Dietary Cocoa Flavanols Enhance Mitochondrial Function in Skeletal Muscle and Modify Whole-Body Metabolism in Healthy Mice
Abstract
1. Introduction
2. Methods
2.1. Animals and Diet
2.2. Tissue Collection
2.3. Metabolic and Physical Activity Assessments
2.4. Total Body and Fat Mass
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. qRT-PCR Analysis
2.7. Enzyme Activities
2.8. Mitochondrial Assessment
2.9. Western Blot Analysis
2.10. NAD Measurement
2.11. Statistics
3. Results
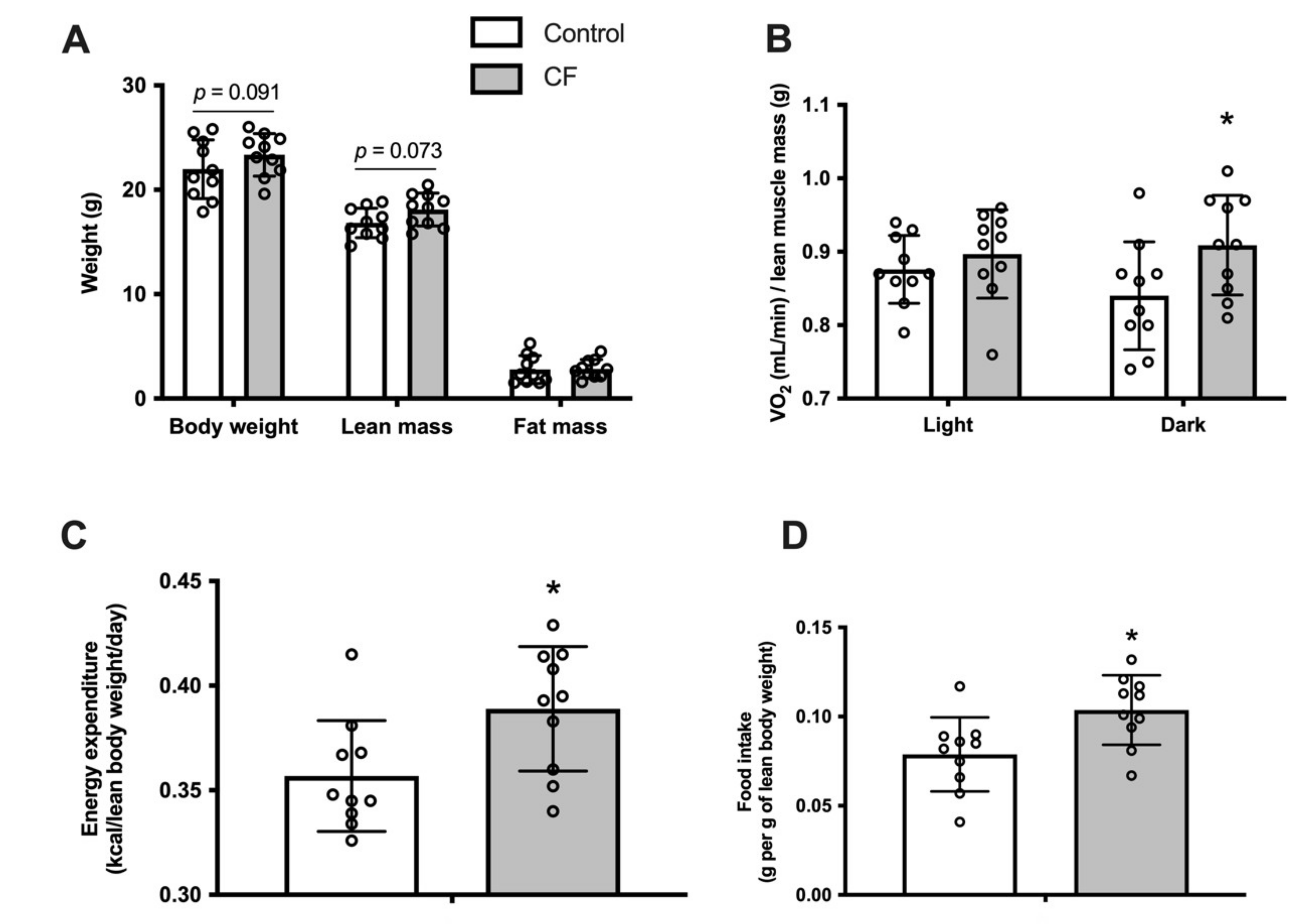
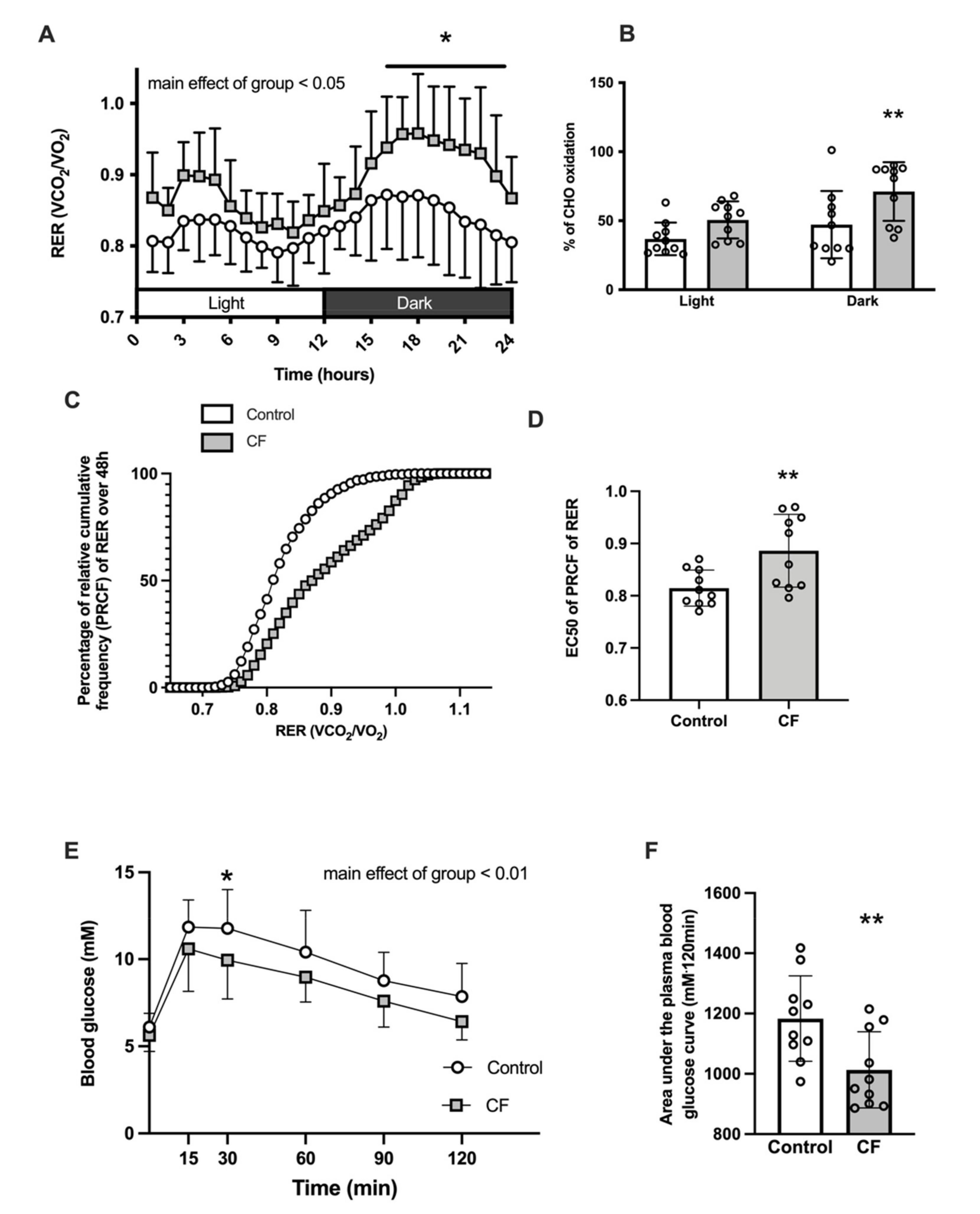
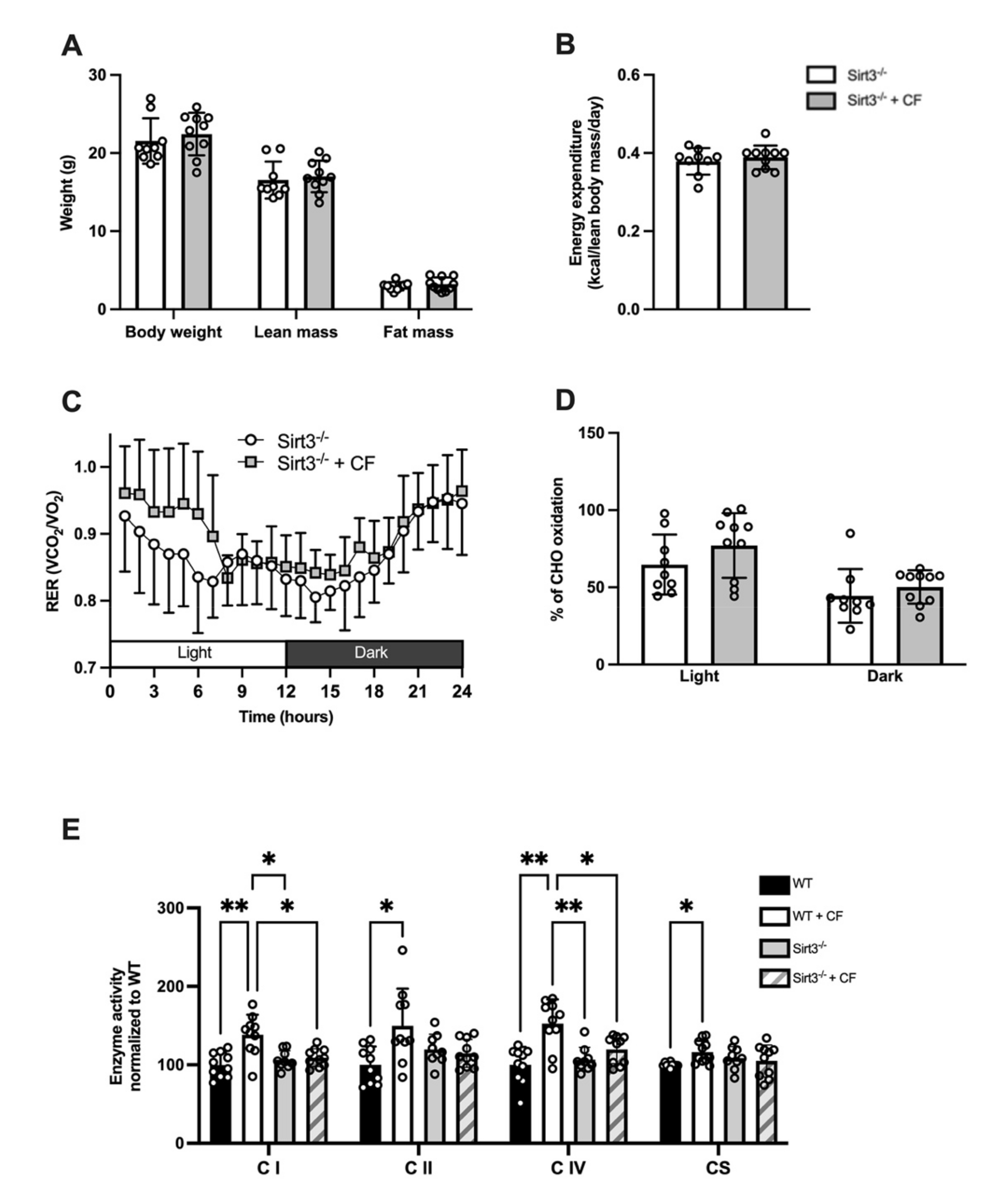
3.1. Body Composition and Whole-Body Metabolism
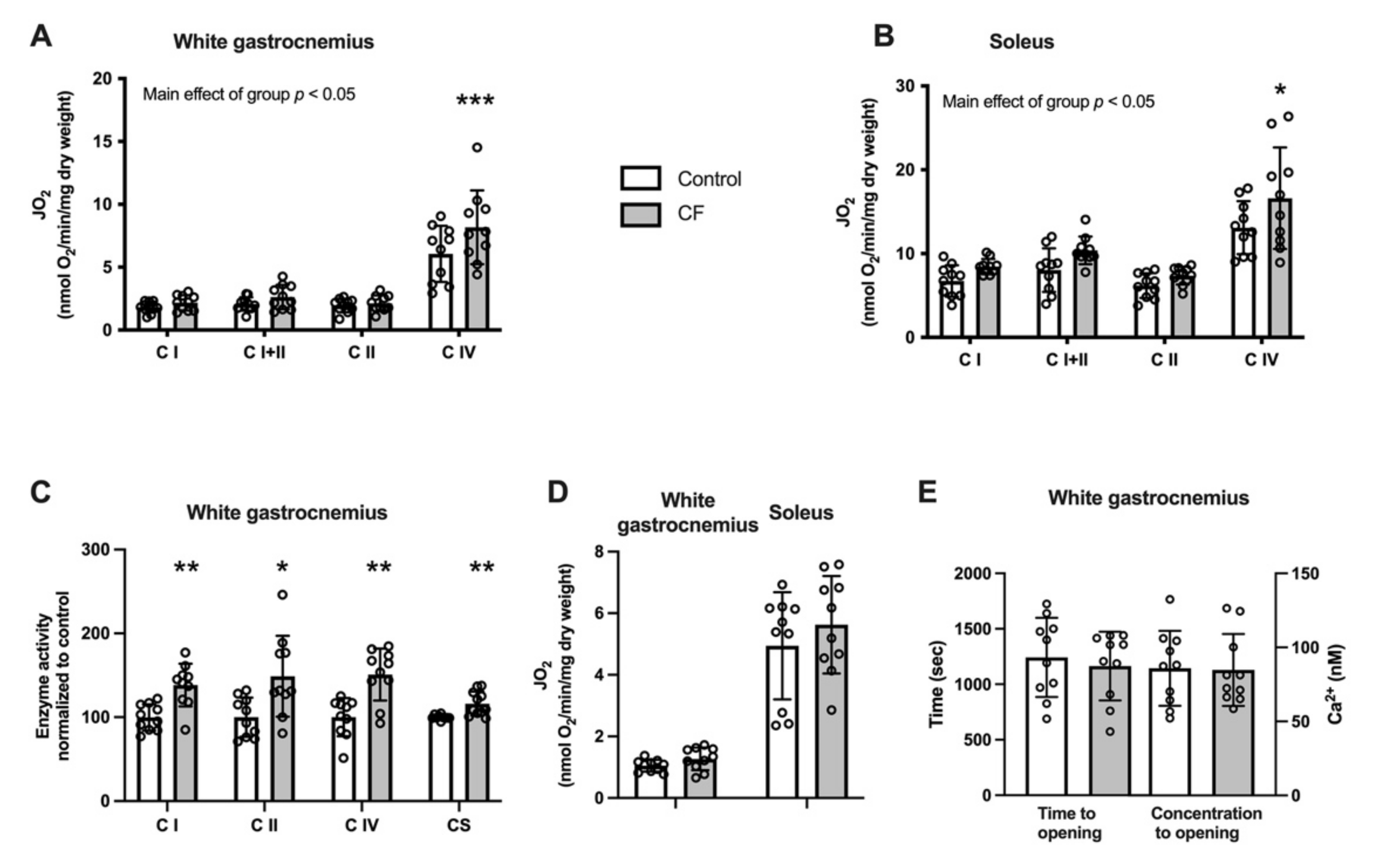
3.2. Mitochondrial Bioenergetics
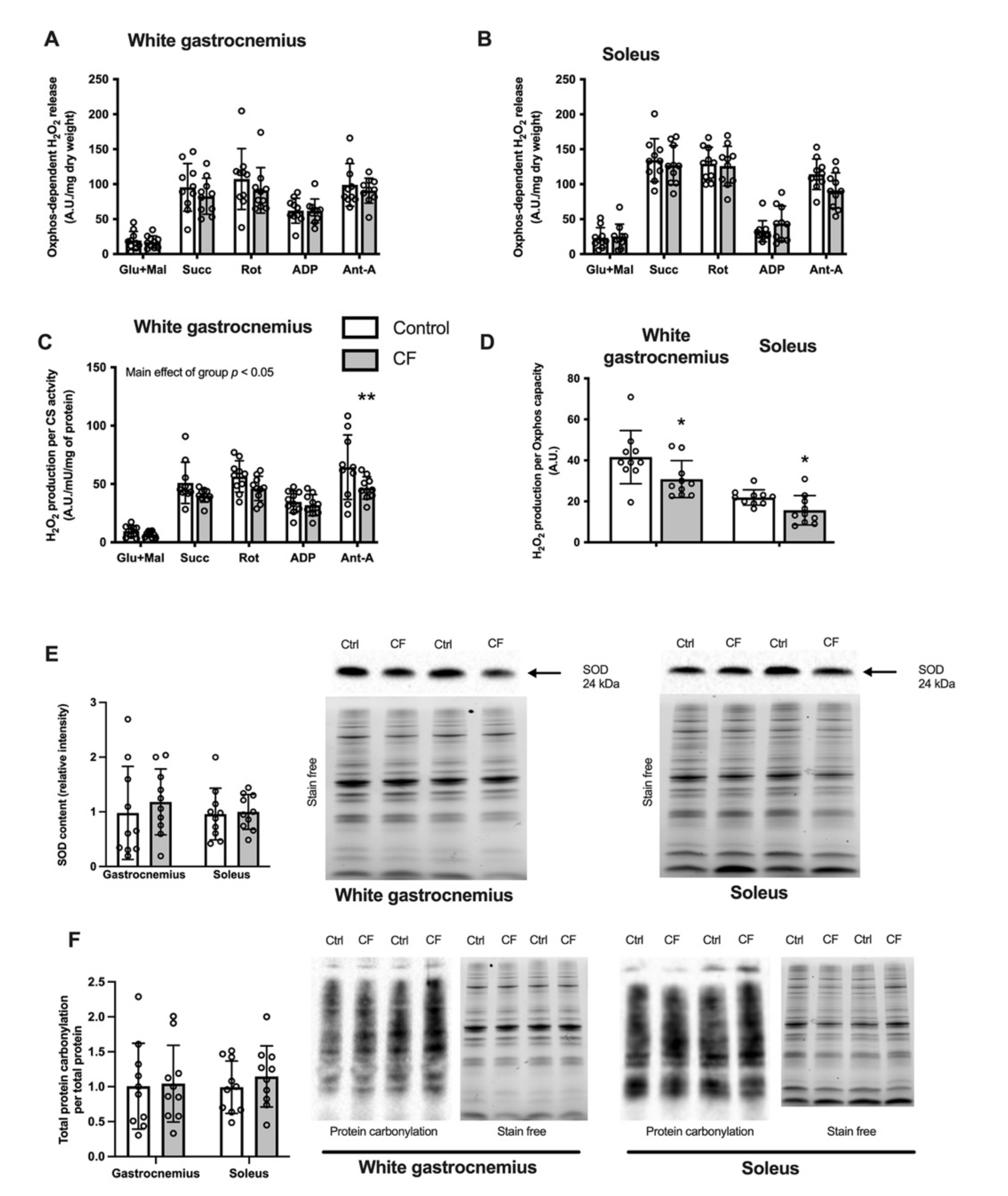
3.3. Oxidative Stress Markers in Skeletal Muscle
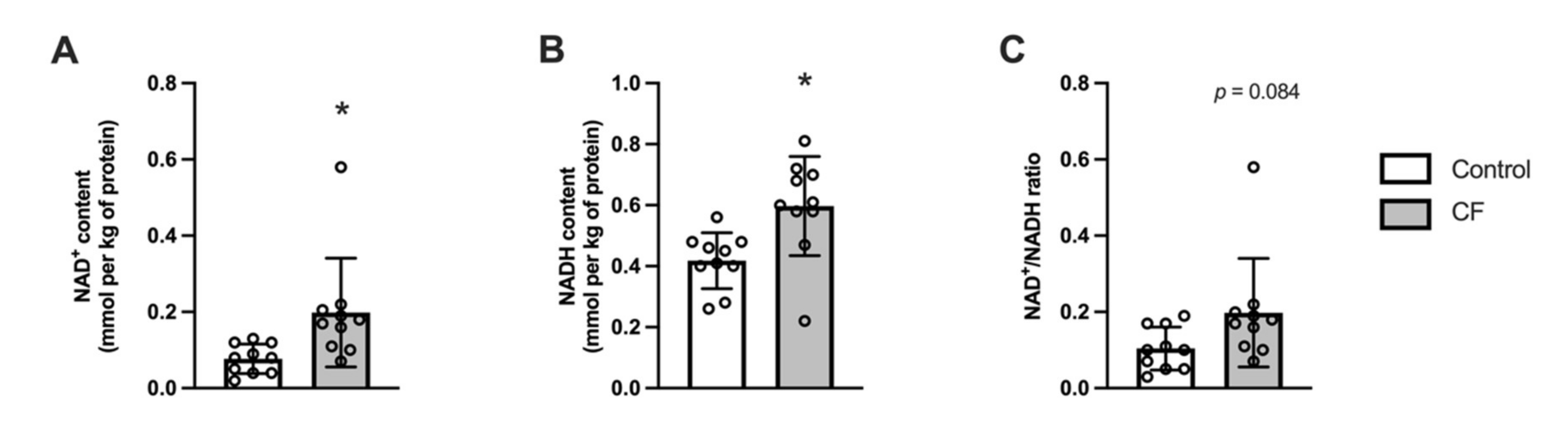
3.4. NAD Metabolism
3.5. Whole-Body and Cellular Metabolism Response Following CF Supplementation in Sirt3-/- Mice
4. Discussion
4.1. Metabolism
4.2. Mitochondrial Function
4.3. Mitochondrial Biogenesis
4.4. Limits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Wacquier, B.; Combettes, L.; Van Nhieu, G.T.; Dupont, G. Interplay Between Intracellular Ca2+ Oscillations and Ca2+-stimulated Mitochondrial Metabolism. Sci. Rep. 2016, 6, srep19316. [Google Scholar] [CrossRef]
- Suomalainen-Wartiovaara, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef]
- Heyman, E.; Daussin, F.; Wieczorek, V.; Caiazzo, R.; Matran, R.; Berthon, P.; Aucouturier, J.; Berthoin, S.; Descatoire, A.; LeClair, E.; et al. Muscle Oxygen Supply and Use in Type 1 Diabetes, From Ambient Air to the Mitochondrial Respiratory Chain: Is There a Limiting Step? Diabetes Care 2020, 43, 209–218. [Google Scholar] [CrossRef]
- Daussin, F.N.; Boulanger, E.; Lancel, S. From mitochondria to sarcopenia: Role of inflammaging and RAGE-ligand axis implication. Exp. Gerontol. 2021, 146, 111247. