Association of Dietary Fish and n-3 Unsaturated Fatty Acid Consumption with Diabetic Nephropathy from a District Hospital in Northern Taiwan
Abstract
1. Introduction
2. Materials and Methods
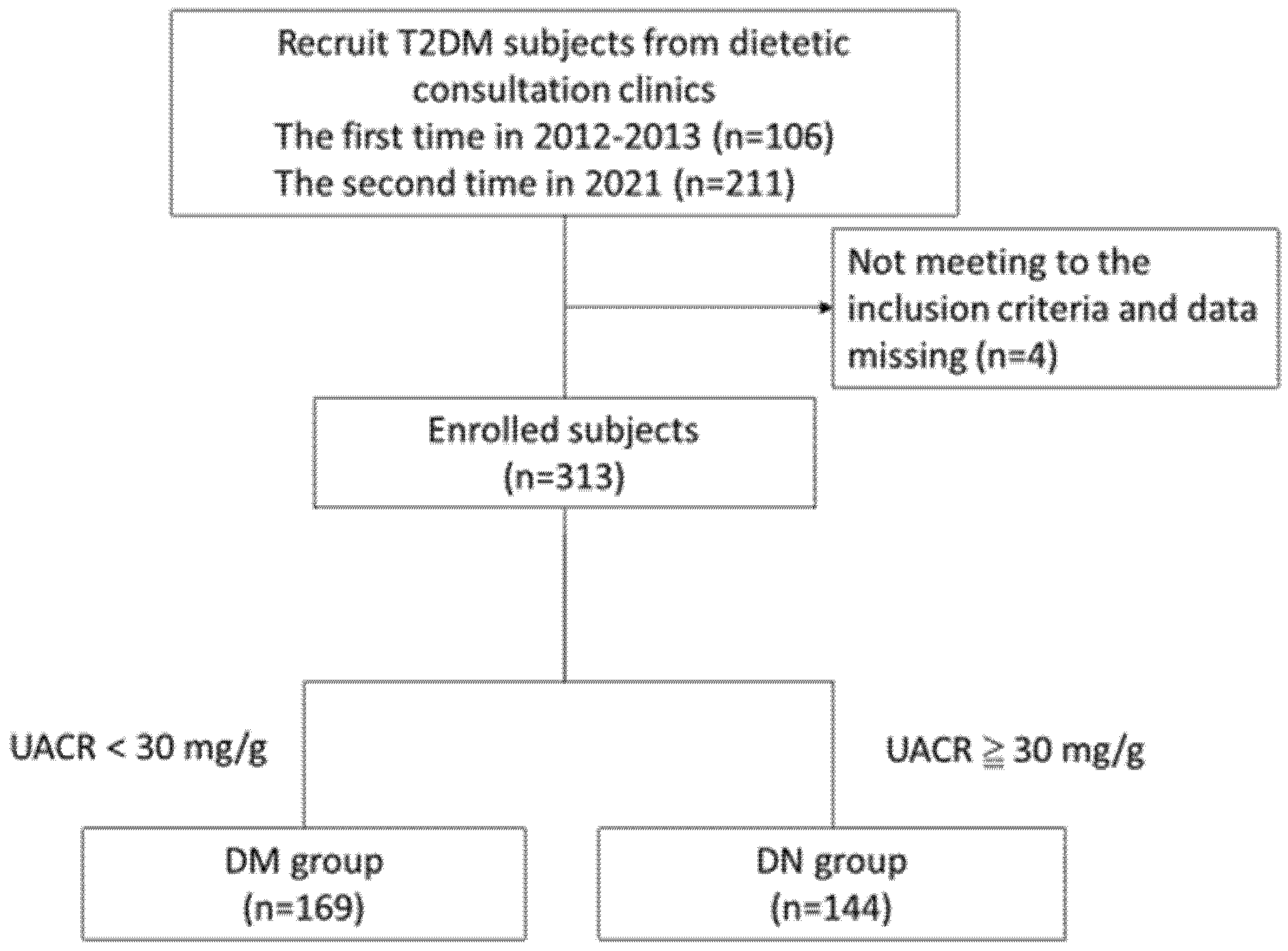
2.1. Study Participants
2.2. Research Design
2.3. Dietary Intake Assessment
2.4. Anthropometry and Blood Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Consumption of Selected Foods as Assessed through SQFFQ
3.3. Association between Selected Foods Consumption and DN
3.4. Dietary Fatty Acid Composition in DM and DN Groups
3.5. Association between Dietary Fatty Acids and DN
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease. Chall. Prog. Possibilities 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Naresh, C.N.; Hayen, A.; Weening, A.; Craig, J.C.; Chadban, S.J. Day-to-Day Variability in Spot Urine Albumin-Creatinine Ratio. Am. J. Kidney Dis. 2013, 62, 1095–1101. [Google Scholar] [CrossRef]
- Wouters, O.J.; O’donoghue, D.J.; Ritchie, J.; Kanavos, P.G.; Narva, A.S. Early chronic kidney disease: Diagnosis, management and models of care. Nat. Rev. Nephrol. 2015, 11, 491–502. [Google Scholar] [CrossRef]
- Narres, M.; Claessen, H.; Droste, S.; Kvitkina, T.; Koch, M.; Kuss, O.; Icks, A. The Incidence of End-Stage Renal Disease in the Diabetic (Compared to the Non-Diabetic) Population: A Systematic Review. PLoS ONE 2016, 11, e0147329. [Google Scholar] [CrossRef]
- Wu, M.Y.; Wu, M.S. Taiwan renal care system: A learning health-care system. Nephrol. 2018, 23 (Suppl. 4), 112–115. [Google Scholar] [CrossRef]
- Hsu, C.L.; Hsu, C.W.; Chang, P.C.; Huang, W.H.; Weng, C.H.; Yang, H.Y.; Liu, S.H.; Chen, K.H.; Weng, S.M.; Chang, C.C. Oral tori in chronic peritoneal dialysis patients. PLoS ONE 2016, 11, e0156988. [Google Scholar] [CrossRef]
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef]
