Abstract
The increased incidence of obesity, diabetes mellitus, aging, and associated comorbidities indicates the interplay between genetic and environmental influences. Several dietary components have been identified to play a role in the pathogenesis of the so-called “modern diseases”, and their complications including advanced glycation end products (AGEs), which are generated during the food preparation and processing. Diet-derived advanced glycation end products (dAGEs) can be absorbed in the gastrointestinal system and contribute to the total body AGEs’ homeostasis, partially excreted in the urine, while a significant amount accumulates to various tissues. Various in vitro, in vivo, and clinical studies support that dAGEs play an important role in health and disease, in a similar way to those endogenously formed. Animal studies using wild type, as well as experimental, animal models have shown that dAGEs contribute significantly to the pathogenesis of various diseases and their complications, and are involved in the changes related to the aging process. In addition, they support that dAGEs’ restriction reduces insulin resistance, oxidative stress, and inflammation; restores immune alterations; and prevents or delays the progression of aging, obesity, diabetes mellitus, and their complications. These data can be extrapolated in humans and strongly support that dAGEs’ restriction should be considered as an alternative therapeutic intervention.
1. Introduction
The increased incidence of various “modern” diseases such as obesity, insulin resistance, diabetes mellitus, and their complications, as well as the normal aging process and its related morbidities, indicates the interplay between genetic and environmental influences.
A large body of evidence supports that advanced glycation end products (AGEs) are involved in the pathogenesis of those diseases and their complications [1,2,3,4,5]. In addition, it is now largely supported that diet-derived AGEs (dAGEs) play an important role as well [1,2,3,4,5].
Methods of food preparation and processing have a significant effect on the generation of diverse unstable carbonyl derivatives of glyco- and lipoxidation reactions, which can be absorbed in the gastrointestinal system and partially excreted in urine [1,2,3,4,5]. The socioeconomic changes in the past 50 years and new technologies employed in the mass production of food, including heat, dehydration, ionization, irradiation, and flavor, have led to the production of various toxic substances in modern foodstuffs, including dAGEs [6,7,8,9].
The increased consumption of dAGEs contributes to the total AGEs’ body burden, in a similar way to those endogenously formed, and leads to suppression of host defenses and induction of intracellular reactive oxygen species, which can shift basal oxidative stress and lead to inflammation, insulin resistance, obesity, β-cell dysfunction, impaired insulin secretion and insulin resistance, and diabetes, as well as their comorbidities, and mediate the aging process (Figure 1).
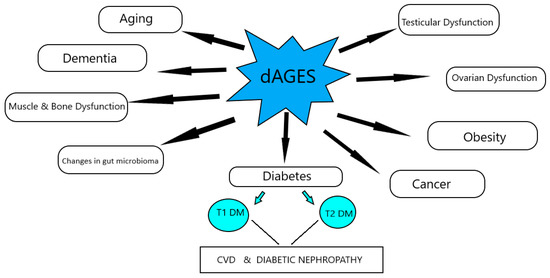
Figure 1.
Effects of dAGEs on health and disease, according to animal studies.
Studies using wild type, as well as experimental, animal models have been very important to clarify the role of dAGEs in various pathologies. These studies emphasize that dAGEs’ restriction reduces basal oxidative stress and inflammation; restores host defenses and increases life span; and prevents or improves dementia, type 1 and type 2 diabetes mellitus, renal disease, and their complications [3].
In this chapter, we will review data emerging from animal studies supporting the role of dAGEs in health and disease.
2. Dietary AGEs’ Homeostasis—Animal Studies
Kinetic studies in normal rats showed that about 10% of dAGEs are absorbed via the gastrointestinal tract. The major "exit" of dAGEs is the kidney, by filtration or by active uptake and secretion, two processes that result in the net excretion of AGEs in the urine [10,11]. Increased dAGEs’ uptake or decreased renal AGEs’ clearance can potentially lead to accumulation of prerenal AGEs and oxidative stress in both renal and extrarenal tissues [10,11]. It has been estimated that two-thirds of dAGEs remain in contact with tissues for 72 h, and that this rate is greater than that of those that are endogenously formed. The actual effect of sustained exposure to dAGEs was evaluated in a series of studies in mice. As described later, several animal studies provide a new framework and an experimental setting from which to revisit the pathogenesis and treatment of various pathologies.
