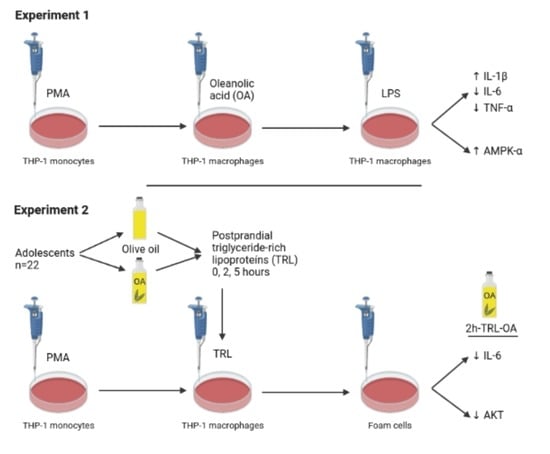
Oleanolic Acid-Enriched Olive Oil Alleviates the Interleukin-6 Overproduction Induced by Postprandial Triglyceride-Rich Lipoproteins in THP-1 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. OA Obtaining and Elaboration of the Functional Olive Oil
2.2.1. OA Obtaining
2.2.2. Analysis of OA by Gas Chromatography (GC)
2.3. Elaboration and Characteristics of the Functional Olive Oil
2.4. Preparation of OA Used in the LPS-Stimulated THP-1 Macrophages
2.5. Postprandial Trial
2.5.1. Design and Implementation of the Study
2.5.2. Setting and Participants
2.5.3. Anthropometric Assessment and Body Composition Analysis
2.5.4. Intervention
2.5.5. Biochemical Determinations and Isolation of TRLs
2.6. THP-1 Monocytes Culture
2.7. Differentiation of THP-1 Monocytes into Macrophages
2.8. Cell Viability Assay in THP-1 Macrophages Pretreated with OA
2.9. Determination of Proinflammatory Cytokines and Kinases in LPS-Stimulated THP-1 Macrophages Pretreated with OA
2.10. TRL Stimulation of THP-1-Derived Macrophages and Determination of Proinflammatory Cytokines and Kinases
2.11. Oil Red O Staining
2.12. Intracellular Fatty Acid Composition
2.13. Statistical Analysis
3. Results
3.1. OA Effects on LPS-Stimulated THP-1 Macrophages
3.1.1. Effects of OA Pretreatment on THP-1 Cell Viability
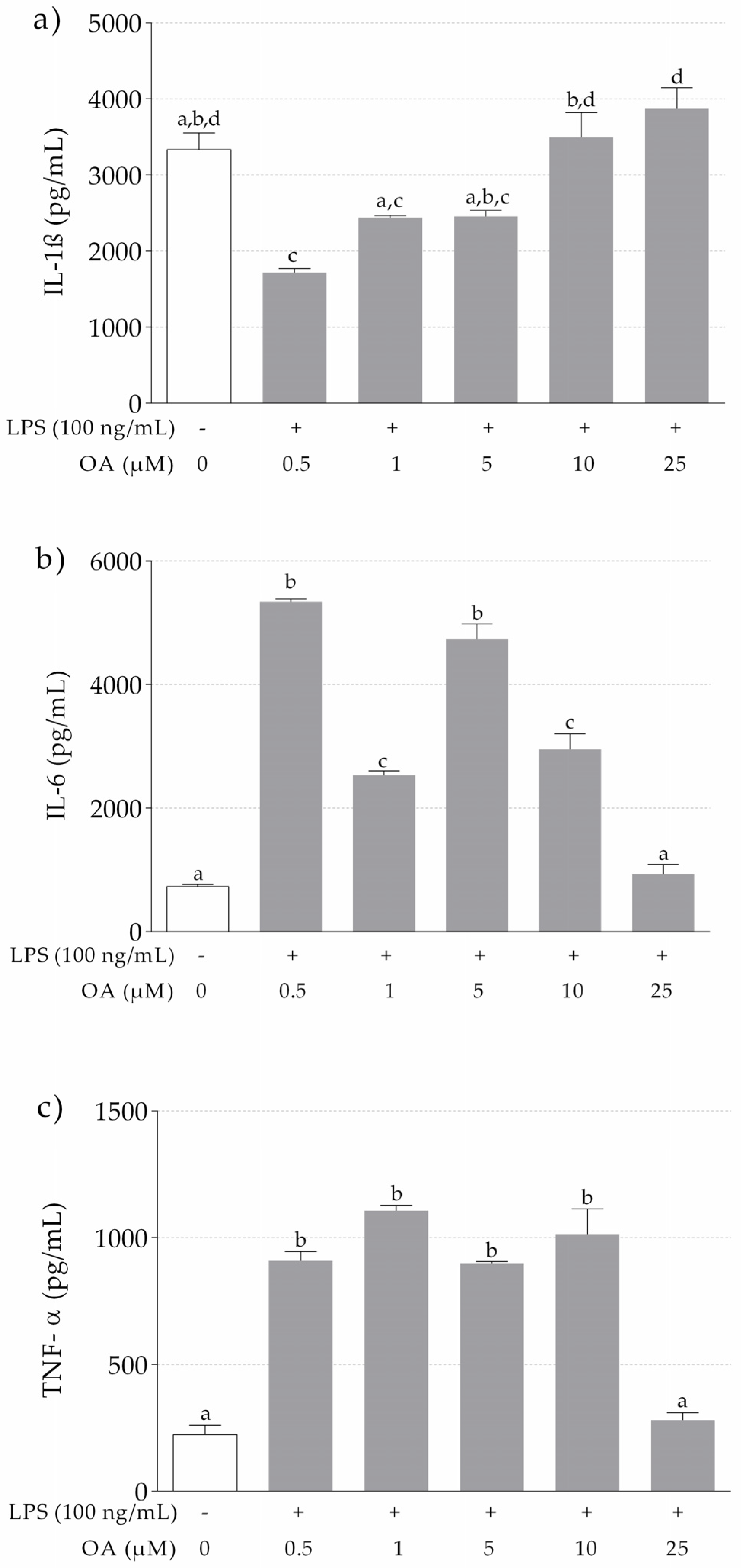
3.1.2. IL-1β, IL-6 and TNF-α Release in LPS-Stimulated THP-1 Macrophages Pretreated with OA
3.1.3. Levels of AMPK-α and Akt in LPS-Stimulated and OA-Pretreated THP-1 Macrophages
3.2. Postprandial Clinical Trial. Activation of THP-1 Macrophages with Human TRL
3.2.1. Anthropometric and Biochemical Data of Adolescents at Baseline
3.2.2. Effects of Human TRLs on the Production of Proinflammatory Cytokines in THP-1 Macrophages
3.2.3. Effects of Human TRLs on the Levels of Kinases and NF-κB in THP-1 Macrophages
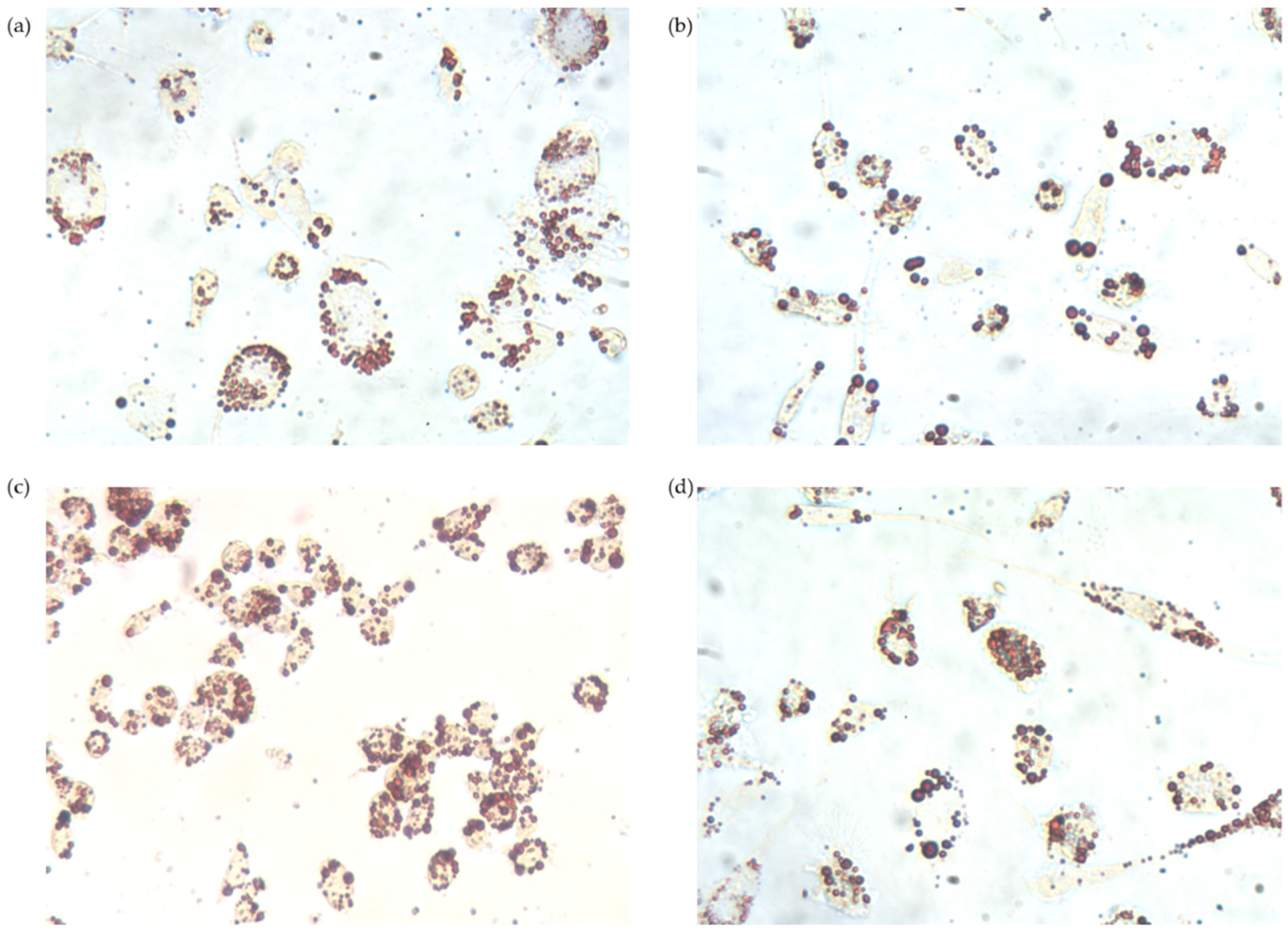
3.2.4. Intracellular Lipid Accumulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 960, pp. 1–17. [Google Scholar]
- Zimmet, P.; Alberti, K.G.M.M.; Serrano Ríos, M. A New International Diabetes Federation Worldwide Definition of the Metabolic Syndrome: The Rationale and the Results. Rev. Española Cardiol. 2005, 58, 1371–1376. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Consensus Worldwide Definition of the Metabolic Syndrome and Tools. Available online: https://www.idf.org/our-activities/advocacy-awareness/resources-and-tools/60:idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 20 June 2019).
