Feasibility of Low-Sodium, High-Potassium Processed Foods and Their Effect on Blood Pressure in Free-Living Japanese Men: A Randomized, Double-Blind Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
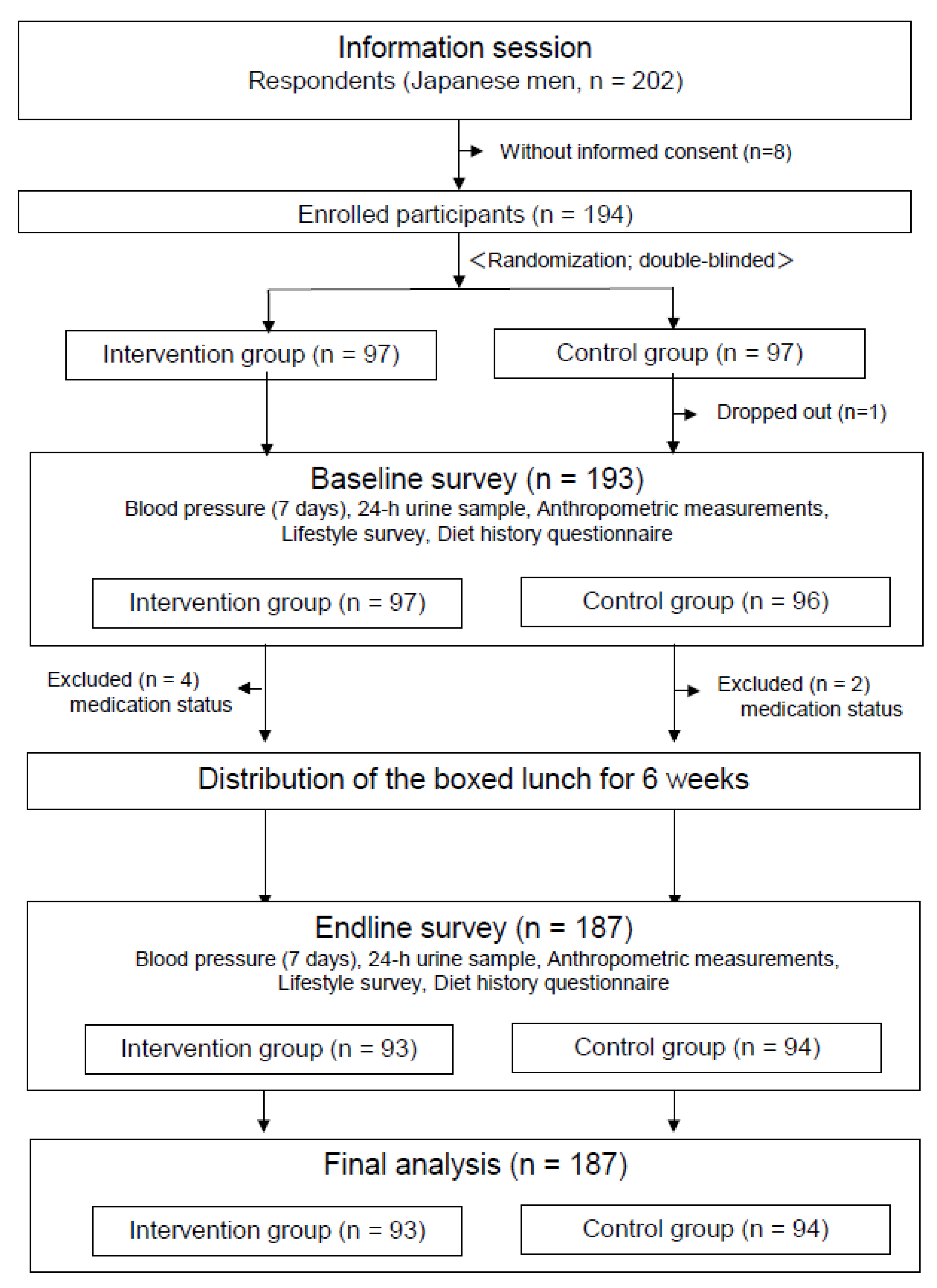
2.1. Study Design and Participants
2.2. New Low-Na, High-K Processed Foods
2.3. Intervention Meals: Boxed Lunches and Instant Miso Soup
2.4. Intervention Schedule
2.5. Blood Pressure Measurement
2.6. Diet History Questionnaire
2.7. Urine Collection
2.8. Anthropological Measurements and Other Variables
2.9. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Compliance and Blinding
3.3. Changes in Dietary Intake and Lifestyle Factors
3.4. Changes in Urinary Excretion of Na and K
3.5. Changes in BP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Seasonings/Processed Foods | Type | Nutritional Value (per 100 g of Ingredients) | Change Rate Compared to Regular Ingredients a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Energy | Protein | Fat | Carbohydrates | Sodium | Potassium | Sodium | Potassium | ||
| (kcal) | (g) | (g) | (g) | (mg) | (mg) | (%) | (%) | ||
| Salt | Regular | 0 | 0.0 | 0.0 | 0.0 | 39,000 | 100 | ||
| New | 4 | 0.7 | 0.0 | 0.3 | 23,334 | 20,672 | −40.2 | 20,572.0 | |
| Soy sauce | Regular | 66 | 8.5 | 0.4 | 7.7 | 5570 | 478 | ||
| New | 70 | 8.6 | 0.1 | 8.6 | 3330 | 4460 | −40.2 | 833.1 | |
| Miso | Regular | 163 | 12.8 | 5.8 | 14.8 | 3955 | 474 | ||
| New | 182 | 13.3 | 5.2 | 20.4 | 3028 | 2934 | −23.4 | 519.0 | |
| Seasoning soy sauce | Regular | 187 | 4.3 | 0.1 | 42.2 | 4170 | 200 | ||
| New | 72 | 4.2 | 0.1 | 13.5 | 2800 | 1840 | −32.9 | 820.0 | |
| Japanese stock powder | Regular | 242 | 27.0 | 0.0 | 33.0 | 13,700 | 180 | ||
| New | 311 | 34.0 | 0.0 | 43.0 | 8100 | 294 | −40.9 | 63.3 | |
| Chicken stock powder | Regular | 184 | 16.0 | 1.5 | 26.4 | 18,920 | 3080 | ||
| New | 260 | 13.4 | 1.4 | 49.6 | 11,200 | 2980 | −40.8 | −3.2 | |
| Seasoning mix for rice, shiso | Regular | 209 | 8.7 | 1.3 | 40.6 | 17,700 | 738 | ||
| New | 201 | 8.8 | 2.3 | 36.3 | 11,000 | 8900 | −37.9 | 1106.0 | |
| Seasoning mix for rice, wakame | Regular | 184 | 15.4 | 2.2 | 32.0 | 18,000 | 56 | ||
| New | 183 | 14.4 | 1.8 | 34.8 | 11,000 | 7900 | −38.9 | 14,007.1 | |
| Seasoned bonito flakes | Regular | 265 | 28.2 | 3.6 | 29.9 | 2670 | 400 | ||
| New | 244 | 32.2 | 4.8 | 17.9 | 1920 | 2020 | −28.1 | 405.0 | |
| Seasoned white sesame seeds | Regular | 493 | 17.7 | 37.5 | 29.5 | 3995 | 284 | ||
| New | 516 | 18.4 | 38.8 | 27.4 | 2500 | 2300 | −37.4 | 709.9 | |
| Seasoned black sesame seeds | Regular | 509 | 18.2 | 39.6 | 28.5 | 3442 | 300 | ||
| New | 535 | 18.7 | 42.2 | 26.1 | 2200 | 1900 | −36.1 | 533.3 | |
| Pork and chicken sausage | Regular | 274 | 14.9 | 21.8 | 2.5 | 722 | 312 | ||
| New | 264 | 14.8 | 21.1 | 3.8 | 445 | 490 | −38.4 | 57.1 | |
| Teriyaki chicken | Regular | 170 | 19.9 | 8.0 | 4.7 | 950 | 25 | ||
| New | 173 | 19.8 | 8.3 | 4.8 | 588 | 350 | −38.1 | 1300.0 | |
| Roast pork | Regular | 256 | 17.3 | 20.6 | 0.4 | 730 | 263 | ||