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and Chocolate in Human Health and Disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood Pressure Is Reduced and Insulin Sensitivity Increased in Glucose-Intolerant, Hypertensive Subjects after 15 Days of Consuming High-Polyphenol Dark Chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Heyman, E.; Burelle, Y. Effects of (−)-epicatechin on mitochondria. Nutr. Rev. 2020, 79, 25–41. [Google Scholar] [CrossRef]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary proanthocyanidins boost hepatic NAD+ metabolism and SIRT1 expression and activity in a dose-dependent manner in healthy rats. Sci. Rep. 2016, 6, 24977. [Google Scholar] [CrossRef]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Perkins, G.; Murphy, A.N.; Naviaux, R.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Alterations in Skeletal Muscle Indicators of Mitochondrial Structure and Biogenesis in Patients with Type 2 Diabetes and Heart Failure: Effects of Epicatechin Rich Cocoa. Clin. Transl. Sci. 2011, 5, 43–47. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Nogueira, L.; Rodriguez, A.; Barboza, J.; Hogan, M.C.; Ceballos, G.; Villarreal, F.; Ramirez-Sanchez, I. Recovery of Indicators of Mitochondrial Biogenesis, Oxidative Stress, and Aging With (−)-Epicatechin in Senile Mice. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2014, 70, 1370–1378. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.; Rodríguez, A.; Moreno-Ulloa, A.; Ceballos, G.; Villarreal, F. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes Vasc. Dis. Res. 2016, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Tishkoff, D.X.; Bao, J. Mitochondrial Sirtuins in the Regulation of Mitochondrial Activity and Metabolic Adaptation. Histone Deacetylases Biol. Clin. Implic. 2011, 206, 163–188. [Google Scholar] [CrossRef]
- Schwer, B.; Bunkenborg, J.; Verdin, R.O.; Andersen, J.S.; Verdin, E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. USA 2006, 103, 10224–10229. [Google Scholar] [CrossRef]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.; Grueter, C.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef]
- Jing, E.; Emanuelli, B.; Hirschey, M.D.; Boucher, J.; Lee, K.Y.; Lombard, D.; Verdin, E.M.; Kahn, C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2011, 108, 14608–14613. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, Z.; Zhang, W.; Deng, Z.; Wan, Y.; Zhang, Y.; An, S.; Huang, Q.; Chen, Z. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free Radic. Res. 2018, 53, 139–149. [Google Scholar] [CrossRef]