Animal studies using wild type, as well as different experimental, animals of diabetes, obesity, insulin resistance, aging, and anti-AGEs agents (Table 1) showed that dAGEs are absorbed, contribute to the total AGEs’ homeostasis, and are implicated in the pathogenesis of various diseases and their complications, in a similar way to their endogenously formed counterparts. The existing studies have used parenteral administration of AGEs mixtures or high AGEs (H-AGEs) and low AGEs (L-AGEs) diets. Both diets were nutritionally equivalent and were pelleted by the manufacturer and kept at 4 °C. The H-AGEs diet was exposed to an autoclaving cycle (exposure to vacuum, autoclaving, drying), as per standard procedure, and fortified with supplements to offset heat-depleted micronutrients. The L-AGEs diet was prepared without exposure to heat. The diets differed in the AGEs content, which was 3- to 5-fold lower in the L-AGEs diet, based on direct assessment of AGEs by ELISA, using specific monoclonal antibodies. The data from animal studies were further confirmed by clinical studies [1,2,3,4,5,12] using diets with different amounts of AGEs according to a large database of different foods, which were subjected to standard cooking methods (boiling, broiling, deep-frying, oven-frying, and roasting) [13,14].

Table 1.
Summary of dAGEs-related animal studies.
A significantly reduced intake of dAGEs was achieved by increasing the consumption of fish, legumes, low-fat milk products, vegetables, fruits, and whole grains and by reducing intake of solid fats, fatty meats, full-fat dairy products, and highly processed foods. Based on this database, a number of clinical studies have used H-AGEs diet (>15,000 kU AGEs) and L-AGEs diet (<15,000 kU AGEs) in subjects with different pathologies, such as insulin resistance, diabetes mellitus, chronic kidney disease, aging, and obesity, showing that decreased dAGEs intake improves the diseases and their complications. [13,14].
3. Dietary AGEs’ Effects on Health
Intraperitoneal injections of AGEs in healthy male Sprague–Dawley rats for 16 weeks resulted in impaired insulin secretion, decreased islet preproinsulin gene expression, and a characteristic decline in first-phase insulin secretion in response to glucose. These changes were attributed to the stimulation of the superoxide production within mitochondria and the interruption of several critical sequential steps, including changes in cellular glucose uptake, alteration of cellular calcium flux, and loss of mitochondrial ATP content, prevented by alagebrium [22,39].
In addition, parenteral or oral administration of AGEs in healthy mice leads to a cycle of increased cell and tissue AGEs, oxidative stress, inflammation, and tissue injury similar to the diabetic vascular or renal complications, without hyperglycemia, reversed by anti-AGE agents [31,40,41,42,43,44]. These studies indicate that dAGEs contribute significantly to the total body AGEs burden and induce oxidant stress, impaired insulin secretion, insulin resistance, and inflammation in healthy mice [3,38]. They also demonstrate that these changes can be diminished, and further pathology can be prevented in several mouse models by restricting the intake of dAGEs without reducing energy intake [29,32,33,34,38,45].
4. Dietary AGEs’ Effects in Diabetes Mellitus
4.1. Type 1 Diabetes Mellitus (T1D)
A large body of evidence in experimental animals supports that AGEs and dAGEs are involved in the pathogenesis of T1D, either by a direct effect on the β-cell, causing defective insulin secretion and cell apoptosis, or by induction of systemic inflammation, oxidative stress, and alterations in the immune cell activation [22,29,34,39,46].
