Association of Serum Phosphate with Low Handgrip Strength in Patients with Advanced Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Biochemical Profiles
2.3. Body Composition and BIA
2.4. Assessment of Sarcopenia
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Clinical Characteristics and Sarcopenia Indicators of CKD Patients Stratified by Serum Pi Tertiles
3.3. Association of Serum Pi Level with Body Composition and Sarcopenia Indicators
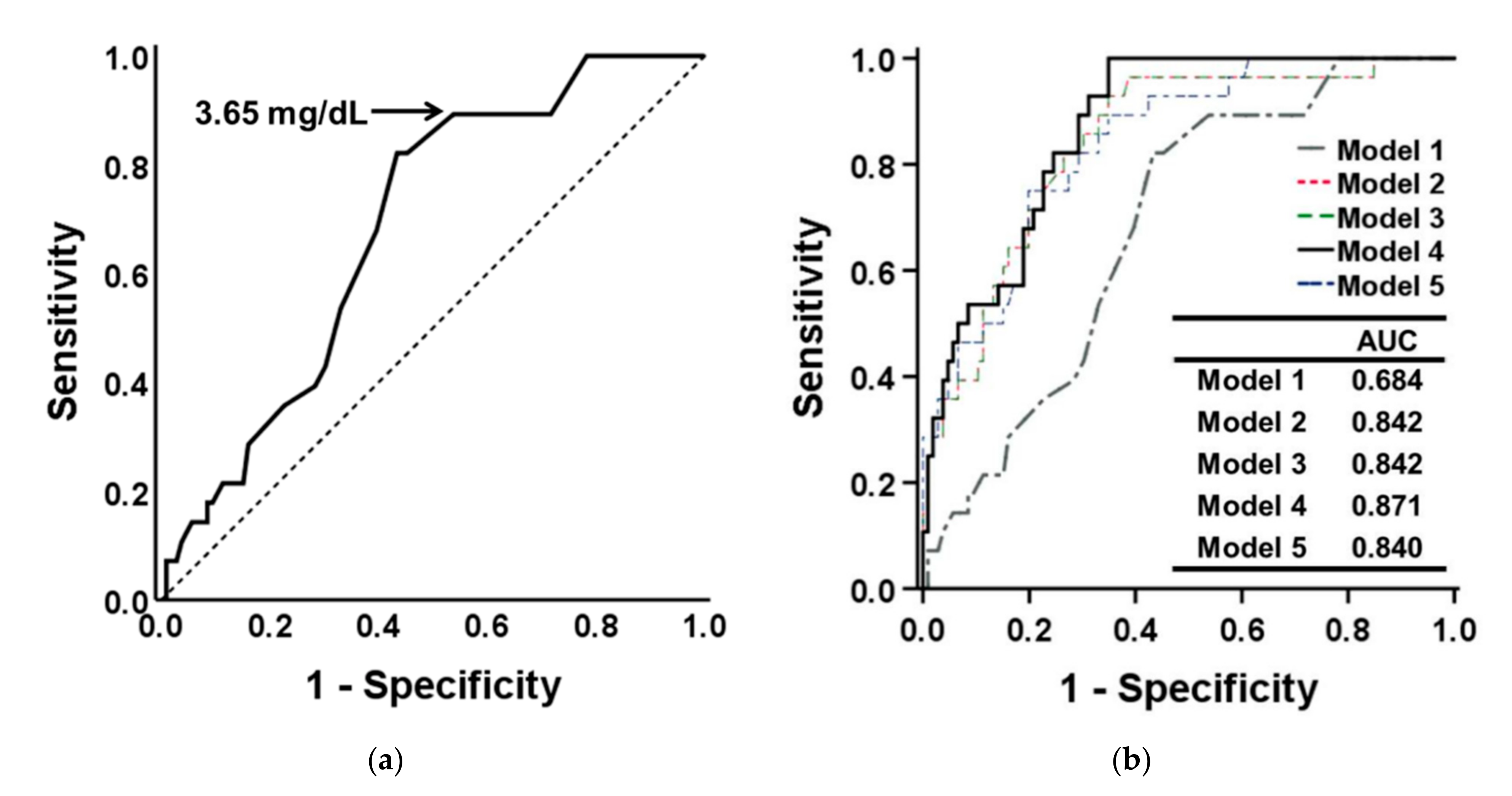
3.4. Determination of Optimal Serum Pi Cutoff Point for Low Handgrip Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised european consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Hawkins, M.; Abramowitz, M.K. Association of sarcopenia with egfr and misclassification of obesity in adults with ckd in the united states. Clin. J. Am. Soc. Nephrol. 2014, 9, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.A.; Oliveira, D.; Barbosa, S.R.; Corrêa, J.O.d.A.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.; Bastos, M.G. Sarcopenia in patients with chronic kidney disease not yet on dialysis: Analysis of the prevalence and associated factors. PLoS ONE 2017, 12, e0176230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziolkowski, S.L.; Long, J.; Baker, J.F.; Simard, J.F.; Chertow, G.M.; Leonard, M.B. Sarcopenia, relative sarcopenia and excess adiposity in chronic kidney disease. JCSM Clin. Rep. 2018, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Giglio, J.; Kamimura, M.A.; Lamarca, F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Association of sarcopenia with nutritional parameters, quality of life, hospitalization, and mortality rates of elderly patients on hemodialysis. J. Ren. Nutr. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the prospective urban rural epidemiology (pure) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.T.