- Jing, E.; O’Neill, B.T.; Rardin, M.J.; Kleinridders, A.; Ilkeyeva, O.R.; Ussar, S.; Bain, J.R.; Lee, K.Y.; Verdin, E.M.; Newgard, C.B.; et al. Sirt3 Regulates Metabolic Flexibility of Skeletal Muscle Through Reversible Enzymatic Deacetylation. Diabetes 2013, 62, 3404–3417. [Google Scholar] [CrossRef]
- Sebaa, R.; Johnson, J.; Pileggi, C.; Norgren, M.; Xuan, J.; Sai, Y.; Tong, Q.; Krystkowiak, I.; Bondy-Chorney, E.; Davey, N.E.; et al. SIRT3 controls brown fat thermogenesis by deacetylation regulation of pathways upstream of UCP1. Mol. Metab. 2019, 25, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Alert, O. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2005. [Google Scholar]
- Riachi, M.; Himms-Hagen, J.; Harper, M.-E. Percent relative cumulative frequency analysis in indirect calorimetry: Application to studies of transgenic mice. Can. J. Physiol. Pharmacol. 2004, 82, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Péronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Overmyer, K.A.; Evans, C.; Qi, N.R.; Minogue, C.E.; Carson, J.J.; Chermside-Scabbo, C.J.; Koch, L.G.; Britton, S.L.; Pagliarini, D.J.; Coon, J.J.; et al. Maximal Oxidative Capacity during Exercise Is Associated with Skeletal Muscle Fuel Selection and Dynamic Changes in Mitochondrial Protein Acetylation. Cell Metab. 2015, 21, 468–478. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Jarreta, D.; Orús, J.; Barrientos, A.; Miró, O.; Roig, E.; Heras, M.; Moraes, C.T.; Cardellach, F.; Casademont, J. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc. Res. 2000, 45, 860–865. [Google Scholar] [CrossRef]
- Picard, M.; Taivassalo, T.; Ritchie, D.; Wright, K.J.; Thomas, M.M.; Romestaing, C.; Hepple, R.T. Mitochondrial Structure and Function Are Disrupted by Standard Isolation Methods. PLoS ONE 2011, 6, e18317. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Csukly, K.; Robillard, M.-E.; Godin, R.; Ascah, A.; Bourcier-Lucas, C.; Burelle, Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R659–R668. [Google Scholar] [CrossRef]