- McCullough, A.J. Epidemiology of the Metabolic Syndrome in the USA. J. Dig. Dis. 2011, 12, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lira, J.C.G.; Xavier, M.D.A.; Borges, J.W.P.; Araújo, M.F.M.D.; Damasceno, M.M.C.; Freitas, R.W.J.F.D. Prevalence of Metabolic Syndrome in Individuals with Type 2 Diabetes Mellitus. Rev. Bras. Enferm. 2017, 70, 265–270. [Google Scholar] [CrossRef]
- Weiss, R.; Bremer, A.A.; Lustig, R.H. What Is Metabolic Syndrome, and Why Are Children Getting It? Ann. N. Y. Acad. Sci. 2013, 1281, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Lee, J. Adipose Tissue Macrophages in the Development of Obesity-Induced Inflammation, Insulin Resistance and Type 2 Diabetes. Arch. Pharm. Res. 2013, 36, 208–222. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J. Role of Obesity-Induced Inflammation in the Development of Insulin Resistance and Type 2 Diabetes: History of the Research and Remaining Questions. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 1–13. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Herrada, A.A.; Olate-Briones, A.; Rojas, A.; Liu, C.; Escobedo, N.; Piesche, M. Adipose Tissue Macrophages as a Therapeutic Target in Obesity-associated Diseases. Obes. Rev. 2021, 22, e13200. [Google Scholar] [CrossRef]
- Scarano, F.; Gliozzi, M.; Zito, M.C.; Guarnieri, L.; Carresi, C.; Macrì, R.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. Potential of Nutraceutical Supplementation in the Modulation of White and Brown Fat Tissues in Obesity-Associated Disorders: Role of Inflammatory Signalling. Int. J. Mol. Sci. 2021, 22, 3351. [Google Scholar] [CrossRef]
- Nandipati, K.C.; Subramanian, S.; Agrawal, D.K. Protein Kinases: Mechanisms and Downstream Targets in Inflammation-Mediated Obesity and Insulin Resistance. Mol. Cell. Biochem. 2017, 426, 27–45. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; pp. 1–176. [Google Scholar]
- Rask Larsen, J.; Dima, L.; Correll, C.U.; Manu, P. The Pharmacological Management of Metabolic Syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E. How the seven countries study contributed to the definition and development of the Mediterranean diet concept: A 50-year journey. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 245–252. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Martínez-Hovelman, N.; Martínez-Huélamo, M.; Lamuela-Raventos, R.M.; Moreno, J.J. Extra Virgin Olive Oil Minor Compounds Modulate Mitogenic Action of Oleic Acid on Colon Cancer Cell Line. J. Agric. Food Chem. 2019, 67, 11420–11427. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A.; et al. Mediterranean Products as Promising Source of Multi-Target Agents in the Treatment of Metabolic Syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.