| New | 234 | 17.6 | 16.7 | 3.2 | 593 | 610 | −18.8 | 131.9 | |
| Hamburger steak | Regular | 213 | 17.7 | 12.0 | 8.5 | 505 | 501 | ||
| New | 217 | 16.9 | 12.9 | 8.3 | 361 | 758 | −28.5 | 51.3 | |
| Chicken meatball | Regular | 144 | 17.9 | 3.9 | 9.3 | 575 | 222 | ||
| New | 147 | 17.2 | 4.5 | 9.4 | 384 | 424 | −33.2 | 91.0 | |
| Salted salmon | Regular | 238 | 21.0 | 14.8 | 0.5 | 1250 | 195 | ||
| New | 238 | 21.0 | 14.8 | 0.5 | 580 | 965 | −53.6 | 394.9 | |
| Roasted salted roe with red hot pepper powder | Regular | 125 | 24.0 | 1.4 | 4.1 | 2000 | 110 | ||
| New | 112 | 20.8 | 1.2 | 4.4 | 1000 | 1100 | −50.0 | 900.0 | |
| Imitation crab meat made from surimi | Regular | 98 | 9.0 | 2.0 | 11.0 | 870 | 8 | ||
| New | 90 | 10.4 | 0.8 | 10.4 | 450 | 540 | −48.3 | 6650.0 | |
| Fried surimi | Regular | 132 | 9.6 | 3.5 | 15.6 | 890 | 27 | ||
| New | 132 | 9.9 | 3.8 | 14.6 | 448 | 630 | −49.7 | 2233.3 | |
| Dried plum paste | Regular | 44 | 0.9 | 0.4 | 9.2 | 7880 | 440 | ||
| New | 44 | 0.9 | 0.4 | 9.2 | 4700 | 4400 | −40.4 | 900.0 | |
| Dried plum | Regular | 72 | 1.3 | 0.2 | 16.3 | 4100 | 130 | ||
| New | 72 | 1.3 | 0.2 | 16.3 | 2100 | 2230 | −48.8 | 1615.4 | |
| Pickled cucumber in soy sauce | Regular | 56 | 3.4 | 0.4 | 9.8 | 1600 | 64 | ||
| New | 41 | 3.4 | 0.4 | 7.3 | 1200 | 670 | −25.0 | 946.9 | |
| Shibazuke: Kyoto-style pickles | Regular | 44 | 2.3 | 0.3 | 8.0 | 1900 | 38 | ||
| New | 36 | 2.2 | 0.3 | 7.3 | 1300 | 620 | −31.6 | 1531.6 | |
| Pickled radish | Regular | 50 | 1.0 | 0.2 | 12.6 | 2100 | 8 | ||
| New | 48 | 1.0 | 0.1 | 12.0 | 1300 | 1200 | −38.1 | 14,900.0 | |
| Pickled mustard leaf | Regular | 29 | 1.6 | 0.7 | 4.1 | 1800 | 59 | ||
| New | 26 | 1.9 | 0.6 | 5.2 | 1300 | 770 | −27.8 | 1205.1 | |
References
- Appel, L.J.; Brands, M.W.; Daniels, S.R.; Karanja, N.; Elmer, P.J.; Sacks, F.M. Dietary approaches to prevent and treat hypertension: A scientific statement from the American Heart Association. Hypertension 2006, 47, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tuomilehto, J.; Jousilahti, P.; Rastenyte, D.; Moltchanov, V.; Tanskanen, A.; Pietinen, P.; Nissinen, A. Urinary sodium excretion and cardiovascular mortality in Finland: A prospective study. Lancet 2001, 357, 848–851. [Google Scholar] [CrossRef]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium intake and all-cause mortality over 20 years in the trials of hypertension prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Segawa, H.; Kadota, A.; Ohkubo, T.; Arima, H.; Miura, K. Epidemiology of hypertension in Japan: Beyond the new 2019 Japanese guidelines. Hypertens. Res. 2020, 43, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Community Health, Health Promotion and Nutrition Section. Special Committee on Planning of next National Health Promotion Campaign, Health Science Council. “Promotion of Health Japan 21 (Second Term)”. Available online: https://www.mhlw.go.jp/bunya/kenkou/dl/kenkounippon21_02.pdf (accessed on 12 August 2021). (In Japanese).