- Strat, K.M.; Rowley, T.J.; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilson, A.P. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Cacao liquor procyanidin extract improves glucose tolerance by enhancing GLUT4 translocation and glucose uptake in skeletal muscle. J. Nutr. Sci. 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Inagawa, K.; Shibata, M.; Osakabe, N. Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids Health Dis. 2014, 13, 64. [Google Scholar] [CrossRef][Green Version]
- Tomaru, M.; Takano, H.; Osakabe, N.; Yasuda, A.; Inoue, K.-I.; Yanagisawa, R.; Ohwatari, T.; Uematsu, H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007, 23, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Galgani, J.E.; Moro, C.; Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1009–E1017. [Google Scholar] [CrossRef]
- Kellogg, D.L.; McCammon, K.M.; Hinchee-Rodriguez, K.S.; Adamo, M.L.; Roman, L.J. Neuronal nitric oxide synthase mediates insulin- and oxidative stress-induced glucose uptake in skeletal muscle myotubes. Free Radic. Biol. Med. 2017, 110, 261–269. [Google Scholar] [CrossRef]
- Deshmukh, A.; Long, Y.C.; de Castro Barbosa, T.; Karlsson, H.; Glund, S.; Zavadoski, W.J.; Gibbs, E.M.; Koistinen, H.; Wallberg-Henriksson, H.; Zierath, J.R. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)Alpha1-specific activity and glucose transport in human skeletal muscle. Diabetologia 2010, 53, 1142–1150. [Google Scholar] [CrossRef]
- Smith, A.C.; Bruce, C.R.; Dyck, D.J. AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle. J. Physiol. 2005, 565, 537–546. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.; Wu, G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, M.; Ali, S.S.; Finley, J.C.; Kellerhals, S.E.; Migita, M.Y.; Head, B.P.; Patel, P.M.; Roth, D.M.; Patel, H. Epicatechin regulation of mitochondrial structure and function is opioid receptor dependent. Mol. Nutr. Food Res. 2013, 57, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Hüttemann, M.; Zielske, S.P. Epicatechin Stimulates Mitochondrial Activity and Selectively Sensitizes Cancer Cells to Radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef]