Peppa et al. showed that early exposure of NOD mice to increased dAGEs had a role in the pathogenesis of T1D. The mice were exposed to a high-AGEs diet (H-AGEs) and to a nutritionally similar diet with approximately fivefold lower levels of Nε-carboxymethyllysine and methylglyoxal-derivatives (L-AGEs), owing to the shorter heat exposure during processing. The suppressive effects of diabetes under L-AGEs feeding were significant across three generations in founder mice, initiated at 3 or 6 weeks of age, and were extended throughout two generations of offspring, F1 and F2, if kept on the L-AGEs maternal regimen. L-AGEs fed mice exhibited a diabetes-free rate of 86%, greatly increased survival rates by 76% for up to 56 weeks of age, and displayed a delay in disease onset (4-month lag). Survival for L-AGEs fed mice was 76 versus 0% after 44 weeks of H-AGEs fed mice. T1D reduction (33 to 14%) coincided with initiation to the L-AGEs diet during the perinatal period. The greatest disease prevention (14%) was associated with exposure to a low AGEs maternal environment, suggesting that toxic AGEs are transportable via the placenta. However, the protective maternal effect of the L-AGEs diet was reversed after crossing over to the H-AGEs diet, as readily as the normally H-AGEs maternal effect was reversed by the L-AGE diet, when applied at weaning (3 or 6 weeks). These data confirmed the plasticity inherent in this period and reinforced the importance of “dose”, “time”, and “duration” of exposure to toxic factor(s). The majority of mice exposed to the L-AGEs environment maternally or at weaning displayed modest insulitis and no diabetes for 1 year. A milder insulitis and a milder diabetes characterized L-AGEs fed mice that did become diabetic, as compared with those with severely damaged islets and overt diabetes seen in H-AGEs fed mice by 25 weeks [29]. Furthermore, the 16-week-old prediabetic L-AGEs fed NOD mice exhibited near-normal glucose and insulin responses to glucose challenge compared with the typically dysfunctional pattern of age-matched H-AGEs fed mice. Reduced insulitis in L-AGEs versus H-AGEs fed mice was marked by GAD- and insulin-unresponsive pancreatic interleukin (IL)-4-positive CD4+ cells compared with the GAD and insulin-responsive interferon (IFN)-γ positive T cells from H-AGEs fed mice. Spleenocytes from L-AGEs fed mice consisted of GAD- and insulin-responsive IL-10-positive CD4+ cells compared with the IFN-γ positive T-cells from H-AGEs fed mice [29]. A marked suppression of total pancreatic lymphocytes was observed in the L-AGEs fed mice (15-fold) compared with the H-AGEs NOD group fed a regular diet. In addition, the L-AGEs fed mice displayed a pattern of predominantly IL-4-positive CD4+ cells. There were virtually no infiltrates in islets from two generations of mice exposed to L-AGEs nutrients during the fetal stage or early in life. In these mice, there was also a marked unresponsiveness of pancreatic T-cells to β-cell antigens, insulin, and GAD. This might indicate blocked autoreactive T-cell recruitment owing to low local expression of these antigens under a “safer” AGEs-poor diet [29].
Borg et al. studied parental male and female NOD mice that followed a low or high in AGEs diet maintained throughout gestation and lactation. From conception to early postnatal life, an L-AGEs diet decreased circulating AGEs’ concentration in female offspring compared with an H-AGEs diet [24]. Insulin gene, glucagon, AGEs, and the AGEs receptor-1 (AGER-1) expression were greater in islet cells isolated from offspring in the L-AGEs diet group than in age-matched nondiabetic control mice. Insulin, proinsulin, and glucagon secretion were greater and infiltration of islet immune cells was lower in the L-AGEs group [24].
In another study, Borg et al. showed that only one-third of NOD mice on an L-AGEs diet developed T1D. In addition, the incidence of T1D decreased to less than 15% over the next two generations. The AGEs-restrictive NOD mice had minimal insulitis, while the NOD mice fed an H-AGEs diet had the typical hyperresponsive, interferon-γ-positive, interleukin-4-negative islet T cells that do not respond to common islet antigens such as glutamic acid decarboxylase or insulin [22,24].