- Yokoyama, T. National health promotion measures in Japan: Health Japan 21 (the second term). J. Natl. Inst. Public Health 2020, 69, 14–24. [Google Scholar] [CrossRef]
- Intersalt Cooperative Research Group. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988, 297, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, M.R.; Frost, C.D.; Wald, N.J. By how much does dietary salt reduction lower blood pressure? I–Analysis of observational data among populations. BMJ 1991, 302, 811–815. [Google Scholar] [CrossRef] [Green Version]
- Frost, C.D.; Law, M.R.; Wald, N.J. By how much does dietary salt reduction lower blood pressure? II–Analysis of observational data within populations. BMJ 1991, 302, 815–818. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.R.; Bolt, R.J.; Borhani, N.O.; Cook, N.R.; Cohen, J.D.; Cutler, J.A.; Hollis, J.F.; Kuller, L.H.; Lasser, N.L.; Trials of Hypertension Prevention (TOHP) Collaborative Research Group; et al. Design of a multicenter trial to evaluate long-term life-style intervention in adults with high-normal blood pressure levels: Trials for hypertension prevention (phase II). Ann. Epidemiol. 1995, 5, 130–139. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; DASH-Sodium Collaborative Research Group; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Meneton, P.; Jeunemaitre, X.; de Wardener, H.E.; MacGregor, G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef] [PubMed]
- Karppanen, H.; Mervaala, E. Sodium intake and hypertension. Prog. Cardiovasc. Dis. 2006, 49, 59–75. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrogué, H.J.; Madias, N.E. Sodium and potassium in the pathogenesis of hypertension. N. Engl. J. Med 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Gu, D.; Chen, J.; Bazzano, L.A.; Rao, D.C.; Hixson, J.E.; Jaquish, C.E.; Cao, J.; Chen, J.; Li, J.; et al. Correlation between blood pressure responses to dietary sodium and potassium intervention in a Chinese population. Am. J. Hypertens. 2009, 22, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Campbell, N.R. Salt and high blood pressure. Clin. Sci. 2009, 117, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Liu, Z.; Liu, F.; Xu, X.; Liang, Y.; Zhu, D. Family-based randomized trial to detect effects on blood pressure of a salt substitute containing potassium and calcium in hypertensive adolescents. Am. J. Hypertens. 2009, 22, 943–947. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabara, Y.; Takahashi, Y.; Kumagai, K.; Setoh, K.; Kawaguchi, T.; Takahashi, M.; Muraoka, Y.; Tsujikawa, A.; Gotoh, N.; Nagahama Study Group; et al. Descriptive epidemiology of spot urine sodium-to-potassium ratio clarified close relationship with blood pressure level: The Nagahama study. J. Hypertens. 2015, 33, 2407–2413. [Google Scholar] [CrossRef]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; PURE Investigators; et al. Association of urinary sodium and potassium excretion with blood pressure. New Engl. J. Med 2014, 371, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.L.; Cogswell, M.E.; Zhao, L.; Terry, A.L.; Wang, C.Y.; Wright, J.; Coleman King, S.M.; Bowman, B.; Chen, T.C.; Merritt, R.; et al. Association between urinary sodium and potassium excretion and blood pressure among adults in the United States: National Health and Nutrition Examination Survey, 2014. Circulation 2018, 137, 237–246. [Google Scholar] [CrossRef]
- Higo, Y.; Nagashima, S.; Tabara, Y.; Setoh, K.; Kawaguchi, T.; Takahashi, Y.; Kosugi, S.; Nakayama, T.; Matsuda, F.; Wakamura, T.; et al. Association of the spot urine sodium-to-potassium ratio with blood pressure is independent of urinary Na and K levels: The Nagahama study. Hypertens. Res. 2019, 42, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Kogure, M.; Nakaya, N.; Hirata, T.; Tsuchiya, N.; Nakamura, T.; Narita, A.; Suto, Y.; Honma, Y.; Sasaki, H.; Miyagawa, K.; et al. Sodium/potassium ratio change was associated with blood pressure change: Possibility of population approach for sodium/potassium ratio reduction in health checkup. Hypertens. Res. 2021, 44, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Shoda, W.; Nomura, N.; Ando, F.; Tagashira, H.; Iwamoto, T.; Ohta, A.; Isobe, K.; Mori, T.; Susa, K.; Sohara, E.; et al. Sodium-calcium exchanger 1 is the key molecule for urinary potassium excretion against acute hyperkalemia. PLoS ONE 2020, 15, 1–23. [Google Scholar] [CrossRef]
- Nomura, N.; Shoda, W.; Uchida, S. Clinical importance of potassium intake and molecular mechanism of potassium regulation. Clin. Exp. Nephrol. 2019, 23, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Mickelsen, O.; Makdani, D.; Gill, J.L.; Frank, R.L. Sodium and potassium intakes and excretions of normal men consuming sodium chloride or a 1:1 mixture of sodium and potassium chlorides. Am. J. Clin. Nutr. 1977, 30, 2033–2040. [Google Scholar] [CrossRef] [Green Version]
- Hayabuchi, H. Tasty recipe and foods with reduced salt content. Blood Press 2012, 19, 798–804. (In Japanese) [Google Scholar]