- Rowley, T.J.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bitner, B.F.; Ray, J.D.; Kener, K.B.; Herring, J.A.; Tueller, J.A.; Johnson, D.K.; Freitas, C.M.T.; Fausnacht, D.W.; Allen, M.E.; Thomson, A.H.; et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in β-cells and skeletal muscle cells. J. Nutr. Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kener, K.B.; Munk, D.J.; Hancock, C.R.; Tessem, J.S. High-resolution Respirometry to Measure Mitochondrial Function of Intact Beta Cells in the Presence of Natural Compounds. J. Vis. Exp. 2018, e57053. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M.A.; et al. Supercomplex Assembly Determines Electron Flux in the Mitochondrial Electron Transport Chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- Acin-Perez, R.; Enriquez, J.A. The function of the respiratory supercomplexes: The plasticity model. Biochim. Biophys. Acta BBA Bioenerg. 2014, 1837, 444–450. [Google Scholar] [CrossRef]
- Milenkovic, D.; Blaza, J.N.; Larsson, N.-G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Muller, F. The nature and mechanism of superoxide production by the electron transport chain: Its relevance to aging. J. Am. Aging Assoc. 2000, 23, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Talbert, E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; De los Santos, S.; Gonzalez-Basurto, S.; Canto, P.; Mendoza-Lorenzo, P.; Palma-Flores, C.; Ceballos-Reyes, G.; Villarreal, F.; Zentella-Dehesa, A.; Coral-Vazquez, R. (-)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic δ-sarcoglycan null mouse striated muscle. FEBS J. 2014, 281, 5567–5580. [Google Scholar] [CrossRef]
- Wiegand, H.; Boesch-Saadatmandi, C.; Regos, I.; Treutter, D.; Wolffram, S.; Rimbach, G. Effects of Quercetin and Catechin on Hepatic Glutathione-S Transferase (GST), NAD(P)H Quinone Oxidoreductase 1 (NQO1), and Antioxidant Enzyme Activity Levels in Rats. Nutr. Cancer 2009, 61, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Taub, P.R.; Ramirez-Sanchez, I.; Patel, M.; Higginbotham, E.; Moreno-Ulloa, A.; Román-Pintos, L.M.; Phillips, P.; Perkins, G.; Ceballos, G.; Villarreal, F. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: Underlying mechanisms. A double blind, randomized, placebo controlled trial. Food Funct. 2016, 7, 3686–3693. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.