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jin, H.; Lim, J.Y.; Lee, S.Y. Relationship between low handgrip strength and chronic kidney disease: Knhanes 2014–2017. J. Ren. Nutr. 2021, 31, 57–63. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Wu, H.-L.; Guo, H.-R.; Cheng, Y.-Y.; Tseng, C.-C.; Wang, M.-C.; Lin, C.-Y.; Sung, J.-M. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol. Dial. Transplant. 2011, 26, 3588–3595. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin d, pth, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, D.M.; Brady, K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012, 81, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Adeney, K.L.; Siscovick, D.S.; Ix, J.H.; Seliger, S.L.; Shlipak, M.G.; Jenny, N.S.; Kestenbaum, B.R. Association of serum phosphate with vascular and valvular calcification in moderate ckd. J. Am. Soc. Nephrol. 2009, 20, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010, 24, 3562–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbs, J.R.; Liu, S.; Tang, W.; Zhou, J.; Wang, Y.; Yao, X.; Quarles, L.D. Role of hyperphosphatemia and 1,25-dihydroxyvitamin d in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 2007, 18, 2116–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peri-Okonny, P.; Baskin, K.K.; Iwamoto, G.; Mitchell, J.H.; Smith, S.A.; Kim, H.K.; Szweda, L.I.; Bassel-Duby, R.; Fujikawa, T.; Castorena, C.M.; et al. High-phosphate diet induces exercise intolerance and impairs fatty acid metabolism in mice. Circulation 2019, 139, 1422–1434. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Biomarkers profile of people with sarcopenia: A cross-sectional analysis from uk biobank. J. Am. Med. Dir. Assoc. 2020, 21, 2017.e1–2017.e9. [Google Scholar] [CrossRef]
- Mori, K.; Nishide, K.; Okuno, S.; Shoji, T.; Emoto, M.; Tsuda, A.; Nakatani, S.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2020, 34, 1347–1372. [Google Scholar] [CrossRef]