-I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of Type 2 Diabetes in Prediabetic Patients by Using Functional Olive Oil Enriched in Oleanolic Acid: The Prediabole Study, a Randomized Controlled Trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Cert, A. Quantitative Determination of Hydroxy Pentacyclic Triterpene Acids in Vegetable Oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef]
- Fernández-Aparicio, Á.; Schmidt-RioValle, J.; Perona, J.S.; Correa-Rodríguez, M.; Castellano, J.M.; González-Jiménez, E. Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. J. Clin. Med. 2019, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, Z.; Bei, W.; Rong, X.; Guo, J.; Hu, X. Oleanolic Acid Attenuates Insulin Resistance via NF- κ B to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 643102. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Albi, T.; Guinda, A.; Lanzón, A. Obtaining Procedure and Determination of Terpenic Acids of Olive Leaf (Olea Europaea). Grasas Aceites 2001, 52, 275–278. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; Ridder, H. International Standards for Anthropometric Assessment, 3rd ed.; International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for Blood Pressure Measurement in Humans and Experimental Animals: Part 1: Blood Pressure Measurement in Humans: A Statement for Professionals from the Subcommittee of Professional and Public Education of the American Heart Association Cou. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef]
- Tanner, J.M. Growth at Adolescence, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1962. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Glaser, C.; Demmelmair, H.; Koletzko, B. High-Throughput Analysis of Total Plasma Fatty Acid Composition with Direct in Situ Transesterification. PLoS ONE 2010, 5, e12045. [Google Scholar] [CrossRef] [PubMed]
- Yeisley, D.J.; Arabiyat, A.S.; Hahn, M.S. Cannabidiol-Driven Alterations to Inflammatory Protein Landscape of Lipopolysaccharide-Activated Macrophages In Vitro May Be Mediated by Autophagy and Oxidative Stress. Cannabis Cannabinoid Res. 2021, 6, 253–263. [Google Scholar] [CrossRef]
- Zheng, S.; Li, W.; Liao, W.; Huang, C.; Zhou, M.; Zheng, Y.; Zou, Z.; He, Z. Silencing of LncRNA-PVT1 Ameliorates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages via Inhibition of the P38 MAPK Signaling Pathway. Ann. Palliat. Med. 2021, 10, 6410–6418. [Google Scholar] [CrossRef]