- Sulaiman, M.K. Diabetic nephropathy: Recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, T.G.; Irving, G.F.; Axelsson, J. To eat or not to eat: Dietary fat in uremia is the question. Semin. Dial. 2010, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A.L. Chronic kidney disease: Considerations for nutrition interventions. J. Parenter. Enter. Nutr. 2014, 38, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Bloom, R.; Muntner, P.; Rahman, M.; Saland, J.M.; Wilson, P.W.F.; Fried, L. KDOQI US Commentary on the 2013 KDIGO Clinical Practice Guideline for Lipid Management in CKD. Am. J. Kidney Dis. 2015, 65, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Belardo, D.; Michos, E.D.; Blankstein, R.; Blumenthal, R.S.; Ferdinand, K.C.; Hall, K.; Klatt, K.; Natajaran, P.; Ostfeld, R.J.; Reddy, K. Practical, Evidence-Based Approaches to Nutritional Modifications to Reduce Atherosclerotic Cardiovascular Disease: An American Society For Preventive Cardiology Clinical Practice Statement. Am. J. Prev. Cardiol. 2022, 10, 100323. [Google Scholar] [CrossRef]
- Grande, J.P.; Donadio, J.V., Jr. Dietary fish oil supplementation in IgA nephropathy: A therapy in search of a mechanism? Nutrition 1998, 14, 240–242. [Google Scholar] [CrossRef]
- Han, E.; Yun, Y.; Kim, G.; Lee, Y.H.; Wang, H.J.; Lee, B.W.; Cha, B.S.; Kim, B.S.; Kang, E.S. Effects of omega-3 fatty acid supplementation on diabetic nephropathy progression in patients with diabetes and hypertriglyceridemia. PLoS ONE 2016, 11, e0154683. [Google Scholar] [CrossRef]
- MOllsten, A.V.; Dahlquist, G.G.; Stattin, E.L.; Rudberg, S. Higher intakes of fish protein are related to a lower risk of microalbuminuria in young Swedish type 1 diabetic patients. Diabetes Care 2001, 24, 805–810. [Google Scholar] [CrossRef]
- Kutner, N.G.; Clow, P.W.; Zhang, R.; Aviles, X. Association of fish intake and survival in a cohort of incident dialysis patients. Am. J. Kidney Dis. 2002, 39, 1018–1024. [Google Scholar] [CrossRef]
- Friedman, A.N.; Saha, C.; Watkins, B.A. Feasibility study of erythrocyte long-chain omega-3 polyunsaturated fatty acid content and mortality risk in hemodialysis patients. J. Ren. Nutr. 2008, 18, 509–512. [Google Scholar] [CrossRef]
- Food and Drug Administration of Taiwan. Available online: https://consumer.fda.gov.tw/food/tfnd.aspx?nodeID=178 (accessed on 18 May 2022).