- Ishikawa, S.; Naito, S.; Iimori, S.; Takahashi, D.; Zeniya, M.; Sato, H.; Nomura, N.; Sohara, E.; Okado, T.; Uchida, S.; et al. Loop diuretics are associated with greater risk of sarcopenia in patients with non-dialysis-dependent chronic kidney disease. PLoS ONE 2018, 13, e0192990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Hellberg, M.; Svensson, P.; Höglund, P.; Clyne, N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol. Dial. Transplant. 2018, 33, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vettoretti, S.; Caldiroli, L.; Armelloni, S.; Ferrari, C.; Cesari, M.; Messa, P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced ckd. Nutrients 2019, 11, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-L.; Chen, S.-Y.; Lai, Y.-H.; Wang, C.-H.; Kuo, C.-H.; Liou, H.-H.; Hsu, B.-G. Serum creatinine to cystatin c ratio predicts skeletal muscle mass and strength in patients with non-dialysis chronic kidney disease. Clin. Nutr. 2020, 39, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Metter, E.J.; Lynch, N.; Conwit, R.; Lindle, R.; Tobin, J.; Hurley, B. Muscle quality and age: Cross-sectional and longitudinal comparisons. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, B207–B218. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.Z.; Caturegli, G.; Metter, E.J.; Makrogiannis, S.; Resnick, S.M.; Harris, T.B.; Ferrucci, L. Difference in muscle quality over the adult life span and biological correlates in the baltimore longitudinal study of aging. J. Am. Geriatr. Soc. 2014, 62, 230–236. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Barany, P.; Heimburger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Isen, J.; McGue, M.; Iacono, W. Genetic influences on the development of grip strength in adolescence. Am. J. Phys. Anthropol. 2014, 154, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternang, O.; Reynolds, C.A.; Finkel, D.; Ernsth-Bravell, M.; Pedersen, N.L.; Aslan, A.K.D. Factors associated with grip strength decline in older adults. Age Ageing 2015, 44, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-Y.; Kao, T.