- Yu, S.-H.; Kim, S.; Kim, Y.; Lee, S.-E.; Park, J.H.; Cho, G.; Ha, J.-C.; Jung, H.; Lim, S.-M.; Han, K.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Mitochondria (PN-101) Attenuate LPS-Induced Inflammatory Responses by Inhibiting NFκB Signaling Pathway. BMB Rep. 2021. [Google Scholar]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic Acid Exerts a Neuroprotective Effect Against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Remick, D.G. Mechanisms of Dimethyl Sulfoxide Augmentation of IL-1β Production. J. Immunol. 2005, 174, 6195–6202. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhao, M.M.; Sun, S.; Guo, X.L.; Wang, Q.; Li, S.A.; Lee, W.H.; Zhang, Y. A High Concentration of DMSO Activates Caspase-1 by Increasing the Cell Membrane Permeability of Potassium. Cytotechnology 2018, 70, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Yang, S.; Sun, Y.; Zhang, L.; Bo, F.; Li, L. Development and Evaluation of Oleanolic Acid Dosage Forms and Its Derivatives. BioMed Res. Int. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Zhou, H.; Feng, Y.; Tang, F.; Hoi, M.P.M.; He, C.; Ma, D.; Zhao, C.; Lee, S.M.Y. Inhibitory Effects of Betulinic Acid on LPS-Induced Neuroinflammation Involve M2 Microglial Polarization via CaMKKβ-Dependent AMPK Activation. Front. Mol. Neurosci. 2018, 11, 98. [Google Scholar] [CrossRef]
- Fan, K.; Lin, L.; Ai, Q.; Wan, J.; Dai, J.; Liu, G.; Tang, L.; Yang, Y.; Ge, P.; Jiang, R.; et al. Lipopolysaccharide-Induced Dephosphorylation of AMPK-Activated Protein Kinase Potentiates Inflammatory Injury via Repression of ULK1-Dependent Autophagy. Front. Immunol. 2018, 9, 1464. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Hu, K.; Xu, Q.; Wen, X.; Cheng, K.; Chen, C.; Yuan, H.; Dai, L.; Sun, H. Synthesis and Anti-Inflammatory Activity of Saponin Derivatives of δ-Oleanolic Acid. Eur. J. Med. Chem. 2021, 209, 112932. [Google Scholar] [CrossRef]
- Matumba, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Chegou, N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. [Google Scholar] [CrossRef]
- Ou-Yang, Q.; Xuan, C.; Wang, X.; Luo, H.; Liu, J.-E.; Wang, L.; Li, T.; Chen, Y.; Liu, J. 3-Acetyl-Oleanolic Acid Ameliorates Non-Alcoholic Fatty Liver Disease in High Fat Diet-Treated Rats by Activating AMPK-Related Pathways. Acta Pharmacol. Sin. 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Ha, D.T.; Tuan, D.T.; Thu, N.B.; Nhiem, N.X.; Ngoc, T.M.; Yim, N.H.; Bae, K.H. Palbinone and Triterpenes from Moutan Cortex (Paeonia Suffruticosa, Paeoniaceae) Stimulate Glucose Uptake and Glycogen Synthesis via Activation of AMPK in Insulin-Resistant Human HepG2 Cells. Bioorg. Med. Chem. Lett. 2009, 19, 5556–5559. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Chen, X.; He, G. PI3K and ERK/Nrf2 Pathways Are Involved in Oleanolic Acid-Induced Heme Oxygenase-1 Expression in Rat Vascular Smooth Muscle Cells. J. Cell. Biochem. 2011, 112, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Xue, C.; Zhang, L.; Zhang, T.; Wang, C.; Bi, C.; Shan, A. Oleanolic Acid Enhances Tight Junctions and Ameliorates Inflammation in Salmonella Typhimurium -Induced Diarrhea in Mice via the TLR4/NF-ΚB and MAPK Pathway. Food Funct. 2020, 11, 1122–1132. [Google Scholar] [CrossRef]
- Choi, J.K.; Kim, S.-W.; Kim, D.-S.; Lee, J.Y.; Lee, S.; Oh, H.-M.; Ha, Y.S.; Yoo, J.; Park, P.-H.; Shin, T.-Y.; et al. Oleanolic Acid Acetate Inhibits Rheumatoid Arthritis by Modulating T Cell Immune Responses and Matrix-Degrading Enzymes. Toxicol. Appl. Pharmacol. 2016, 290, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deeb, D.; Jiang, H.; Liu, Y.; Dulchavsky, S.A.; Gautam, S.C. Synthetic Triterpenoids Inhibit Growth and Induce Apoptosis in Human Glioblastoma and Neuroblastoma Cells through Inhibition of Prosurvival Akt, NF-ΚB and Notch1 Signaling. J. Neurooncol. 2007, 84, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; He, H.; Zhang, X.; Wu, R.; Gan, L.; Li, D.; Lu, Y.; Wu, P.; Wong, W.-L.; Zhang, K. The in Vitro and in Vivo Study of Oleanolic Acid Indole Derivatives as Novel Anti-Inflammatory Agents: Synthesis, Biological Evaluation, and Mechanistic Analysis. Bioorg. Chem. 2021, 113, 104981. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; Zhao, Y.; Qiao, P.; Tang, D.; Chen, Y.; Xue, C.; Li, C.; Liu, S.; Wang, J.; et al. Oleanolic Acid Exerts Bone Protective Effects in Ovariectomized Mice by Inhibiting Osteoclastogenesis. J. Pharmacol. Sci. 2018, 137, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The Activation of Akt/PKB Signaling Pathway and Cell Survival. J. Cell. Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, H.; Basson, M.D. Akt2, but Not Akt1 or Akt3 Mediates Pressure-Stimulated Serum-Opsonized Latex Bead Phagocytosis through Activating MTOR and P70 S6 Kinase. J. Cell. Biochem. 2007, 102, 353–367. [Google Scholar] [CrossRef]
- Jin, L.; Chen, C.; Li, Y.; Yuan, F.; Gong, R.; Wu, J.; Zhang, H.; Kang, B.; Yuan, G.; Zeng, H.; et al. A Biodegradable Mg-Based Alloy Inhibited the Inflammatory Response of THP-1 Cell-Derived Macrophages Through the TRPM7–PI3K–AKT1 Signaling Axis. Front. Immunol. 2019, 10, 2798. [Google Scholar] [CrossRef]