- Varley, H. Practical Clinical Biochemistry; Williain Heinemann Medical Books Ltd.: London, UK, 1954; pp. 145–147. [Google Scholar]
- Abdelhafiz, A.H.; Ahmed, S.; El Nahas, M. Microalbuminuria: Marker or maker of cardiovascular disease. Nephron Exp. Nephrol. 2011, 119, e6–e10. [Google Scholar] [CrossRef] [PubMed]
- Boulatov, V.A.; Stenehjem, A.; Os, I. Association between albumin: Creatinine ratio and 24-hour ambulatory blood pressure in essential hypertension. Am. J. Hypertens. 2001, 14, 338–344. [Google Scholar] [CrossRef]
- Gu, S.; Wang, A.; Ning, G.; Zhang, L.; Mu, Y. Insulin resistance is associated with urinary albumin-creatinine ratio in normal weight individuals with hypertension and diabetes: The REACTION study. J. Diabetes 2020, 12, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Kamran, M. Association of high levels of spot urine protein with high blood pressure, mean arterial pressure and pulse pressure with the development of diabetic chronic kidney dysfunction or failure among diabetic patients. statistical regression modeling to predict diabetic proteinuria. Curr. Diabetes Rev. 2019, 15, 486–496. [Google Scholar]
- Schernthaner, G.; Schernthaner, G.H. Diabetic nephropathy: New approaches for improving glycemic control and reducing risk. J. Nephrol. 2013, 26, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Fadem, S.Z. Staying Healthy with Kidney Disease; Springer: Berlin/Heidelberg, Germany, 2022; pp. 23–33. [Google Scholar]
- Van Huffel, L.; Tomson, C.R.; Ruige, J.; Nistor, I.; Van Biesen, W.; Bolignano, D. Dietary restriction and exercise for diabetic patients with chronic kidney disease: A systematic review. PLoS ONE 2014, 9, e113667. [Google Scholar] [CrossRef]
- Jheng, H.F.; Hirotsuka, M.; Goto, T.; Shibata, M.; Matsumura, Y.; Kawada, T. Dietary low-fat soy milk powder retards diabetic nephropathy progression via inhibition of renal fibrosis and renal inflammation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Aziz, M.; Jalilpiran, Y.; Nekouimehr, M.; Fattahi, S.; Mokhtari, P.; Jayedi, A.; Yekaninejad, M.S.; Mirzaei, K. Dietary protein sources and risk of diabetic nephropathy in women: A case-control study. BMC Endocr. Disord. 2021, 21, 174. [Google Scholar] [CrossRef]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary protein sources and risk for incident chronic kidney disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef]
- Câmara, N.O.; Martins, J.O.; Landgraf, R.G.; Jancar, S. Emerging roles for eicosanoids in renal diseases. Curr. Opin. Nephrol. Hypertens. 2009, 18, 21–27. [Google Scholar] [CrossRef]