-W.; Chou, C.-W.; Wu, C.-J.; Yang, H.-F.; Lai, C.-H.; Wu, L.-W.; Chen, W.-L. Exploring the link between serum phosphate levels and low muscle strength, dynapenia, and sarcopenia. Sci. Rep. 2018, 8, 3573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Yang, M.; Bao, J.-F.; Gu, L.-J.; Yu, H.-L.; Yuan, W.-J. Phosphate stimulates myotube atrophy through autophagy activation: Evidence of hyperphosphatemia contributing to skeletal muscle wasting in chronic kidney disease. BMC Nephrol. 2018, 19, 45. [Google Scholar] [CrossRef]
- Sosa, P.; Alcalde-Estevez, E.; Plaza, P.; Troyano, N.; Alonso, C.; Martinez-Arias, L.; Aroeira, A.E.d.M.; Rodriguez-Puyol, D.; Olmos, G.; Lopez-Ongil, S.; et al. Hyperphosphatemia promotes senescence of myoblasts by impairing autophagy through ilk overexpression, a possible mechanism involved in sarcopenia. Aging Dis. 2018, 9, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Sonou, T.; Ohya, M.; Kawakami, K.; Yashiro, M.; Mima, T.; Negi, S.; Shigematsu, T. High phosphate levels promote muscle atrophy via myostatin expression in differentiated l6 myotubes. Res. Sq. 2020. in preprint. [Google Scholar] [CrossRef]
- Chung, L.-H.; Liu, S.-T.; Huang, S.-M.; Salter, D.M.; Lee, H.-S.; Hsu, Y.-J. High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating nrf2 signaling. Aging 2020, 12, 21446–21468. [Google Scholar] [CrossRef]
- Allen, D.G.; Westerblad, H. Role of phosphate and calcium stores in muscle fatigue. J. Physiol. 2001, 536, 657–665. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Hostrup, M.; Bangsbo, J.; Cairns, S.P. Inorganic phosphate, protons and diprotonated phosphate may contribute to the exacerbated muscle fatigue in older adults. J. Physiol. 2019, 597, 4865–4866. [Google Scholar] [CrossRef]