- Cabello-Moruno, R.; Sinausia, L.; Botham, K.M.; Montero, E.; Avella, M.; Perona, J.S. Postprandial Phase Time Influences the Uptake of TAG from Postprandial TAG-Rich Lipoproteins by THP-1 Macrophages. Br. J. Nutr. 2014, 112, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Botham, K.M.; Bravo, E. Postprandial Human Triglyceride-Rich Lipoproteins Increase Chemoattractant Protein Secretion in Human Macrophages. Cytokine 2013, 63, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Graham, V.S.; Lawson, C.; Wheeler-Jones, C.P.D.; Perona, J.S.; Ruiz-Gutierrez, V.; Botham, K.M. Triacylglycerol-Rich Lipoproteins Derived from Healthy Donors Fed Different Olive Oils Modulate Cytokine Secretion and Cyclooxygenase-2 Expression in Macrophages: The Potential Role of Oleanolic Acid. Eur. J. Nutr. 2012, 51, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Domoto, K.; Taniguchi, T.; Takaishi, H.; Takahashi, T.; Fujioka, Y.; Takahashi, A.; Ishikawa, Y.; Yokoyama, M. Chylomicron Remnants Induce Monocyte Chemoattractant Protein-1 Expression via P38 MAPK Activation in Vascular Smooth Muscle Cells. Atherosclerosis 2003, 171, 193–200. [Google Scholar] [CrossRef]
- Takahashi, Y.; Fujioka, Y.; Takahashi, T.; Domoto, K.; Takahashi, A.; Taniguchi, T.; Ishikawa, Y.; Yokoyama, M. Chylomicron Remnants Regulate Early Growth Response Factor-1 in Vascular Smooth Muscle Cells. Life Sci. 2005, 77, 670–682. [Google Scholar] [CrossRef]
- Botham, K.M.; Wheeler-Jones, C.P.D. Postprandial Lipoproteins and the Molecular Regulation of Vascular Homeostasis. Prog. Lipid Res. 2013, 52, 446–464. [Google Scholar] [CrossRef]
- De Pascale, C.; Graham, V.; Fowkes, R.C.; Wheeler-Jones, C.P.D.; Botham, K.M. Suppression of Nuclear Factor-ΚB Activity in Macrophages by Chylomicron Remnants: Modulation by the Fatty Acid Composition of the Particles. FEBS J. 2009, 276, 5689–5702. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; Altman, R.; Norman, J.E.; Rutledge, J.C. Postprandial VLDL Lipolysis Products Increase Monocyte Adhesion and Lipid Droplet Formation via Activation of ERK2 and NFκB. Am. J. Physiol. Circ. Physiol. 2014, 306, H109–H120. [Google Scholar] [CrossRef]
- Perona, J.S.; Avella, M.; Botham, K.M.; Ruiz-Gutierrez, V. Differential Modulation of Hepatic Very Low-Density Lipoprotein Secretion by Triacylglycerol-Rich Lipoproteins Derived from Different Oleic-Acid Rich Dietary Oils. Br. J. Nutr. 2008, 99, 29–36. [Google Scholar] [CrossRef][Green Version]
- Cabello-Moruno, R.; Perona, J.S.; Osada, J.; Garcia, M.; Ruiz-Gutierrez, V. Modifications in Postprandial Triglyceride-Rich Lipoprotein Composition and Size after the Intake of Pomace Olive Oil. J. Am. Coll. Nutr. 2007, 26, 24–31. [Google Scholar] [CrossRef]