- Qi, Z.; Cai, H.; Morrow, J.D.; Breyer, M.D. Differentiation of cyclooxygenase 1-and 2–derived prostanoids in mouse kidney and aorta. Hypertension 2006, 48, 323–328. [Google Scholar] [CrossRef]
- Hao, C.M.; Breyer, M. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007, 71, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Kim, H.J.; Cho, K.H.; Vaziri, N.D. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am. J. Physiol. -Ren. Physiol. 2009, 297, F895–F903. [Google Scholar] [CrossRef] [PubMed]
- Diaz Encarnacion, M.M.; Warner, G.M.; Gray, C.E.; Cheng, J.; Keryakos, H.K.; Nath, K.A.; Grande, J.P. Signaling pathways modulated by fish oil in salt-sensitive hypertension. Am. J. Physiol. -Ren. Physiol. 2008, 294, F1323–F1335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glatz, J.; Soffers, A.; Katan, M.B. Fatty acid composition of serum cholesteryl esters and erythrocyte membranes as indicators of linoleic acid intake in man. Am. J. Clin. Nutr. 1989, 49, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.F.; Long, K.Z.; Hsu, C.C.; Al Mamun, A.; Jhang, H.R.; Shin, S.J.; Hwang, S.J.; Huang, M.C. Association of n-3 polyunsaturated fatty acids and inflammatory indicators with renal function decline in type 2 diabetes. Clin. Nutr. 2015, 34, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Jardine, M.J.; Mahaffey, K.W.; Neal, B.; Agarwal, R.; Bakris, G.L.; Brenner, B.M.; Bull, S.; Cannon, C.P.; Charytan, D.M.; De Zeeuw, D. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am. J. Nephrol. 2017, 46, 462–472. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Polidori, D.; Sha, S.; Ghosh, A.; Plum-Mörschel, L.; Heise, T.; Rothenberg, P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2013, 98, E867–E871. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Del Prato, S.; Chilton, R.; DeFronzo, R.A. SGLT2 inhibitors and cardiovascular risk: Lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016, 39, 717–725. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | DM b (n = 169) | DN (n = 144) | p-Value |
|---|---|---|---|
| Sex (M/F) | 89/80 | 73/71 | 0.735 |
| Age (years) | 62.7 ± 12.5 | 66.0 ± 11.1 | 0.017 * |
| Diabetes duration (years) | 9.3 ± 7.4 | 12.8 ± 8.6 | <0.001 * |
| Body Height (cm) | 161.1 ± 9.1 | 159.9 ± 8.2 | 0.201 |
| Body Weight (kg) | 68.7 ± 13.4 | 69.5 ± 15.6 | 0.614 |
| Body mass index (kg/m2) | 26.4 ± 4.3 | 27.0 ± 5.6 | 0.309 |
| Waist circumference(inch) | 33.7 ± 5.2 | 34.8 ± 5.2 | 0.068 |