Therefore, high AGEs intake may provide excess antigenic stimulus for T-cell-mediated diabetes or direct β-cell injury in NOD mice being ameliorated by maternal or neonatal exposure to L-AGEs nutrition. The above data indicate that dAGEs are involved in the pathogenesis of T1D [29]. Dietary AGEs restriction results in suppression of islet infiltration by β-cell cytotoxic T-cells and islet toxicity, significantly delayed onset, reduced severity of disease, and marked increase in overall survival [29,38]. However, future studies are required to define how exposure to excess AGEs specifically contributes to T1D development and autoimmunity, and whether this is via direct or indirect pathways. Taken together, the existing data suggest dAGEs as a modifiable risk factor and therapeutic target for the prevention of T1D.
4.2. Type 2 Diabetes Mellitus (T2D)
AGEs have been linked to the pathogenesis of insulin resistance (IR), which is the main underlying mechanism of type 2 diabetes (T2D) [47]. A large body of experimental evidence shows that dAGEs are also involved in the development of IR.
Hofmann et al. showed that already obese and diabetic C57/BL/KsJ db/db mice fed with L-AGEs diet ceased to gain weight within 10 weeks of study and, by 20 weeks, they exhibited a significant weight loss and better glycemic profile. L-AGEs fed mice exhibited lower fasting insulin levels, a nearly complete restoration of glycemic response to intravenous glucose, and insulin tolerance tests, indicating a well-preserved pancreatic islet morphology and function [33,48]. L-AGEs fed mice showed an improved glucose uptake in abdominal adipose tissue and increased HDL cholesterol levels [33]. This study supported that dAGEs contribute to the pathogenesis of IR and dietary AGE restriction seems to be effective in the prevention of this process [33].
Sandu et al. showed that, after 6 months on high-fat (HF) and H-AGEs (H-AGEs-HF) diet, 75% of normal C57/BL6 mice were diabetic and exhibited higher body weight, fasting glucose, insulin, serum AGEs, impaired glucose and insulin responses during glucose tolerance tests, euglycemic and hyperglycemic clamps, altered pancreatic islet structure and function, higher plasma 8-isoprostanes, and lower adiponectin levels, compared with those on L-AGEs-HF diet. This group also showed more visceral fat (by two- and fourfold) and more AGEs modified fat (by two- and fivefold) than the L-AGEs-HF and control mice, respectively, despite similar dietary fat intake and body weight gain by both groups. None of the mice on the L-AGEs-HF diet were diabetic, despite a similar rise in body weight and plasma lipids. This study emphasizes the protective role for a low-AGEs diet, even if the fat content is elevated [34].
Cai et al. performed a study in four generations of F3 mice that exhibit increased adiposity and premature IR, marked by severe deficiency of AGEs receptor 1 (AGER-1) and of survival factor sirtuin 1 in white adipose tissue, skeletal muscle, and liver. The mice were fed isocaloric diets either with or without AGEs (H-AGEs and L-AGEs, respectively) [35]. F3-H-AGEs mice exhibited increased adiposity, glucose intolerance, hyperinsulinemia, elevated leptin, and decreased adiponectin levels, and appeared prematurely, namely 6 months before its onset in control mice and 12 months before onset in F3-LAGEs mice. They also showed increased lipids, enriched with AGEs, expanded adipose tissue, a marked increase in NF-κB acetylated-p65 in adipocytes, and high TNF-α and CD11c in peritoneal macrophages, as well as suppressed AGES receptor 1, survival factor sirtuin 1, PPARγ, IL-10, and CD26 mRNA levels, compared with the control mice. This study indicates that the increased dAGEs produced in thermally treated food can profoundly alter the inflammation/insulin axis [35].