- Hayabuchi, H. Approach to salt reduction in dietary education for the next generation—succession of Japanese traditional dietary culture and salt reduction. Health Sci. 2013, 56, 179–183. (In Japanese) [Google Scholar]
- Melander, O.; von Wowern, F.; Frandsen, E.; Burri, P.; Willsteen, G.; Aurell, M.; Hulthen, U.L. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J. Hypertens. 2007, 25, 619–627. [Google Scholar] [CrossRef]
- Takahashi, A.; Hayabuchi, H.; Youko, U.; Uehara, A.; Adachi, H.; Sasaki, S.; Nishioka, K.; Ohkubo, T. Adherence to multiple measurements of home blood pressure and selection bias of recorded blood pressure values. J. Blood. Press 2013, 20, 630–635. (In Japanese) [Google Scholar]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Yanagibori, R.; Amano, K. Validity of a self-administered diet history questionnaire for assessment of sodium and potassium: Comparison with single 24-hour urinary excretion. Jpn. Circ. J. 1998, 62, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Ushio, F.; Amano, K.; Morihara, M.; Todoriki, O.; Uehara, Y.; Toyooka, E. Serum biomarker-based validation of a self-administered diet history questionnaire for Japanese subjects. J. Nutr. Sci. Vitaminol. 2000, 46, 285–296. [Google Scholar] [CrossRef]
- Okubo, H.; Sasaki, S.; Rafamantanantsoa, H.H.; Ishikawa-Takata, K.; Okazaki, H.; Tabata, I. Validation of self-reported energy intake by a self-administered diet history questionnaire using the doubly labeled water method in 140 Japanese adults. Eur. J. Clin. Nutr. 2008, 62, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Yamasaki, M.; Hayabuchi, H.; Goda, T.; Oka, J.; Baba, K.; Ohki, K.; et al. Misreporting of dietary energy, protein, potassium and sodium in relation to body mass index in young Japanese women. Eur. J. Clin. Nutr. 2008, 62, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Ljungman, S.; Granerus, G. The Evaluation of Kidney Function in Hypertensive Patients. In Hypertension: Pathophysiology, Diagnosis, and Management, 2nd ed.; Larah, J.H., Brenner, B.M., Eds.; Raven Press: New York, NY, USA, 1995; pp. 1987–2004. [Google Scholar]
- Cook, N.R.; Obarzanek, E.; Cutler, J.A.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K.; Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The Trials of Hypertension Prevention follow-up study. Arch. Intern. Med. 2009, 169, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Sasaki, S.; Okubo, S.; Hayashi, M.; Tsugane, S. Blood pressure change in a free-living population-based dietary modification study in Japan. J. Hypertens. 2006, 24, 451–458. [Google Scholar] [CrossRef]
- Nakamura, K.; Okamura, T.; Hayakawa, T.; Hozawa, A.; Kadowaki, T.; Murakami, Y.; Kita, Y.; Okayama, A.; Ueshima, H.; NIPPON DATA90 Research Group. The proportion of individuals with alcohol-induced hypertension among total hypertensives in a general Japanese population: NIPPON DATA90. Hypertens. Res. 2007, 30, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmot, M.G.; Elliott, P.; Shipley, M.J.; Dyer, A.R.; Ueshima, H.U.; Beevers, D.G.; Stamler, R.; Kesteloot, H.; Rose, G.; Stamler, J. Alcohol and blood pressure: The INTERSALT study. BMJ 1994, 308, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Yoshita, K.; Miura, K.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; Soyama, Y.; Suwazono, Y.; Nogawa, K.; Nakagawa, H. Relationship of alcohol consumption to 7-year blood pressure change in Japanese men. J. Hypertens. 2005, 23, 1485–1490. [Google Scholar] [CrossRef]
- Ueshima, H.; Mikawa, K.; Baba, S.; Sasaki, S.; Ozawa, H.; Tsushima, M.; Kawaguchi, A.; Omae, T.; Katayama, Y.; Kayamori, Y. Effect of reduced alcohol consumption on blood pressure in untreated hypertensive men. Hypertension 1993, 21, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Ueshima, H.; Stamler, J.; Elliott, P.; Chan, Q.; Brown, I.J.; Carnethon, M.R.; Daviglus, M.L.; He, K.; Moag-Stahlberg, A.; INTERMAP Research Group; et al. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension 2007, 50, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.K.; Nyantika, A.N.; Kauhanen, J.; Voutilainen, S.; Tuomainen, T.P. Serum long-chain n-3 polyunsaturated fatty acids, methylmercury and blood pressure in an older population. Hypertens. Res. 2012, 35, 1000–1004. [Google Scholar] [CrossRef] [Green Version]
- Cabo, J.; Alonso, R.; Mata, P. Omega-3 fatty acids and blood pressure. Br. J. Nutr. 2012, 107, S195–S200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebenhofer, A.; Jeitler, K.; Berghold, A.; Waltering, A.; Hemkens, L.G.; Semlitsch, T.; Pachler, C.; Strametz, R.; Horvath, K. Long-term effects of weight-reducing diets in hypertensive patients. Cochrane Database Syst. Rev. 2011, 9, CD008274. [Google Scholar] [CrossRef]
- Mertens, I.L.; Van Gaal, L.F. Overweight, obesity, and blood pressure: The effects of modest weight reduction. Obes. Res. 2000, 8, 270–278. [Google Scholar] [CrossRef]
- Whelton, S.P.; Hyre, A.D.; Pedersen, B.; Yi, Y.; Whelton, P.K.; He, J. Effect of dietary fiber intake on blood pressure: A meta-analysis of randomized, controlled clinical trials. J. Hypertens. 2005, 23, 475–481. [Google Scholar] [CrossRef]
