insulin Sensitivity In Vivo. Methods Mol. Biol. 2009, 560, 221–238. [Google Scholar] [CrossRef]
- Association, A.D. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. Clin. Diabetes 2020, 38, 10–38. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of Metformin versus Glipizide on Cardiovascular Outcomes in Patients with Type 2 Diabetes and Coronary Artery Disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef] [Green Version]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Therap. Adv. Cardiovasc. Disease 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Hafizur, R.M.; Hameed, A.; Shukrana, M.; Raza, S.A.; Chishti, S.; Kabir, N.; Siddiqui, R.A. Cinnamic Acid Exerts Anti-Diabetic Activity by Improving Glucose Tolerance in Vivo and by Stimulating Insulin Secretion in Vitro. Phytomedicine 2015, 22, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, Characterization, and In Vitro Cancer Cell Growth Inhibition Evaluation of Novel Phosphatidylcholines with Anisic and Veratric Acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, F.L.; Chen, Y.C.; Cheng, J.T. Caffeic Acid as Active Principle from the Fruit of Xanthium Strumarium to Lower Plasma Glucose in Diabetic Rats. Planta Med. 2000, 66, 228–230. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Roengsamran, S.; Hsu, W.H.; Yibchok-anun, S. Mechanisms of Antihyperglycemic Effect of P-Methoxycinnamic Acid in Normal and Streptozotocin-Induced Diabetic Rats. Life Sci. 2005, 78, 406–412. [Google Scholar] [CrossRef]
- Yibchok-anun, S.; Adisakwattana, S.; Moonsan, P.; Hsu, W.H. Insulin-Secretagogue Activity of p-Methoxycinnamic Acid in Rats, Perfused Rat Pancreas and Pancreatic Beta-Cell Line. Basic Clin. Pharmacol. Toxicol. 2008, 102, 476–482. [Google Scholar] [CrossRef]
- Insulin-Releasing Properties of a Series of Cinnamic Acid Derivatives In Vitro and In Vivo-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18651742/ (accessed on 13 August 2021).
- Adisakwattana, S.; Hsu, W.H.; Yibchok-anun, S. Mechanisms of P-Methoxycinnamic Acid-Induced Increase in Insulin Secretion. Horm. Metab. Res. 2011, 43, 766–773. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis and Biological Evaluation of Phosphatidylcholines with Cinnamic and 3-Methoxycinnamic Acids with Potent Antiproliferative Activity. RSC Adv. 2018, 8, 35744–35752. [Google Scholar] [CrossRef]
- Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegała, Ł.; Gliszczyńska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Cierzniak, A.; Kaliszewski, K.; Dobosz, T. PPARG Hypermethylation as the First Epigenetic Modification in Newly Onset Insulin Resistance in Human Adipocytes. Genes 2021, 12, 889. [Google Scholar] [CrossRef]
- Malodobra-Mazur, M.; Cierzniak, A.; Dobosz, T. Oleic Acid Influences the Adipogenesis of 3T3-L1 Cells via DNA Methylation and May Predispose to Obesity and Obesity-Related Disorders. Lipids Health Dis. 2019, 18, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dordevic, A.L.; Konstantopoulos, N.; Cameron-Smith, D. 3T3-L1 Preadipocytes Exhibit Heightened Monocyte-Chemoattractant Protein-1 Response to Acute Fatty Acid Exposure. PLoS ONE 2014, 9, e99382. [Google Scholar] [CrossRef] [Green Version]
- Shinjo, S.; Jiang, S.; Nameta, M.; Suzuki, T.; Kanai, M.; Nomura, Y.; Goda, N. Disruption of the Mitochondria-Associated ER Membrane (MAM) Plays a Central Role in Palmitic Acid-Induced Insulin Resistance. Exp. Cell Res. 2017, 359, 86–93. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.-P.; Jouve, C.; Capel, F. Modulation of Insulin Resistance and the Adipocyte-Skeletal Muscle Cell Cross-Talk by LCn-3PUFA. Int. J. Mol. Sci. 2018, 19, 2778. [Google Scholar] [CrossRef] [Green Version]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-Glucoside and Protocatechuic Acid Exert Insulin-like Effects by Upregulating PPARγ Activity in Human Omental Adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [Green Version]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors Affecting Intake, Metabolism and Health Benefits of Phenolic Acids: Do We Understand Individual Variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved Bioavailability of Dietary Phenolic Acids in Whole Grain Barley and Oat Groat Following Fermentation with Probiotic Lactobacillus Acidophilus, Lactobacillus Johnsonii, and Lactobacillus Reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Czarnecka, M.; Świtalska, M.; Wietrzyk, J. Synthesis and Biological Evaluation of Novel Phosphatidylcholine Analogues Containing Monoterpene Acids as Potent Antiproliferative Agents. PLoS ONE 2016, 11, e0157278. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Świtalska, M.; Wietrzyk, J. Isoprenoid-Phospholipid Conjugates as Potential Therapeutic Agents: Synthesis, Characterization and Antiproliferative Studies. PLoS ONE 2017, 12, e0172238. [Google Scholar] [CrossRef]
- Drzazga, A.; Kamińska, D.; Gliszczyńska, A.; Gendaszewska-Darmach, E. Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs. Int. J. Mol. Sci. 2021, 22, 5748. [Google Scholar] [CrossRef] [PubMed]
- Palko-Łabuz, A.; Gliszczyńska, A.; Skonieczna, M.; Poła, A.; Wesołowska, O.; Środa-Pomianek, K. Conjugation with Phospholipids as a Modification Increasing Anticancer Activity of Phenolic Acids in Metastatic Melanoma-In Vitro and In Silico Studies. Int. J. Mol. Sci. 2021, 22, 8397. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małodobra-Mazur, M.; Lewoń, D.; Cierzniak, A.; Okulus, M.; Gliszczyńska, A. Phospholipid Derivatives of Cinnamic Acid Restore Insulin Sensitivity in Insulin Resistance in 3T3-L1 Adipocytes. Nutrients 2021, 13, 3619. https://doi.org/10.3390/nu13103619
Małodobra-Mazur M, Lewoń D, Cierzniak A, Okulus M, Gliszczyńska A. Phospholipid Derivatives of Cinnamic Acid Restore Insulin Sensitivity in Insulin Resistance in 3T3-L1 Adipocytes. Nutrients. 2021; 13(10):3619. https://doi.org/10.3390/nu13103619
Chicago/Turabian StyleMałodobra-Mazur, Małgorzata, Dominika Lewoń, Aneta Cierzniak, Marta Okulus, and Anna Gliszczyńska. 2021. "Phospholipid Derivatives of Cinnamic Acid Restore Insulin Sensitivity in Insulin Resistance in 3T3-L1 Adipocytes" Nutrients 13, no. 10: 3619. https://doi.org/10.3390/nu13103619
APA StyleMałodobra-Mazur, M., Lewoń, D., Cierzniak, A., Okulus, M., & Gliszczyńska, A. (2021). Phospholipid Derivatives of Cinnamic Acid Restore Insulin Sensitivity in Insulin Resistance in 3T3-L1 Adipocytes. Nutrients, 13(10), 3619. https://doi.org/10.3390/nu13103619