- Rodriguez-Benot, A.; Martin-Malo, A.; Alvarez-Lara, M.A.; Rodriguez, M.; Aljama, P. Mild hyperphosphatemia and mortality in hemodialysis patients. Am. J. Kidney Dis. 2005, 46, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Gao, Z.; Curhan, G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005, 112, 2627–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease Improving Global Outcomes. KDIGO CKD-MBD Update Work Group: KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Available online: https://kdigo.org/wp-content/uploads/2017/02/2017-KDIGO-CKD-MBD-GL-Update.pdf (accessed on 20 July 2021).
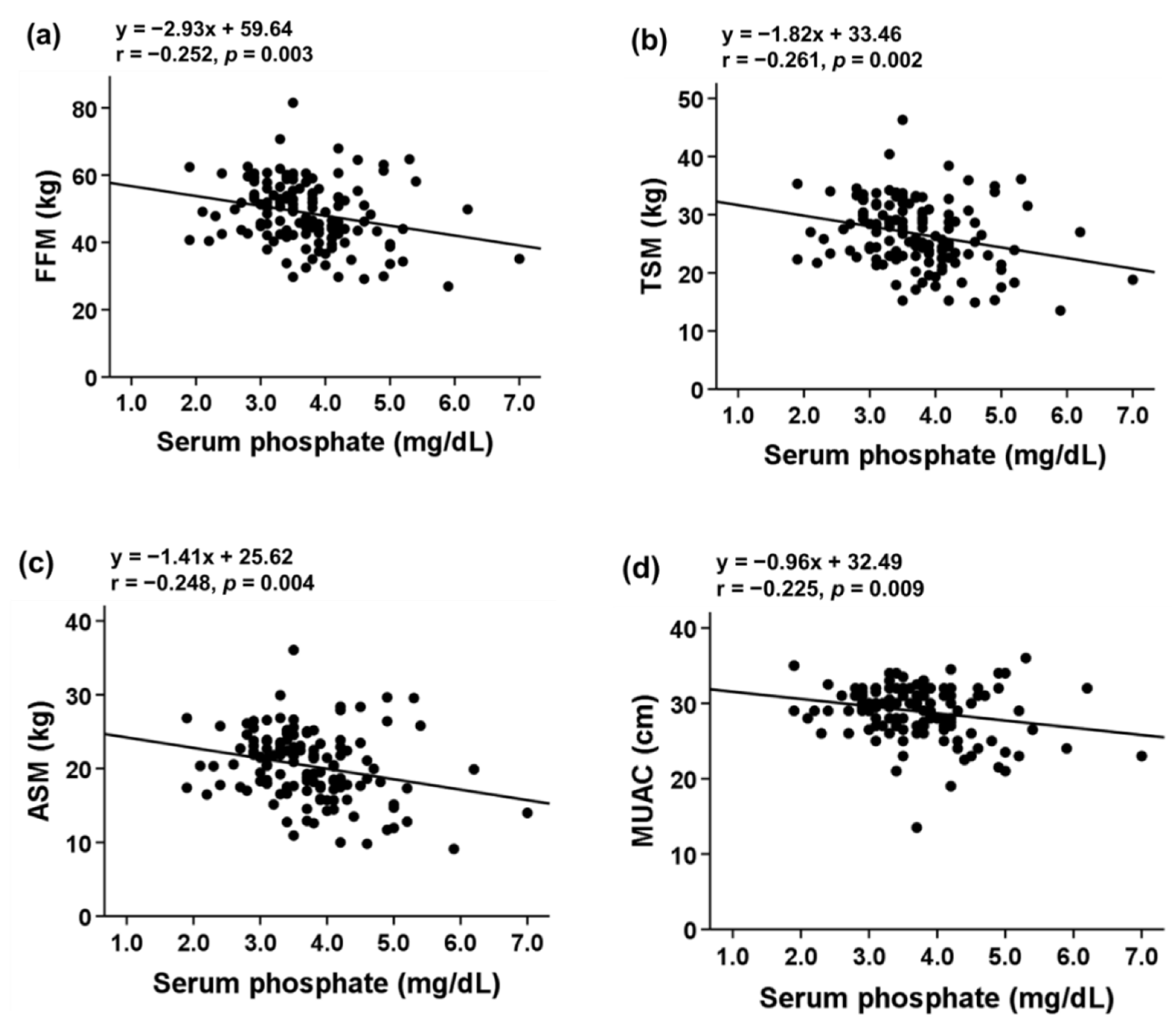
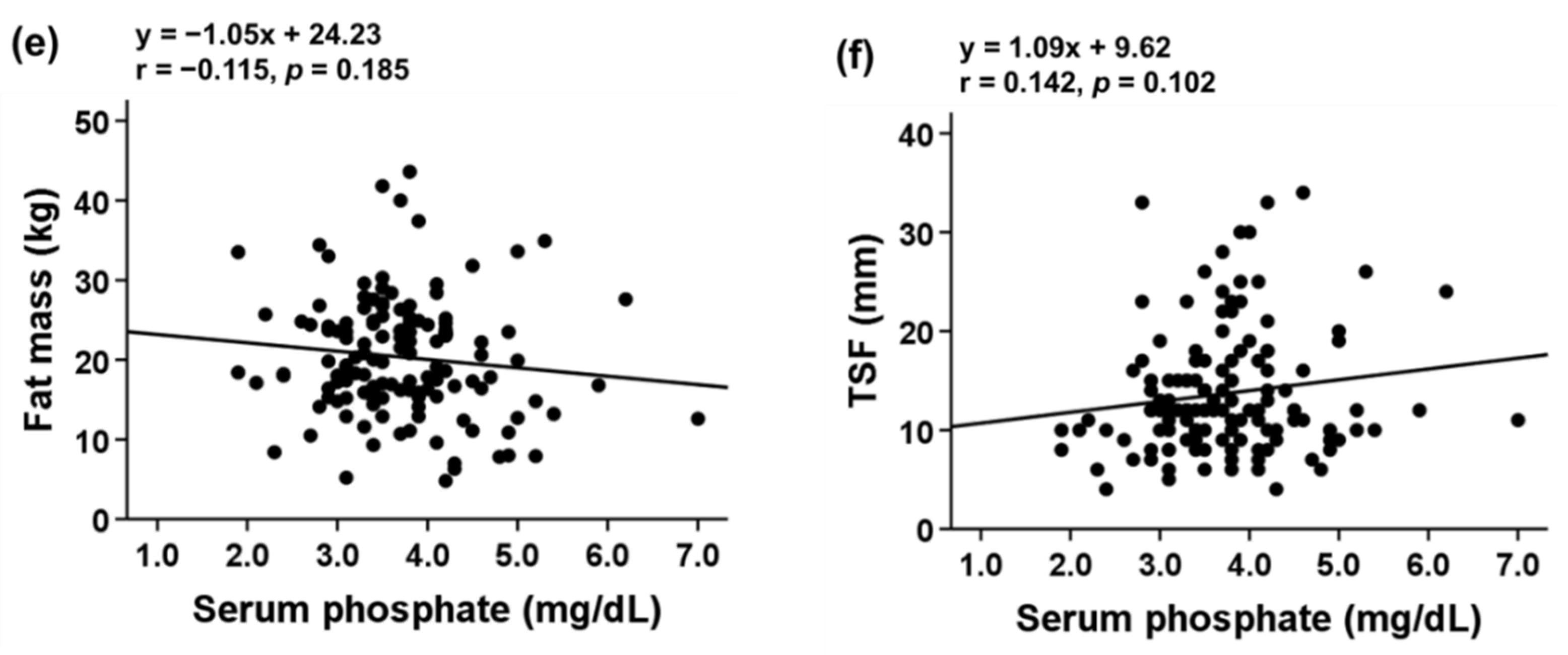
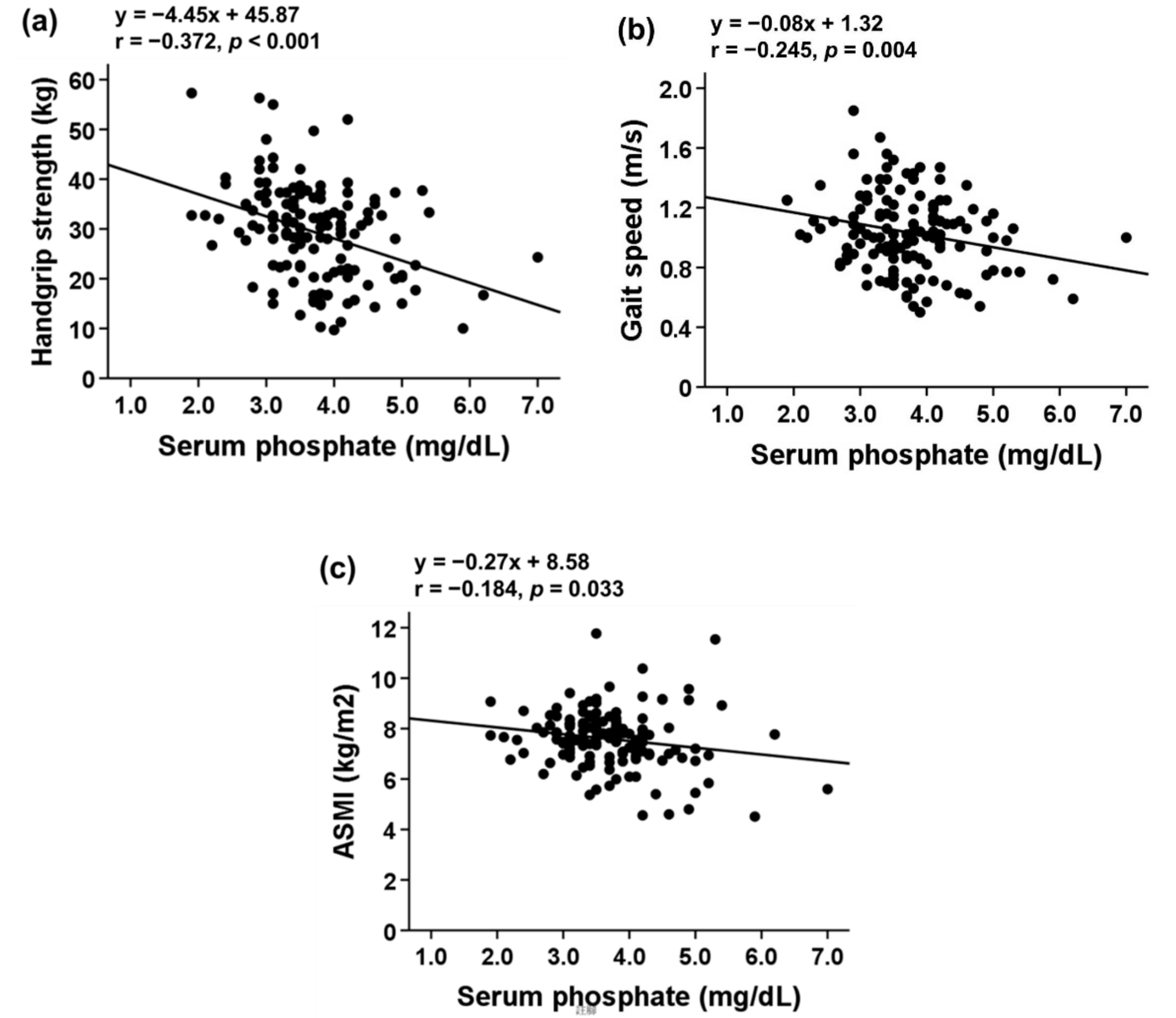
| Parameters | Total (n = 134) | Sarcopenia Phenotype (n = 70) | Control (n = 64) | p Value |
|---|---|---|---|---|
| Age (years) | 65.34 ± 12.96 | 68.87± 12.19 | 61.48 ± 12.75 | 0.001 |
| Male sex, n (%) | 93 (69.4) | 40 (57.1) | 53 (82.8) | 0.001 |
| Height (cm) | 162.83 ± 8.93 | 160.54 ± 10.02 | 165.33 ± 6.79 | 0.002 |
| Weight (kg) | 68.13 ± 13.23 | 64.87 ± 14.47 | 71.70 ± 10.74 | 0.003 |
| BMI (kg/m2) | 25.60 ± 4.11 | 25.06 ± 4.66 | 26.19 ± 3.33 | 0.114 |
| MUAC (cm) | 28.93 ± 3.46 | 27.74 ± 3.67 | 30.23 ± 2.69 | <0.001 |
| TSF (mm) | 13.68 ± 6.26 | 13.67 ± 5.63 | 13.69 ± 6.93 | 0.988 |
| eGFR | 35.33 ± 16.61 | 34.18 ± 16.29 | 36.59 ± 17.00 | 0.404 |
| Diabetes mellitus, n (%) | 63 (47.0) | 34 (48.6) | 29 (45.3) | 0.706 |
| Hypertension, n (%) | 99 (73.9) | 54 (77.1) | 45 (70.3) | 0.369 |
| Coronary artery disease, n (%) | 31 (23.1) | 17 (24.3) | 14 (21.9) | 0.741 |
| Hyperlipidemia, n (%) | 91 (67.9) | 44 (62.9) | 47 (73.4) | 0.190 |
| Laboratory measurements | ||||
| Hemoglobin (g/dL) | 12.37 ± 2.02 | 11.99 ± 1.93 | 12.79 ± 2.05 | 0.030 |
| Platelet count (×109/L) | 221.31 ± 58.51 | 214.31 ± 64.53 | 227.05 ± 53.25 | 0.365 |
| Total cholesterol (mg/dL) | 166.96 ± 37.73 | 162.72 ± 33.90 | 171.36 ± 41.17 | 0.227 |
| HDL cholesterol (mg/dL) | 46.28 ± 18.92 | 46.00 ± 21.38 | 46.50 ± 17.14 | 0.557 |