Mastrocola et al. have shown that diets high in AGEs promote liver AGEs’ accumulation, affecting liver enzymes’ production and action, leading to high plasma levels of the sphingolipid intermediates (e.g., ceramide and sphingosine-1-phosphate) and IR [18]. This was further supported by the beneficial effects of pyridoxamine supplementation, which diminished hepatic AGEs and prevented alterations of sphingolipid metabolism and the development of IR in H-AGEs fed FD mice [18].
Verboven et al. showed that Sprague–Dawley rats fed with a “Western” diet for 18 weeks exhibited increased AGEs levels, dyslipidemia, and hyperglycemia in combination with altered heart function, suggesting that a “Western” diet that is rich in AGEs might be a cause of DM and cardiovascular disease, which often present together [19].
4.3. Diabetic Complications
4.3.1. Cardiovascular Disease
Several studies in experimental animals have shown that dAGEs play an important role in the pathogenesis of micro- and macroangiopathy, in a similar way to those endogenously formed.
Lin et al. showed that genetically hypercholesterolemic apolipoprotein E-deficient (apoE−/−); streptozotocin-induced diabetic mice fed with an L-AGEs diet exhibited lower AGEs levels; 50% smaller lesions at the aortic root; markedly suppressed tissue AGEs, AGE-R- 1, AGER- 2, and AGER expression; and reduced numbers of inflammatory cells, tissue factor, vascular cell adhesion molecule-1, and MCP-1 levels. This study indicated an important link between dAGEs, tissue AGEs’ accumulation, and diabetes-related vascular disease, and supported a beneficial role of dAGEs’ restriction [49]. Furthermore, Lin et al. showed that apoE−/−, streptozotocin-induced diabetic mice, on an L-AGEs diet, showed a significant decrease in circulating AGEs levels, intimal formation, neointimal area, intima/media ratio, and stenotic luminal area, at 4 weeks after femoral artery injury. A marked reduction (56%) in macrophages in the neointimal lesions, as well as an obvious reduction in smooth muscle cell content, and a reduced deposition of AGEs in the endothelia, SMC, and macrophages in neointimal lesions, were also observed [50].
Deng et al. investigated male apoE−/− mice, divided into four groups based on the feeding periods (0, 2, 4, and 6 months), and further divided them into three subgroups corresponding to the following diet treatments: regular diet, high-fat diet + normal saline injection, and high-fat diet + aminoguanidine injection [17]. A high fat diet, meaning high AGEs intake, was associated with increased total and low-density cholesterol levels, transaortic valve velocity, calcification, and AGEs’ deposition around the aortic valve surfaces, mediated through AGEs’ interaction with AGER-1 and the counteraction of BMPR2 and TGF-β1 signaling. These changes were alleviated and attenuated with the AGE inhibitor aminoguanidine, which inhibited the AGEs’ interaction with AGER-1 [17].
Haesen et al. showed that adult male Sprague–Dawley rats, under daily injections with high molecular weight AGEs, for six weeks, showed intracardiac pressure overload, characterized by increased systolic and mean pressures, increased contraction response in aortic rings to phenylephrine, impaired relaxation in response to acetylcholine, increased collagen deposition, and intima-media thickness of the aortic vessel wall, compared with the control mice group [51]. This study showed an important contribution of high molecular weight AGEs, which are present in the Western diet, in the development of cardiovascular disease.
Peppa et al. showed that female db/db mice, after 3 months on an L-AGEs diet, displayed lower circulating and skin AGEs deposits, increased epithelialization, angiogenesis, inflammation, granulation tissue deposition, enhanced collagen organization, and more rapid wound closure time, compared with H-AGEs fed mice. This study suggests that dAGEs’ restriction might improve impaired diabetic wound healing [30,52].
Verboven et al. showed that Sprague–Dawley rats fed a “Western” diet for 18 weeks exhibited increased AGEs levels and altered heart function with increased end-diastolic pressure and left ventricle hypertrophy, in addition to hyperglycemia and dyslipidemia. This study highlights the importance of the “Western” diet, a diet rich in AGEs, as a cause of cardiovascular disease and DM [19].