| Systolic blood pressure (mm Hg) | 137.4 ± 13.8 | 136.2 ± 14.6 | 0.450 |
| Diastolic blood pressure (mm Hg) | 74.4 ± 9.6 | 74.0 ± 10.1 | 0.722 |
| Use of antihypertensive drugs, n (%) | 0.735 | ||
| No | 64 (37.9) | 43 (29.9) | |
| One type | 67 (39.6) | 62 (43.1) | |
| Two-type combination | 26 (15.4) | 25 (17.4) | |
| More than two type combination | 12 (7.1) | 14 (9.7) | |
| Use of oral hypoglycemic drugs, n (%) | 0.023 * | ||
| No | 12 (11.0) | 13 (13.0) | |
| One type | 71 (65.1) | 47 (47.0) | |
| Two-type combination | 26 (23.9) | 40 (40.0) | |
| More than two type combination | 26 (23.9) | 35 (35.0) | |
| Use of Insulin, n (%) | 26 (23.9) | 35 (35.0) | 0.077 |
| UACR (mg/g) | 12.2 ± 8.0 | 349.9 ± 692.6 | <0.001 * |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 1.2 ± 0.8 | <0.001 * |
| eGFR (mL/min/1.73 m2) | 85.6 ± 22.8 | 72.7 ± 30.7 | <0.001 * |
| Fasting plasma glucose (mg/dL) | 142.9 ± 46.2 | 153.1 ± 47.6 | 0.046 * |
| HbA1c (%) | 7.3 ± 1.2 | 7.9 ± 1.4 | <0.001 * |
| Triglycerides (mg/dL) | 139.6 ± 98.1 | 164.3 ± 100.6 | 0.031 * |
| Cholesterol (mg/dL) | 157.8 ± 31.6 | 158.6 ± 59.2 | 0.876 |
| Food Items | DM (n = 169) | DN (n = 144) | p-Value |
|---|---|---|---|
| Eggs | 2.2 ± 1.5 | 2.1 ± 1.5 | 0.682 |
| Meat and offal | 7.2 ± 2.8 | 7.4 ± 2.7 | 0.527 |
| Marine water fishes | |||
| Low-fat | 2.7 ± 2.3 | 2.2 ± 2.3 | 0.055 |
| Moderate-fat | 2.5 ± 2.4 | 2.1 ± 2.2 | 0.095 |
| High-fat | 3.0 ± 2.3 | 2.3 ± 2.3 | 0.012 * |
| Freshwater fishes | 1.5 ± 2.0 | 1.7 ± 2.2 | 0.519 |
| Shellfish | 0.7 ± 1.2 | 0.5 ± 0.9 | 0.035 * |
| Processed fish products | 0.3 ± 0.8 | 0.2 ± 0.6 | 0.145 |
| Dairy products | |||
| Whole milk | 0.9 ± 1.6 | 0.7 ± 1.3 | 0.137 |
| low-fat milk | 0.3 ± 0.9 | 0.2 ± 0.7 | 0.147 |
| skim milk | 0.2 ± 0.7 | 0.0 ± 0.3 | 0.053 |
| Soybean products | 2.5 ± 2.6 | 1.9 ± 2.0 | 0.013 * |
| Fats and oils | |||
| Animal fat | 1.4 ± 3.1 | 1.6 ± 3.3 | 0.634 |
| Soybean oil and sunflower oil (n-6 PUFA mainly) | 5.7 ± 4.5 | 6.1 ± 4.6 | 0.431 |
| Olive oil (n-9 MUFA mainly) | 3.1 ± 3.9 | 2.3 ± 3.6 | 0.044 * |
| Other oils | 1.4 ± 2.9 | 0.7 ± 2.3 | 0.031 * |
| Nuts and seeds | 1.7 ± 2.7 | 1.2 ± 2.1 | 0.120 |
| Food Items | Coefficients | SE a | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| Eggs | |||||
| Model 1 | −0.026 | 0.077 | 0.975 | 0.837–1.135 | 0.742 |
| Model 2 | −0.021 | 0.082 | 0.979 | 0.834–1.149 | 0.794 |
| Meat, Offal | |||||
| Model 1 | 0.026 | 0.042 | 1.027 | 0.946–1.114 | 0.525 |
| Model 2 | 0.066 | 0.045 | 1.069 | 0.978–1.167 | 0.142 |