| Triglyceride (mg/dL) | 161.98 ± 118.42 | 168.66 ± 139.64 | 155.19 ± 92.71 | 0.524 |
| Blood urea nitrogen (mg/dL) | 36.25 ± 19.90 | 39.67 ± 22.39 | 32.58 ± 16.25 | 0.053 |
| Creatinine (mg/dL) | 2.56 ± 1.83 | 2.79 ± 2.17 | 2.30 ± 1.34 | 0.123 |
| Uric acid (mg/dL) | 6.10 ± 1.71 | 6.28 ± 1.72 | 5.90 ± 1.69 | 0.197 |
| Total calcium (mg/dL) | 9.33 ± 0.54 | 9.33 ± 0.57 | 9.33 ± 0.51 | 0.959 |
| Glucose (mg/dL) | 110.48 ± 31.24 | 116.22 ± 37.17 | 105.22 ± 23.72 | 0.059 |
| Alanine aminotransferase (U/L) | 22.00 ± 13.75 | 22.17 ± 13.69 | 21.85 ± 13.92 | 0.907 |
| Albumin (g/dL) | 4.16 ± 0.41 | 4.11 ± 0.40 | 4.21 ± 0.41 | 0.191 |
| Phosphate (mg/dL) | 3.72 ± 0.81 | 3.88 ± 0.86 | 3.54 ± 0.73 | 0.016 |
| Bioelectrical impedance analysis | ||||
| FM% | 29.01 ± 7.83 | 29.74 ± 8.92 | 28.22 ± 6.40 | 0.260 |
| FM (kg) | 20.32 ± 7.41 | 19.83 ± 8.12 | 20.86 ± 6.56 | 0.424 |
| FFM (kg) | 48.73 ± 9.49 | 45.67 ± 10.42 | 52.07 ± 7.04 | <0.001 |
| TSM (kg) | 26.69 ± 5.67 | 24.75 ± 6.11 | 28.81 ± 4.26 | <0.001 |
| ASM (kg) | 20.36 ± 4.64 | 18.97 ± 5.22 | 21.88 ± 3.33 | <0.001 |
| Sarcopenia indicator | ||||
| Handgrip strength (kg) | 29.33 ± 9.73 | 24.01 ± 7.96 | 35.15 ± 8.04 | <0.001 |
| Gait speed (m/s) | 1.03 ± 0.26 | 0.87 ± 0.20 | 1.21 ± 0.19 | <0.001 |
| ASMI (kg/m2) | 7.59 ± 1.18 | 7.23 ± 1.34 | 7.97 ± 0.84 | <0.001 |
| Parameters | Serum Phosphate Tertiles | p | ||
|---|---|---|---|---|
| Lower (n = 43) | Middle (n = 47) | Upper (n = 44) | ||
| Age (years) | 66.98 ± 10.76 | 64.36 ± 14.38 | 64.80 ± 13.45 | 0.600 |
| Male sex, n (%) | 39 (90.7) | 32 (68.1) | 22 (50.0) | <0.001 |
| Height (cm) | 166.79 ± 6.82 | 162.28 ± 9.02 | 159.55 ± 9.33 | 0.001 |
| Weight (kg) | 71.05 ± 10.60 | 70.19 ± 12.42 | 63.07 ± 15.07 | 0.007 |
| BMI (kg/m2) | 25.46 ± 2.78 | 26.67 ± 4.30 | 24.59 ± 4.74 | 0.051 |
| MUAC (cm) | 29.78 ± 2.22 | 29.19 ± 3.61 | 27.83 ± 4.04 | 0.025 |
| TSF (mm) | 12.14 ± 5.26 | 14.62 ± 6.03 | 14.18 ± 7.18 | 0.139 |
| eGFR | 39.76 ± 14.89 | 34.35 ± 16.85 | 32.05 ± 17.36 | 0.084 |