4.3.2. Diabetic Nephropathy
Many studies support the role of AGEs in diabetic nephropathy development and progression to end stage renal disease. Among them, animal studies give some impressive results for dAGEs.
L- AGEs fed NOD mice showed lower serum and renal AGEs, a minimal glomerular pathology, a modest increase in urine albumin/creatinine ratio, and significantly prolonged survival, compared with the H-AGEs fed mice [38].
Yuan et al. showed that intraperitoneal injections of a high-fat, high-sugar diet in streptozocin induced male Sprague-Dawley diabetic rats resulted in strong staining of Oil Red O in the renal tubules, increased serum and renal AGEs’ deposition, upregulation of both mRNA and protein expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase, LDL receptor, sterol regulatory element binding protein-2, and decreased urine protein and u-NGAL. It is noteworthy that these changes were reversed or prevented by the simultaneous administration of aminoguanidine and anti-AGER agents [23]. This study indicates, in addition to the harmful effects of an H-AGEs diet, that AGEs induce DN via disruption of intracellular feedback regulation of cholesterol, while an anti-AGEs strategy decreases lipid accumulation, offering a renoprotective role in the progression of DN [23].
5. Dietary AGEs’ Effects on Obesity
AGEs are involved in the pathogenesis of obesity, and dAGEs seem to also be involved in a similar manner.
Rajan et al., using a proteome-cytokine array kit, evaluated 40 circulatory proinflammatory cytokines and chemokines in experimental Swiss albino mice fed with H-AGEs and regular diet in the presence and absence of curcumin and gallic acid for 6 months. He showed that H-AGEs fed mice displayed increased body weight. In addition, five cytokines (IL-16, IL-1α, ICAM, TIMP-1, and C5a) were found to be highly expressed (3.5-fold), eleven cytokines were moderately expressed (2-fold), three chemokines (BLC, SDF-1 and MCP-1) were highly expressed (4-fold), and ten showed moderate expression (2-fold), in H-AGEs fed mice compared with control mice. These effects were prevented by the co-treatment with curcumin and gallic acid. This study indicates that dAGEs induce obesity and systemic inflammation, which are rather parallel phenomena [16].
Sandu et al. showed that, after 6 months on an HF and H-AGEs (H-AGEs-HF) diet, 75% of normal C57/BL6 mice exhibited higher body weight, in addition to changes in glucose homeostasis, compared with an HF and L-AGEs diet, indicating the weight promoting effect of increased dAGEs’ consumption [34].
Hofmann et al. showed that already obese and diabetic C57/BL/KsJ db/db mice fed an L-AGEs diet ceased to gain weight within 10 weeks of study and, by 20 weeks, they exhibited a significant weight loss and better glycemic profile, compared with the H-AGEs fed mice. This study supported that dAGEs contribute to the pathogenesis of obesity in combination with other components of the metabolic syndrome [33].
6. Dietary AGEs Effects on Aging
Studies have shown that AGEs are involved in the aging process and its complications, and the same seems to be the case for dAGES.
Cai et al. showed that C57BL6 male mice on a lifelong exposure to an L-AGEs diet exhibited higher than baseline tissue AGER-1 levels and glutathione/oxidized glutathione ratio, as well as decreased plasma 8-isoprostanes, tissue AGER-1, and p66shc levels, compared with the control group. This was associated with decreased systemic AGEs’ accumulation, amelioration of IR, albuminuria, glomerulosclerosis, and extended lifespan. This study showed that dAGEs’ restriction may preserve from life-long exposure to high levels of glycoxidants that exceed AGER-1 and antioxidant reserve capacity, resulting in decreased tissue damage and a longer lifespan [38].
In another study, Cai et al. showed that pair-fed mice on a calorie-restricted H-AGEs diet developed high levels of 8-isoprostanes, AGEs, AGER-1, and p66shc, coupled with low AGER-1 and glutathione/oxidized glutathione ratio, IR, marked myocardial and renal fibrosis, and shortened lifespan, compared with those on a calorie-restricted diet alone. This study showed that H-AGEs intake competed against the benefits of calorie-restriction and that healthspan seems to be increased by calorie-restriction in terms of consumption of foods containing L-AGEs [36].