| Marine fishes | |||||
| Low-fat | |||||
| Model 1 | −0.101 | 0.051 | 0.904 | 0.818–0.999 | 0.047 * |
| Model 2 | −0.110 | 0.054 | 0.896 | 0.807–0.995 | 0.041 * |
| Moderate-fat | |||||
| Model 1 | −0.102 | 0.051 | 0.903 | 0.817–0.998 | 0.045 * |
| Model 2 | −0.098 | 0.053 | 0.906 | 0.816–1.006 | 0.064 |
| High-fat | |||||
| Model 1 | −0.132 | 0.051 | 0.876 | 0.792–0.969 | 0.010 * |
| Model 2 | −0.141 | 0.054 | 0.868 | 0.781–0.965 | 0.009 * |
| Freshwater fishes | |||||
| Model 1 | 0.037 | 0.055 | 1.038 | 0.933–1.155 | 0.493 |
| Model 2 | 0.070 | 0.058 | 1.072 | 0.957–1.201 | 0.228 |
| Shellfish | |||||
| Model 1 | −0.301 | 0.118 | 0.740 | 0.588–0.933 | 0.011 * |
| Model 2 | −0.343 | 0.126 | 0.709 | 0.554–0.908 | 0.007 * |
| Processed fish products | |||||
| Model 1 | −0.262 | 0.174 | 0.770 | 0.547–1.083 | 0.133 |
| Model 2 | −0.354 | 0.190 | 0.702 | 0.483–1.018 | 0.062 |
| Dairy products | |||||
| Whole milk | |||||
| Model 1 | −0.095 | 0.082 | 0.910 | 0.774–1.069 | 0.249 |
| Model 2 | −0.088 | 0.086 | 0.916 | 0.774–1.085 | 0.310 |
| Low-fat milk | |||||
| Model 1 | −0.232 | 0.144 | 0.793 | 0.598–1.051 | 0.106 |
| Model 2 | −0.199 | 0.148 | 0.820 | 0.614–1.095 | 0.178 |
| Skim milk | |||||
| Model 1 | −0.497 | 0.264 | 0.608 | 0.362–1.021 | 0.060 |
| Model 2 | −0.578 | 0.289 | 0.561 | 0.319–0.988 | 0.045 * |
| Soybean products | |||||
| Model 1 | −0.136 | 0.057 | 0.873 | 0.781–0.975 | 0.016 * |
| Model 2 | −0.112 | 0.056 | 0.894 | 0.800–0.998 | 0.046 * |
| Fats/oils | |||||
| Animal fat | |||||
| Model 1 | 0.012 | 0.036 | 1.012 | 0.943–1.086 | 0.747 |
| Model 2 | 0.003 | 0.038 | 1.003 | 0.931–1.081 | 0.933 |
| Soybean oil and sunflower oil (n−6 PUFA mainly) | |||||
| Model 1 | 0.026 | 0.025 | 1.026 | 0.977–1.079 | 0.304 |
| Model 2 | 0.064 | 0.053 | 1.066 | 0.960–1.183 | 0.235 |
| Olive oil (n-9 MUFA mainly) | |||||
| Model 1 | −0.074 | 0.030 | 0.928 | 0.875–0.985 | 0.014 * |
| Model 2 | −0.060 | 0.032 | 0.942 | 0.884–1.003 | 0.062 |
| Nuts and seeds | |||||
| Model 1 | −0.057 | 0.049 | 0.945 | 0.859–1.040 | 0.244 |
| Model 2 | −0.042 | 0.051 | 0.958 | 0.867–1.059 | 0.404 |
| Variables | DM b (n = 169) | DN (n = 144) | p-Value |
|---|---|---|---|
| Total saturated fatty acids (g) | 33.8 ± 13.3 | 30.1 ± 13.1 | 0.016 * |
| Total monounsaturated fatty acids (g) | 44.1 ± 15.7 | 39.6 ± 15.8 | 0.012 * |
| Total polyunsaturated fatty acids (g) | 31.5 ± 10.8 | 28.6 ± 11.0 | 0.020 * |
| Σn-6 (g) b | 22.1 ± 7.3 | 20.8 ± 7.1 | 0.114 |
| Σn-3 (g) | 7.6 ± 4.3 | 6.4 ± 4.4 | 0.011 * |
| EPA (g) | 1.9 ± 1.4 | 1.5 ± 1.3 | 0.011 * |