| Diabetes mellitus, n (%) | 17 (39.5) | 24 (51.1) | 22 (50.0) | 0.489 |
| Hypertension, n (%) | 28 (65.1) | 34 (72.3) | 37 (84.1) | 0.126 |
| Coronary artery disease, n (%) | 9 (20.9) | 8 (17.0) | 14 (31.8) | 0.226 |
| Hyperlipidemia, n (%) | 30 (69.8) | 33 (70.2) | 28 (63.6) | 0.759 |
| Laboratory tests | ||||
| Hemoglobin (g/dL) | 13.63 ± 1.89 | 12.44 ± 1.75 | 11.19 ± 1.72 | <0.001 |
| Platelet count (×109/L) | 214.73 ± 59.90 | 213.35 ± 44.60 | 236.61 ± 69.61 | 0.456 |
| Total cholesterol (mg/dL) | 165.16 ± 35.03 | 174.07 ± 40.99 | 160.97 ± 35.97 | 0.287 |
| HDL Cholesterol (mg/dL) | 43.12 ± 14.42 | 43.10 ± 13.17 | 55.31 ± 28.11 | 0.461 |
| Triglyceride (mg/dL) | 149.80 ± 121.16 | 188.27 ± 126.18 | 146.31 ± 104.59 | 0.189 |
| Blood urea nitrogen (mg/dL) | 22.89 ± 7.14 | 31.15 ± 14.03 | 53.83 ± 20.63 | <0.001 |
| Creatinine (mg/dL) | 1.69 ± 0.74 | 1.92 ± 0.84 | 4.07 ± 2.34 | <0.001 |
| Uric acid (mg/dL) | 5.89 ± 1.60 | 5.79 ± 1.72 | 6.63 ± 1.72 | 0.043 |
| Total calcium (mg/dL) | 9.37 ± 0.52 | 9.46 ± 0.39 | 9.15 ± 0.64 | 0.019 |
| Glucose (mg/dL) | 104.56 ± 18.10 | 118.62 ± 39.42 | 108.14 ± 31.46 | 0.119 |
| Alanine aminotransferase (U/L) | 23.05 ± 13.53 | 23.47 ± 15.49 | 19.00 ± 11.86 | 0.377 |
| Albumin (g/dL) | 4.21 ± 0.33 | 4.22 ± 0.31 | 4.04 ± 0.53 | 0.077 |
| Bioelectrical impedance analysis | ||||
| FM% | 27.88 ± 6.87 | 30.67 ± 8.26 | 28.35 ± 8.11 | 0.190 |
| FM (kg) | 20.22 ± 6.39 | 22.12 ± 7.87 | 18.50 ± 7.54 | 0.065 |
| FFM (kg) | 51.50 ± 7.49 | 49.38 ± 9.47 | 45.33 ± 10.38 | 0.008 |
| TSM (kg) | 28.41 ± 4.49 | 27.09 ± 5.67 | 24.58 ± 6.12 | 0.005 |
| ASM (kg) | 21.66 ± 3.41 | 20.57 ± 4.52 | 18.87 ± 5.41 | 0.017 |
| Sarcopenia indicator | ||||
| Handgrip strength (kg) | 34.13 ± 9.45 | 28.18 ± 8.97 | 25.86 ± 9.09 | <0.001 |
| Gait speed (m/s) | 1.11 ± 0.24 | 1.01 ± 0.28 | 0.98 ± 0.23 | 0.042 |
| ASMI (kg/m2) | 7.74 ± 0.75 | 7.73 ± 1.13 | 7.28 ± 1.51 | 0.120 |
| Std. β | Unstd. β (95% CI) | p Value | |