7. Dietary AGEs’ Effects on Dementia
Dementia has been associated with increased oxidant stress and AGEs [53,54,55], while dAGEs seem to play an important role as well.
Cai et al. studied MG+/MG− mice exposed throughout life to an L-AGEs diet (MG-), a supplemented AGEs diet (MG+), and regular chow. The MG+ fed mice developed metabolic syndrome, increased brain amyloid-β42 and AGEs’ deposits, gliosis, and cognitive deficits, accompanied by suppressed SIRT1, nicotinamide phosphoribosyl transferase, AGER-1, and PPARγ, changes that seem to be prevented in L-AGEs fed mice. This study indicates the importance of dAGEs’ restriction in the development and progression of dementia [53].
Huang et al., using healthy male mice on different diets (a typical diet and free drinking, a standard diet and 300 mg/L lead acetate solution, and a high-fat diet and free drinking) for 10 weeks, showed that the high-fat fed mice exhibited lipid metabolism disorders and impaired cognitive function and central nervous system. This was mediated by promoting the secretion of inflammatory factors in glial cells, inducing the inflammatory reaction of brain tissue, inhibiting glutathione antioxidant status, and increasing the AGEs’ content, which further aggravates the injury of brain tissue [25].
Maciejczyk et al. studied male Wistar rats on high and standard protein diets for 8 weeks. The mice on a high protein diet showed higher uric acid concentration and activity of the glutathione hyperoxidase system, catalase, and lower glutathione levels in the hypothalamus, whereas in the cerebral cortex, these parameters remained unchanged. Both brain structures expressed a higher content of 4-hydroxynonenal and malondialdehyde and increased AGEs in the hypothalamus. The observed differences among the studied brain compartments suggest that the hypothalamus is more susceptible to oxidant stress caused by a high protein diet rich in AGEs [26].
8. Dietary AGEs’ Effects on Muscle and Bone
Diets enriched with AGEs have recently been related to muscle and bone dysfunction processes [56].
Egawa et al. showed that mice on an H-AGEs diet for 16 weeks exhibited higher levels of Nε -(carboxymethyl)-L-lysine, a marker for AGEs, in extensor digitorum longus muscles, as well as lower muscle strength and endurance and decreased mRNA expression levels of myogenic factors, including myogenic factor 5 and myogenic differentiation, in extensor digitorum longus muscle, compared with L-AGEs fed mice. This study indicates that long-term exposure to an increased dAGEs’ consumption disrupts the skeletal muscle growth and contractile muscle function owing to the inhibition of myogenic potential and protein synthesis [56].
IIIien-Junger et al. studied female and male mice on L-AGEs and H-AGEs diets, for 6 and 18 months. The H-AGEs fed female mice exhibited increased AGEs levels in serum and cortical vertebrae, an inferior vertebral trabecular structure with decreased bone mineral density and bone volume fraction, functional deterioration with reduced shear and compression moduli, and maximum load to failure after 6 months. At 18 months, H-AGEs fed mice of both sexes had significant, but small decreases in cortical BMD and thickness, and functional biomechanical behaviors compatible with those observed in aging mice. This study showed that increased dAGEs’ consumption without morbidities such as diabetes or obesity have an adverse effect on vertebral microstructure, mechanical behaviors, and fracture resistance in young female mice, in a manner suggesting accelerated bone aging [28].
9. Dietary AGEs’ Effects on the Gut
Qu et al. showed that Sprague-Dawley rats on an H-AGEs diet for 6, 12, or 18 weeks exhibited a reduction in the diversity and richness of the microbiota, especially saccharolytic bacteria such as Ruminococcaceae and Alloprevotella, which can produce small chain fatty acids (SCFAs), whereas some putatively harmful bacteria (Desulfovibrio and Bacteroides) were increased [27].