-J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute cocoa Flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Santos, L.F.S.; Stolfo, A.; Calloni, C.; Salvador, M. Catechin and epicatechin reduce mitochondrial dysfunction and oxidative stress induced by amiodarone in human lung fibroblasts. J. Arrhythmia 2017, 33, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; De Stefani, D.; De Vivo, I.; Scapagnini, G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020, 31, 536–550. [Google Scholar] [CrossRef]
- Dominy, J.E.; Puigserver, P. Mitochondrial Biogenesis through Activation of Nuclear Signaling Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a015008. [Google Scholar] [CrossRef]
- Schnorr, O.; Brossette, T.; Momma, T.Y.; Kleinbongard, P.; Keen, C.L.; Schroeter, H.; Sies, H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys. 2008, 476, 211–215. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M.; Malek, M.H. (−)-Epicatechin Attenuates Degradation of Mouse Oxidative Muscle Following Hindlimb Suspension. J. Strength Cond. Res. 2016, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Imai, S. Dissecting systemic control of metabolism and aging in the NAD World: The importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011, 585, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (−)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Mansour, C.; Navarrete-Yañez, V.; Ayala-Hernandez, M.; Guevara, G.; Castillo, C.; Loredo, M.; Bustamante, M.; Ceballos, G.; Villarreal, F.J. (−)-Epicatechin induced reversal of endothelial cell aging and improved vascular function: Underlying mechanisms. Food Funct. 2018, 9, 4802–4813. [Google Scholar] [CrossRef]
- Giralt, A.; Villarroya, F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and aging. Biochem. J. 2012, 444, 1–10. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-14C](−)-epicatechin in humans: Implications for the assessment of efficacy, safety and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of Fermentation, Drying, Roasting, and Dutch Processing on Epicatechin and Catechin Content of Cacao Beans and Cocoa Ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef] [PubMed]