- Wang, L.; Manson, J.E.; Buring, J.E.; Lee, I.M.; Sesso, H.D. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008, 51, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Ruidavets, J.B.; Bongard, V.; Simon, C.; Dallongeville, J.; Ducimetière, P.; Arveiler, D.; Amouyel, P.; Bingham, A.; Ferrières, J. Independent contribution of dairy products and calcium intake to blood pressure variations at a population level. J. Hypertens. 2006, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Nicolson, D.; Cook, J.V.; Campbell, F.; Beyer, F.R.; Ford, G.A.; Mason, J. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. 2006, 2, CD004639. [Google Scholar] [CrossRef]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R.; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; OmniHeart Collaborative Research Group; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yancy, W.S., Jr.; Yu, D.; Champagne, C.; Appel, L.J.; Lin, P.H. The relationship between dietary protein intake and blood pressure: Results from the PREMIER study. J. Hum. Hypertens. 2008, 22, 745–754. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wofford, M.R.; Reynolds, K.; Chen, J.; Chen, C.S.; Myers, L.; Minor, D.L.; Elmer, P.J.; Jones, D.W.; Whelton, P.K. Effect of dietary protein supplementation on blood pressure: A randomized, controlled trial. Circulation 2011, 124, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.Y.; Tong, X.; Wu, Z.W.; Xun, P.C.; He, K.; Qin, L.Q. Effect of soya protein on blood pressure: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2011, 106, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyses, L.; Groth, H.; Wetter, W. Acceptability in food of NaCl/KCl mixture. Lancet 1983, 322, 1427–1428. [Google Scholar] [CrossRef]
- Jeffery, R.W.; Pirie, P.L.; Elmer, P.J.; Bjornson-Benson, W.M.; Mullenbach, V.A.; Kurth, C.L.; Johnson, S.L. Low-sodium, high-potassium diet: Feasibility and acceptability in a normotensive population. Am. J. Public Health 1984, 74, 492–494. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.O.; Maller, O.; Cardello, A.V. Consumer acceptance of foods lower in sodium. J. Am. Diet Assoc. 1995, 95, 447–453. [Google Scholar] [CrossRef]
- Karanja, N.; Lancaster, K.J.; Vollmer, W.M.; Lin, P.H.; Most, M.M.; Ard, J.D.; Swain, J.F.; Sacks, F.M.; Obarzanek, E. Acceptability of sodium-reduced research diets, including the Dietary Approaches to Stop Hypertension diet, among adults with prehypertension and stage 1 hypertension. J. Am. Diet. Assoc. 2007, 107, 1530–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Prescott, J.; Wu, Y.; Barzi, F.; Yu, X.; Zhao, L.; Neal, B.; China Salt Substitute Study Collaborative Group. The effects of a reduced-sodium, high-potassium salt substitute on food taste and acceptability in rural northern China. Br. J. Nutr. 2009, 101, 1088–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med 2010, 362, 590–599. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; MacGregor, G.A. Beneficial effects of potassium on human health. Physiol. Plant 2008, 133, 725–735. [Google Scholar] [CrossRef]
- Filippini, T.; Naska, A.; Kasdagli, M.I.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium intake and blood pressure: A dose-response meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef]
- Greer, R.C.; Marklund, M.; Anderson, C.A.M.; Cobb, L.K.; Dalcin, A.T.; Henry, M.; Appel, L.J. Potassium-enriched salt substitutes as a means to lower blood pressure: Benefits and risks. Hypertension 2020, 75, 266–274. [Google Scholar] [CrossRef]
- Peng, W.G.; Li, W.; Wen, X.X.; Li, Y.; Hu, J.H.; Zhao, L.C. Effects of salt substitutes on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Gu, D.; Chen, J.; Jaquish, C.E.; Rao, D.C.; Hixson, J.E.; Chen, J.C.; Duan, X.; Huang, J.F.; GenSalt Collaborative Research Group; et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens 2009, 27, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Montasser, M.E.; Douglas, J.A.; Roy-Gagnon, M.H.; Van Hout, C.V.; Weir, M.R.; Vogel, R.; Parsa, A.; Steinle, N.I.; Snitker, S.; Brereton, N.H.; et al. Mitchell, Determinants of blood pressure response to low-salt intake in a healthy adult population. J. Clin. Hypertens. 2011, 13, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, A.; Elliott, P.; Chee, D.; Stamler, J. Urinary biochemical markers of dietary intake in the INTERSALT study. Am. J. Clin. Nutr. 1997, 65, 1246S–1253S. [Google Scholar] [CrossRef] [PubMed]
- Kubozono, T.; Akasaki, Y.; Kawasoe, S.; Ojima, S.; Kawabata, T.; Makizako, H.; Kuwahata, S.; Takenaka, T.; Maeda, M.; Ohno, M.; et al. The relationship between home blood pressure measurement and room temperature in a Japanese general population. Hypertens. Res. 2021, 44, 454–463. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society of Hypertension. Guidelines for the Management of Hypertension 2019 (JSH2019). 2019. Available online: https://www.jpnsh.jp/data/jsh2019/JSH2019_hp.pdf (accessed on 17 September 2021).