|---|---|---|---|
| Handgrip strength | |||
| Model 1 | −0.372 | −4.446 (−6.357, −2.536) | <0.001 |
| Model 2 | −0.214 | −2.559 (−4.087, −1.031) | 0.001 |
| Model 3 | −0.216 | −2.583 (−4.136, −1.031) | 0.001 |
| Model 4 | −0.184 | −2.197 (−3.698, −0.696) | 0.004 |
| Model 5 | −0.168 | −1.972 (−3.659, −0.285) | 0.022 |
| Gait speed | |||
| Model 1 | −0.245 | −0.078 (−0.131, −0.025) | 0.004 |
| Model 2 | −0.244 | −0.077 (−0.130, −0.024) | 0.005 |
| Model 3 | −0.224 | −0.071 (−0.124, −0.017) | 0.010 |
| Model 4 | −0.197 | −0.062 (−0.115, −0.010) | 0.020 |
| Model 5 | −0.160 | −0.050 (−0.113, 0.013) | 0.121 |
| ASMI | |||
| Model 1 | −0.184 | −0.268 (−0.514, −0.022) | 0.033 |
| Model 2 | 0.008 | 0.011 (−0.211, 0.234) | 0.921 |
| Model 3 | 0.017 | 0.025 (−0.201, 0.250) | 0.829 |
| Model 4 | 0.077 | 0.111 (−0.032, 0.254) | 0.126 |
| Model 5 | 0.001 | 0.002 (−0.158, 0.162) | 0.981 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, P.-H.; Yang, H.-C.; Lin, C.; Sung, C.-C.; Chu, P.; Hsu, Y.-J. Association of Serum Phosphate with Low Handgrip Strength in Patients with Advanced Chronic Kidney Disease. Nutrients 2021, 13, 3605. https://doi.org/10.3390/nu13103605
Tsai P-H, Yang H-C, Lin C, Sung C-C, Chu P, Hsu Y-J. Association of Serum Phosphate with Low Handgrip Strength in Patients with Advanced Chronic Kidney Disease. Nutrients. 2021; 13(10):3605. https://doi.org/10.3390/nu13103605
Chicago/Turabian StyleTsai, Ping-Huang, Hsiu-Chien Yang, Chin Lin, Chih-Chien Sung, Pauling Chu, and Yu-Juei Hsu. 2021. "Association of Serum Phosphate with Low Handgrip Strength in Patients with Advanced Chronic Kidney Disease" Nutrients 13, no. 10: 3605. https://doi.org/10.3390/nu13103605
APA StyleTsai, P.-H., Yang, H.-C., Lin, C., Sung, C.-C., Chu, P., & Hsu, Y.-J. (2021). Association of Serum Phosphate with Low Handgrip Strength in Patients with Advanced Chronic Kidney Disease. Nutrients, 13(10), 3605. https://doi.org/10.3390/nu13103605