- Kofink, M.; Papagiannopoulos, M.; Galensa, R. (-)-Catechin in Cocoa and Chocolate: Occurence and Analysis of an Atypical Flavan-3-ol Enantiomer. Molecules 2007, 12, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ulloa, A.; Cid, A.; Rubio-Gayosso, I.; Ceballos, G.; Villarreal, F.; Ramirez-Sanchez, I. Effects of (−)-epicatechin and derivatives on nitric oxide mediated induction of mitochondrial proteins. Bioorganic Med. Chem. Lett. 2013, 23, 4441–4446. [Google Scholar] [CrossRef]
- Turkmen, K.; Karagoz, A.; Kucuk, A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J. Diabetes 2014, 5, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Lu, N.; Zou, F.; Meng, F.-Z. Sirtuins as novel targets in the pathogenesis of airway inflammation in bronchial asthma. Eur. J. Pharmacol. 2019, 865, 172670. [Google Scholar] [CrossRef] [PubMed]
| Compound | Content |
|---|---|
| Total flavanols (%) | 31.62 |
| (-)-epicatechin (%) | 5.93 |
| (-)-catechin (%) | 1.21 |
| Théobromin (%) | 7.12 |
| Caffeine (%) | 0.92 |
| Target Gene | Official Full Name | GenBank Accession Number | Forward Primer (5′-3′) Reverse Primer (3′-5′) |
|---|---|---|---|
| PGC1α | Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | NM_008904.2 | AAACTTGCTAGCGGTCCTCA TGGCTGGTGCCAGTAAGAG |
| NRF1 | Mus musculus nuclear respiratory factor 1 | NM_001164226 | GCACCTTTGGAGAATGTGGT GGGTCATTTTGTCCACAGAGA |
| TFAM | Mus musculus transcription factor A, mitochondrial | NM_009360 | CCTTCGATTTTCCACAGAACA GCTCACAGCTTCTTTGTATGCTT |
| CS | Citrate synthase | NM_026444.4 |
GGAGCCAAGAACTCATCCTG TCTGGCCTGCTCCTTAGGTA |
| ND1 | NADH dehydrogenase subunit 1 | NC_005089.1 |
ACACTTATTACAACCCAAGAACACAT TCATATTATGGCTATGGGTCAGG |
| ND2 | NADH dehydrogenase subunit 2 | NC_005089.1 |
CCATCAACTCAATCTCACTTCTATG GAATCCTGTTAGTGGTGGAAGG |
| SDHa | Succinate dehydrogenase complex, subunit A | NM_023281.1 |
GGAACACTCCAAAAACAGACCT CCACCACTGGGTATTGAGTAGAA |
| Cox2 | Cytochrome c oxidase subunit II | NC_005089.1 | CATCTGAAGACGTCCTCCACTCAT TGCTTGATTTAGTCGGCCTGGGAT |
| Cat | Catalase | NM_009804.2 |
TGAGAAGCCTAAGAACGCAATTC CCCTTCGCAGCCATGTG |
| MnSOD | Superoxide dismutase [Mn] | NM_013671.3 |
TTAACGCGCAGATCATGCA GGTGGCGTTGAGATTGTTCA |
| Sirt1 | Sirtuin 1 | NM_019812 | AAAGGAATTGGTTCATTTATCAGAG TTGTGGTTTTTCTTCCACACA |
| Sirt3 | Sirtuin 3 | NM_022433.2 | AGGTGGAGGAAGCAGTGAGA GCTTGGGGTTGTGAAAGAAA |