| Intervention Group | Control Group | p-Value * | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Boxed lunches a | |||||
| Energy (kcal) | 724 | 32 | 740 | 40 | 0.348 |
| Protein (g) | 28.1 | 2.7 | 28.8 | 2.8 | 0.596 |
| Fat (g) | 20.9 | 4.5 | 21.1 | 4.8 | 0.928 |
| Carbohydrates (g) | 98.1 | 7.0 | 101.7 | 9.0 | 0.323 |
| Sodium (mg) | 783 | 135 | 1654 | 220 | <0.0001 |
| Potassium (mg) | 1156 | 199 | 635 | 142 | <0.0001 |
| Sodium-to-potassium ratio | 0.7 | 0.1 | 2.7 | 0.8 | <0.0001 |
| Instant miso soup b | |||||
| Energy (kcal) | 20 | 5 | 23 | 3 | 0.140 |
| Protein (g) | 1.6 | 0.2 | 1.7 | 0.3 | 0.411 |
| Fat (g) | 0.6 | 0.2 | 0.8 | 0.2 | 0.054 |
| Carbohydrates (g) | 2.2 | 0.5 | 2.3 | 0.2 | 0.587 |
| Sodium (mg) | 392 | 17 | 589 | 20 | <0.0001 |
| Potassium (mg) | 320 | 38 | 68 | 21 | <0.0001 |
| Sodium-to-potassium ratio | 1.2 | 0.2 | 9.7 | 3.7 | <0.0001 |
| Intervention Group (n = 93) | Control Group (n = 94) | p-Value * | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 48.1 | 7.4 | 48.0 | 7.2 | 0.936 c |
| Physiological data | |||||
| Height (cm) | 171.1 | 6.2 | 171.4 | 5.5 | 0.725 c |
| Weight (kg) | 71.1 | 9.3 | 72.7 | 11.1 | 0.303 c |
| BMI (kg/m2) a | 24.3 | 3.0 | 24.7 | 3.1 | 0.399 c |
| SBP (mmHg) | 128.7 | 15.7 | 127.3 | 12.6 | 0.485 c |
| DBP (mmHg) | 83.5 | 10.4 | 82.7 | 10.1 | 0.617 c |
| Smoking status | 0.189 d | ||||
| Current smoker | 38 | (40.9) | 28 | (29.8) | |
| Past smoker | 21 | (22.5) | 20 | (21.3) | |
| Non-smoker | 34 | (36.6) | 46 | (48.9) | |
| Overweight status a | 0.833 d | ||||
| Overweight | 25 | (26.9) | 29 | (30.9) | |
| Obese | 6 | (6.5) | 6 | (6.4) | |
| Presence of diseases | |||||
| Hyperuricemia | 2 | (2.2) | 5 | (5.3) | 0.444 c |
| Hyperlipidemia | 2 | (2.2) | 0 | (0.0) | 0.246 c |
| Diabetes | 0 | (0.0) | 1 | (1.1) | 1000 d |
| Self-reported dietary intake b | |||||
| Energy (kcal/d) | 2203 | 555 | 2224 | 630 | 0.811 c |
| Protein (g/d) | 65.8 | 18.8 | 67.9 | 22.7 | 0.484 c |
| Fat (g/d) | 58.9 | 22.3 | 64.0 | 33.5 | 0.236 c |
| n-3 polyunsaturated fat (g/d) | 2.50 | 0.98 | 2.86 | 1.75 | 0.086 c |
| Carbohydrates (g/d) | 279.8 | 87.3 | 277.5 | 69.4 | 0.841 c |
| Dietary fiber (g/d) | 10.2 | 3.4 | 10.2 | 4.0 | 0.907 c |
| Alcohol (g/d) | 35.9 | 28.7 | 32.6 | 30.4 | 0.448 c |
| Calcium (mg/d) | 406 | 166 | 407 | 200 | 0.950 c |
| Magnesium (mg/d) | 251 | 69 | 256 | 84 | 0.644 c |
| Sodium (mg/d) | 4028 | 1361 | 3950 | 1523 | 0.714 c |
| Potassium (mg/d) | 2134 | 655 | 2231 | 800 | 0.366 c |
| Fruit (g/d) | 54.1 | 57.9 | 78.1 | 95.2 | 0.039 c |
| Vegetables (g/d) | 156.2 | 86.4 | 172.0 | 97.3 | 0.240 c |
| Physical activity (h/weekday) | |||||
| Intermediate activities | 2.30 | 2.06 | 2.50 | 1.90 | 0.504 c |
| Doing sports | 0.65 | 0.61 | 0.66 | 0.46 | 0.881 c |
| Muscle training | 0.08 | 0.17 | 0.07 | 0.17 | 0.678 c |
| Urinary excretion | |||||
| Urinary volume (mL/d) | 1692 | 726 | 1701 | 601 | 0.924 c |
| Sodium (mg/d) | 4776 | 1728 | 5035 | 1686 | 0.299 c |
| Potassium (mg/d) | 1787 | 587 | 1898 | 582 | 0.195 c |
| Sodium-to-potassium excretion ratio | 2.8 | 1.2 | 2.8 | 1.1 | 0.926 c |