| DHA (g) | 3.7 ± 2.5 | 3.0 ± 2.6 | 0.023 * |
| Σn-6/Σn-3 ratio | 4.1 ± 3.0 | 4.9 ± 3.3 | 0.038 * |
| Variables | β | S.E a | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Total saturated fatty acids | |||||
| Model 1 | −0.020 | 0.009 | 0.981 | 0.964–0.998 | 0.027 * |
| Model 2 | −0.018 | 0.009 | 0.982 | 0.964–1.000 | 0.044 * |
| Total monounsaturated fatty acids | |||||
| Model 1 | −0.017 | 0.007 | 0.983 | 0.969–0.998 | 0.023 * |
| Model 2 | −0.015 | 0.008 | 0.985 | 0.970–0.999 | 0.042 * |
| Total polyunsaturated fatty acids | |||||
| Model 1 | −0.022 | 0.011 | 0.978 | 0.958–0.999 | 0.039 * |
| Model 2 | −0.020 | 0.011 | 0.981 | 0.960–1.022 | 0.079 |
| Σn-6 b | |||||
| Model 1 | −0.021 | 0.016 | 0.980 | 0.949–1.011 | 0.194 |
| Model 2 | −0.014 | 0.017 | 0.986 | 0.954–1.018 | 0.390 |
| Σn-3 | |||||
| Model 1 | −0.064 | 0.027 | 0.938 | 0.889–0.989 | 0.017 * |
| Model 2 | −0.065 | 0.028 | 0.937 | 0.887–0.990 | 0.021 * |
| EPA | |||||
| Model 1 | −0.188 | 0.086 | 0.828 | 0.699–0.981 | 0.029 * |
| Model 2 | −0.197 | 0.090 | 0.821 | 0.688–0.979 | 0.029 * |
| DHA | |||||
| Model 1 | −0.098 | 0.046 | 0.907 | 0.829–0.992 | 0.033 * |
| Model 2 | −1.102 | 0.048 | 0.903 | 0.823–0.992 | 0.033 * |
| Σn-6/Σn-3 ratio | |||||
| Model 1 | 0.077 | 0.037 | 1.080 | 1.006–1.161 | 0.035 * |
| Model 2 | 0.084 | 0.038 | 1.088 | 1.010–1.172 | 0.027 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-P.; Chen, C.-M.; Wang, K.-L.; Wu, K.-L.; Li, S.-C. Association of Dietary Fish and n-3 Unsaturated Fatty Acid Consumption with Diabetic Nephropathy from a District Hospital in Northern Taiwan. Nutrients 2022, 14, 2148. https://doi.org/10.3390/nu14102148
Lin S-P, Chen C-M, Wang K-L, Wu K-L, Li S-C. Association of Dietary Fish and n-3 Unsaturated Fatty Acid Consumption with Diabetic Nephropathy from a District Hospital in Northern Taiwan. Nutrients. 2022; 14(10):2148. https://doi.org/10.3390/nu14102148
Chicago/Turabian StyleLin, Shih-Ping, Chiao-Ming Chen, Kang-Ling Wang, Kun-Lin Wu, and Sing-Chung Li. 2022. "Association of Dietary Fish and n-3 Unsaturated Fatty Acid Consumption with Diabetic Nephropathy from a District Hospital in Northern Taiwan" Nutrients 14, no. 10: 2148. https://doi.org/10.3390/nu14102148
APA StyleLin, S.-P., Chen, C.-M., Wang, K.-L., Wu, K.-L., & Li, S.-C. (2022). Association of Dietary Fish and n-3 Unsaturated Fatty Acid Consumption with Diabetic Nephropathy from a District Hospital in Northern Taiwan. Nutrients, 14(10), 2148. https://doi.org/10.3390/nu14102148