| NMNAT1 | Nicotinamide nucleotide adenyltransferase 1 | NM_133435.2 | TGTGCCCAAGGTGAAATTGCT CCACGATTTGCGTGATGTCC |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | NM_008084.3 | ACTCCACTCACGGCAAATTC GTTAGTGGGGTCTCGCTCCT |
| Gene | Control Group | Group | p |
|---|---|---|---|
| PGC1α | 1.00 ± 0.42 | 1.21 ± 1.28 | 0.66 |
| NRF1 | 1.00 ± 0.42 | 1.70 ± 0.72 | 0.04 |
| TFAM | 1.00 ± 0.69 | 1.27 ± 0.52 | 0.45 |
| CS | 1.00 ± 0.20 | 1.34 ± 0.49 | 0.08 |
| ND1 | 1.00 ± 1.02 | 1.23 ± 0.79 | 0.64 |
| ND2 | 1.00 ± 1.16 | 1.32 ± 0.72 | 0.53 |
| SDHa | 1.00 ± 0.37 | 1.06 ± 0.53 | 0.80 |
| Cox2 | 1.00 ± 0.92 | 1.37 ± 0.86 | 0.44 |
| Catalase | 1.00 ± 0.91 | 0.56 ± 0.32 | 0.22 |
| MnSOD | 1.00 ± 0.84 | 0.74 ± 0.32 | 0.45 |
| Sirt1 | 1.00 ± 1.04 | 0.92 ± 0.76 | 0.86 |
| Sirt3 | 1.00 ± 0.46 | 1.93 ± 1.36 | 0.10 |
| NMNAT | 1.00 ± 0.38 | 1.34 ± 0.38 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daussin, F.N.; Cuillerier, A.; Touron, J.; Bensaid, S.; Melo, B.; Al Rewashdy, A.; Vasam, G.; Menzies, K.J.; Harper, M.-E.; Heyman, E.; et al. Dietary Cocoa Flavanols Enhance Mitochondrial Function in Skeletal Muscle and Modify Whole-Body Metabolism in Healthy Mice. Nutrients 2021, 13, 3466. https://doi.org/10.3390/nu13103466
Daussin FN, Cuillerier A, Touron J, Bensaid S, Melo B, Al Rewashdy A, Vasam G, Menzies KJ, Harper M-E, Heyman E, et al. Dietary Cocoa Flavanols Enhance Mitochondrial Function in Skeletal Muscle and Modify Whole-Body Metabolism in Healthy Mice. Nutrients. 2021; 13(10):3466. https://doi.org/10.3390/nu13103466
Chicago/Turabian StyleDaussin, Frédéric Nicolas, Alexane Cuillerier, Julianne Touron, Samir Bensaid, Bruno Melo, Ali Al Rewashdy, Goutham Vasam, Keir J. Menzies, Mary-Ellen Harper, Elsa Heyman, and et al. 2021. "Dietary Cocoa Flavanols Enhance Mitochondrial Function in Skeletal Muscle and Modify Whole-Body Metabolism in Healthy Mice" Nutrients 13, no. 10: 3466. https://doi.org/10.3390/nu13103466
APA StyleDaussin, F. N., Cuillerier, A., Touron, J., Bensaid, S., Melo, B., Al Rewashdy, A., Vasam, G., Menzies, K. J., Harper, M.-E., Heyman, E., & Burelle, Y. (2021). Dietary Cocoa Flavanols Enhance Mitochondrial Function in Skeletal Muscle and Modify Whole-Body Metabolism in Healthy Mice. Nutrients, 13(10), 3466. https://doi.org/10.3390/nu13103466