| Intervention Group (n = 93) | Control Group (n = 94) | Adjusted Difference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endline | Change from Baseline | Baseline | Endline | Change from Baseline | (Intervention vs. Control) | |||||||||||
| Mean | SD | Mean | SD | Mean | (95% CI) | p-Value b | Mean | SD | Mean | SD | Mean | (95% CI) | p-Value b | Mean | (95% CI) | p-Value c | |
| Self-reported dietary intake a | |||||||||||||||||
| Energy (kcal/d) | 2203 | 555 | 2122 | 496 | −81 | (−172, 9) | 0.077 | 2224 | 630 | 2195 | 632 | −28 | (−100, 44) | 0.435 | −59 | (−162, 44) | 0.263 |
| Protein (g/d) | 65.8 | 18.8 | 64.7 | 18.3 | −1.1 | (−4.4, 2.2) | 0.516 | 67.9 | 22.7 | 68.8 | 24.2 | 0.9 | (−2.5, 4.3) | 0.595 | −2.6 | (−7.0, 1.8) | 0.250 |
| Fat (g/d) | 58.9 | 22.3 | 54.3 | 20.6 | −4.6 | (−8.2, −1.0) | 0.013 | 64.0 | 33.5 | 59.9 | 34.2 | −4.1 | (−8.3, 0.1) | 0.057 | −1.9 | (−7.0, 3.2) | 0.462 |
| n-3 polyunsaturated fat (g/d) | 2.50 | 0.98 | 2.44 | 0.98 | −0.06 | (−0.22, 0.09) | 0.414 | 2.86 | 1.75 | 2.79 | 1.77 | −0.07 | (−0.34, 0.21) | 0.625 | −0.10 | (−0.39, 0.20) | 0.523 |
| Carbohydrates (g/d) | 279.8 | 87.3 | 270.5 | 68.5 | −9.3 | (−22.8, 4.2) | 0.176 | 277.5 | 69.4 | 277.8 | 64.1 | 0.3 | (−8.4, 9.1) | 0.940 | −13.9 | (−58.2, 30.3) | 0.535 |
| Dietary fiber (g/d) | 10.2 | 3.4 | 9.5 | 4.0 | −0.8 | (−1.5, 0.1) | 0.032 | 10.2 | 4.0 | 10.1 | 4.0 | −0.1 | (−0.6, 0.5) | 0.805 | −0.6 | (−1.5, 0.2) | 0.110 |
| Alcohol (g/d) | 35.9 | 28.7 | 36.3 | 29.3 | 0.4 | (−3.2, 4.0) | 0.823 | 32.6 | 30.4 | 33.1 | 28.5 | 0.5 | (−2.7, 3.6) | 0.774 | 0.5 | (−0.4, 5.1) | 0.815 |
| Calcium (mg/d) | 406 | 166 | 369 | 150 | −37 | (−59, −16) | <0.001 | 407 | 200 | 397 | 177 | −10 | (−30, 9) | 0.296 | −27 | (−52, −2) | 0.038 |
| Magnesium (mg/d) | 251 | 69 | 242 | 75 | −9 | (−23, 4) | 0.170 | 256 | 84 | 255 | 82 | −1 | (−11, 9) | 0.853 | −10 | (−26, 6) | 0.221 |
| Sodium (mg/d) | 4028 | 1361 | 3777 | 1302 | −251.0 | (−553, 51) | 0.103 | 3950 | 1523 | 4010 | 1730 | 60.0 | (−206, 326) | 0.656 | −278 | (−634, 78) | 0.125 |
| Potassium (mg/d) | 2134 | 655 | 2077 | 705 | −57 | (−173, 59) | 0.331 | 2231 | 800 | 2217 | 812 | −14 | (−116, 88) | 0.792 | −65 | (−212, 81) | 0.382 |
| Fruit (g/d) | 54.1 | 57.9 | 63.8 | 71.8 | 9.6 | (−3.4, 22.6) | 0.145 | 78.1 | 95.2 | 76.4 | 80.6 | −1.7 | (−18.3, 14.9) | 0.837 | 0.4 | (−18.1, 18.9) | 0.966 |
| Vegetables (g/d) | 156.2 | 86.4 | 140.6 | 112.0 | −15.5 | (−34.5, 3.4) | 0.107 | 172.0 | 97.3 | 161.0 | 94.7 | −11.1 | (−26.6, 4.5) | 0.160 | −9.0 | (−32.2, 14.3) | 0.448 |
| Lifestyle characteristics | |||||||||||||||||
| Body weight (kg) | 71.1 | 9.3 | 70.8 | 9.3 | −0.3 | (−0.6, −0.1) | 0.005 | 72.7 | 11.1 | 72.5 | 11.1 | −0.2 | (−0.4, 0.0) | 0.079 | −0.2 | (−0.5, 0.1) | 0.241 |
| Physical activities (h/weekday) | |||||||||||||||||
| Intermediate activities | 2.30 | 2.06 | 2.88 | 2.44 | 0.57 | (0.11, 1.04) | 0.016 | 2.50 | 1.90 | 2.42 | 2.05 | −0.08 | (−0.49, 0.33) | 0.697 | 0.57 | (0.00, 1.13) | 0.049 |
| Doing sports | 0.65 | 0.61 | 1.04 | 3.13 | 0.40 | (−0.22, 1.02) | 0.207 | 0.66 | 0.46 | 0.66 | 0.55 | 0.00 | (−0.09, 0.08) | 0.974 | 0.40 | (−0.22, 1.02) | 0.202 |