- Cabello-Moruno, R.; Martinez-Force, E.; Montero, E.; Perona, J.S. Minor Components of Olive Oil Facilitate the Triglyceride Clearance from Postprandial Lipoproteins in a Polarity-Dependent Manner in Healthy Men. Nutr. Res. 2014, 34, 40–47. [Google Scholar] [CrossRef] [PubMed][Green Version]



| 0 Hour | 2 Hours | 5 Hours | |||||
|---|---|---|---|---|---|---|---|
| Fatty Acids | Control | Olive Oil | OA-Enriched Olive Oil | Olive Oil | OA-Enriched Olive Oil | Olive Oil | OA-Enriched Olive Oil |
| 12:0 | 41.46 ± 3.41 | 41.68 ± 4.26 | 38.93 ± 3.23 | 42.10 ± 5.51 | 41.02 ± 2.21 | 35.63 ± 4.93 | 40.06 ± 6.80 |
| 14:0 | 45.00 ± 3.33 | 48.13 ± 4.26 | 42.41 ± 2.38 | 48.18 ± 4.30 | 40.71 ± 3.10 | 40.94 ± 2.95 | 41.41 ± 4.56 |
| 16:0 | 95.68 ± 16.74 | 158.11 ± 26.64 | 151.96 ± 26.97 | 171.74 ± 19.10 | 148.57 ± 24.76 | 130.98 ± 20.06 | 180.53 ± 48.50 |
| 18:1 | 60.16 ± 6.51 | 119.58 ± 24.85 | 104.55 ± 22.00 | 126.60 ± 13.02 * | 119.88 ± 19.83 | 102.02 ± 14.42 | 145.63 ± 44.69 |
| 18:2 | 46.68 ± 6.07 | 45.22 ± 4.12 | 40.83 ± 3.43 | 56.26 ± 11.56 | 41.65 ± 3.45 | 37.49 ± 2.99 | 40.59 ± 4.35 |
| 20:4 | 41.74 ± 1.30 | 51.22 ± 16.64 | 63.37 ± 17.74 | 46.04 ± 4.73 | 43.44 ± 6.23 | 42.60 ± 11.64 | 35.02 ± 1.65 |
| Others | 79.37 ± 9.00 | ND | 59.97 ± 26.24 | ND | ND | ND | 155.12 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Aparicio, Á.; Perona, J.S.; Castellano, J.M.; Correa-Rodríguez, M.; Schmidt-RioValle, J.; González-Jiménez, E. Oleanolic Acid-Enriched Olive Oil Alleviates the Interleukin-6 Overproduction Induced by Postprandial Triglyceride-Rich Lipoproteins in THP-1 Macrophages. Nutrients 2021, 13, 3471. https://doi.org/10.3390/nu13103471
Fernández-Aparicio Á, Perona JS, Castellano JM, Correa-Rodríguez M, Schmidt-RioValle J, González-Jiménez E. Oleanolic Acid-Enriched Olive Oil Alleviates the Interleukin-6 Overproduction Induced by Postprandial Triglyceride-Rich Lipoproteins in THP-1 Macrophages. Nutrients. 2021; 13(10):3471. https://doi.org/10.3390/nu13103471
Chicago/Turabian StyleFernández-Aparicio, Ángel, Javier S. Perona, José M. Castellano, María Correa-Rodríguez, Jacqueline Schmidt-RioValle, and Emilio González-Jiménez. 2021. "Oleanolic Acid-Enriched Olive Oil Alleviates the Interleukin-6 Overproduction Induced by Postprandial Triglyceride-Rich Lipoproteins in THP-1 Macrophages" Nutrients 13, no. 10: 3471. https://doi.org/10.3390/nu13103471
APA StyleFernández-Aparicio, Á., Perona, J. S., Castellano, J. M., Correa-Rodríguez, M., Schmidt-RioValle, J., & González-Jiménez, E. (2021). Oleanolic Acid-Enriched Olive Oil Alleviates the Interleukin-6 Overproduction Induced by Postprandial Triglyceride-Rich Lipoproteins in THP-1 Macrophages. Nutrients, 13(10), 3471. https://doi.org/10.3390/nu13103471