| Muscle training | 0.08 | 0.17 | 0.11 | 0.23 | 0.03 | (−0.00, 0.07) | 0.051 | 0.07 | 0.17 | 0.08 | 0.19 | 0.01 | (−0.03, 0.06) | 0.511 | 0.02 | (−0.03, 0.08) | 0.348 |
| Urinary excretion | |||||||||||||||||
| Urinary volume (mL/d) | 1692 | 726 | 1423 | 672 | −268 | (−391, −147) | <0.001 | 1701 | 601 | 1497 | 676 | −204 | (−326, −82) | 0.001 | −69 | (−224, 86) | 0.383 |
| Sodium (mg/d) | 4776 | 1728 | 4484 | 2019 | −291 | (−684, 101) | 0.144 | 5035 | 1686 | 4784 | 1783 | −251 | (−604, 102) | 0.161 | −156 | (−635, 324) | 0.522 |
| Potassium (mg/d) | 1787 | 587 | 2222 | 804 | 435 | (291, 577) | <0.001 | 1898 | 582 | 1640 | 528 | −258 | (−375, −140) | <0.001 | 646 | (477, 815) | <0.001 |
| Na-to-K excretion ratio | 2.8 | 1.2 | 2.1 | 0.9 | −0.7 | (−0.9, −0.5) | <0.001 | 2.8 | 1.1 | 3.1 | 1.3 | 0.3 | (−0.0, 0.5) | 0.06 | −1.0 | (−1.3, −0.7) | <0.001 |
| N | Baseline | Endline | Change from Baseline | Adjusted Difference (Intervention vs. Control) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | Model III | |||||||||||||||
| Mean | SD | Mean | SD | Mean | (95% CI) | p-Value | Mean | (95% CI) | p-Value a | Mean | (95% CI) | p-Value b | Mean | (95% CI) | p-Value c | ||
| SBP (mmHg) | |||||||||||||||||
| Intervention group | 93 | 128.7 | 15.7 | 128.3 | 15.0 | −0.5 | (−1.7, 0.8) | 0.472 | −2.1 | (−3.6, −0.6) | 0.007 | −2.0 | (−3.6, −0.5) | 0.008 | −2.0 | (−3.6, −0.4) | 0.011 |
| Control group | 94 | 127.3 | 12.6 | 129.0 | 13.0 | 1.7 | (0.8, 2.6) | <0.001 | |||||||||
| p-value d | 0.485 | 0.724 | |||||||||||||||
| DBP (mmHg) | |||||||||||||||||
| Intervention group | 93 | 83.5 | 10.4 | 83.0 | 10.1 | −0.4 | (−1.2, 0.4) | 0.270 | −0.9 | (−2.0, 0.2) | 0.116 | −0.9 | (−2.0, 0.3) | 0.107 | −0.9 | (−2.1, 0.3) | 0.115 |
| Control group | 94 | 82.7 | 10.1 | 83.2 | 10.5 | 0.5 | (−0.3, 1.3) | 0.226 | |||||||||
| p-value d | 0.617 | 0.902 | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umeki, Y.; Hayabuchi, H.; Adachi, H.; Ohta, M. Feasibility of Low-Sodium, High-Potassium Processed Foods and Their Effect on Blood Pressure in Free-Living Japanese Men: A Randomized, Double-Blind Controlled Trial. Nutrients 2021, 13, 3497. https://doi.org/10.3390/nu13103497
Umeki Y, Hayabuchi H, Adachi H, Ohta M. Feasibility of Low-Sodium, High-Potassium Processed Foods and Their Effect on Blood Pressure in Free-Living Japanese Men: A Randomized, Double-Blind Controlled Trial. Nutrients. 2021; 13(10):3497. https://doi.org/10.3390/nu13103497
Chicago/Turabian StyleUmeki, Yoko, Hitomi Hayabuchi, Hisashi Adachi, and Masanori Ohta. 2021. "Feasibility of Low-Sodium, High-Potassium Processed Foods and Their Effect on Blood Pressure in Free-Living Japanese Men: A Randomized, Double-Blind Controlled Trial" Nutrients 13, no. 10: 3497. https://doi.org/10.3390/nu13103497
APA StyleUmeki, Y., Hayabuchi, H., Adachi, H., & Ohta, M. (2021). Feasibility of Low-Sodium, High-Potassium Processed Foods and Their Effect on Blood Pressure in Free-Living Japanese Men: A Randomized, Double-Blind Controlled Trial. Nutrients, 13(10), 3497. https://doi.org/10.3390/nu13103497