Mao et al. studied hyperlipidemic rats fed diets consisting of fish protein, 6% low level glycated fish protein, and 12% high level glycated fish protein for 4 weeks. The mice fed with the low-level glycated fish protein displayed significantly changed protein fermentation and reduced inflammation markers and blood lipids, but increased AGEs’ plasma accumulation and fecal excretion and butyrate producing Ruminococcus_1 and Roseburia [21]. Furthermore, the high level of glycated fish protein diet significantly decreased Ruminiclostridium_6, Desulfovibrio, Helicobacter, and Lachnospiraceae groups. These changes reflect the effect of dAGEs on the the modulation of gut microbiota composition and fermentation metabolite profiles, emphasizing the beneficial effect of L-AGEs on gut health [21,57].
10. Dietary AGEs’ Effects on Reproductive Function
AGEs have been involved in various parts of reproductive function in both males or females, and dAGEs seem to play an important role as well.
Thornton et al. showed that mice fed an H-AGEs diet spent a significantly longer time in the diestrus phase, had a similar number of oocytes released following ovarian superovulation, showed increasing mRNA expression in genes involved in steroidogenesis (Star) and folliculogenesis, showed increased expression of macrophage markers in the ovary, and had significantly fewer corpora lutea, compared with the L-AGEs group [15]. This study showed that increased dAGEs’ consumption impacts fertility and, in general, ovarian function, regardless of obesity or other morbidities [15].
Kandaraki et al. studied normal nonandrogenized and androgenized prepubertal rats, fed H-AGEs and L-AGEs diets. She showed that H-AGEs fed mice had lower ovarian glyoxalase-1 activity, compared with L-AGEs and control groups. In addition, ovarian glyoxalase-1 activity was positively correlated with body weight gain as well as estradiol and progesterone levels, and negatively correlated with AGEs’ expression in the ovarian granulosa cell layer of all groups [37]. This study indicated that ovarian tissue seems to be a new target of AGEs’ deposition, leading to hyperandrogenic disorders such as PCOS.
Janšáková et al. studied pregnant rats fed a standard or an AGEs-rich diet from gestation day 1, while a third group received a standard diet and AGEs (CML) via gavage. Rats fed an H-AGEs diet exhibited higher plasma AGEs levels. However, this study does not support the speculation that dAGEs might contribute to the pathogenesis of pregnancy complications, and it cannot be speculated whether this is the cause or result, but the short duration of the rodent gestation warrants further studies analyzing the long-term effects of AGEs in preconception nutrition [58].
Regarding testicular function, increased dAGEs’ intake resulted in increased serum AGEs and histopathological damage in the testes and epididymides, a decreased total number of epididymal sperm, and an increased abnormal sperm rate AGER-1 and malondialdehyde levels, compared with the control group. These changes were restored after administration of silymarin, a natural AGEs inhibitor, further emphasizing the role of dAGEs in testicular health [20].
Akbarian et al. studied twenty-five 4-week-old C57BL/6 mice, fed diets differing in fat content (control, 45% high fat, 60% high fat, 45% high fat/AGEs, 60% high fat/AGEs). After 18 months, sperm motility, DNA fragmentation, and protamine deficiency, as well as membrane and cytoplasmic peroxidation, were negatively affected by the high fat and high fat/AGEs diets, in addition to changes in glucose and insulin homeostasis [59].
11. Dietary AGEs Effects on Cancer
AGEs have been associated with tumor development either directly through their increased formation because of increased glucose uptake and glycolysis or indirectly through insulin resistance, oxidative stress, and chronic inflammation induction.
Van Heijst et al. studied the presence of AGEs in human cancer tissues and detected the presence of the AGEs Nε-(carboxymethyl) lysine) (CML) and argpyrimidine in several human tumors using specific antibodies, suggesting that AGEs could be implicated in the biology of human cancer [60]. Jiao et al., in a study of 2193 pancreatic cancer cases (1407 men and 786 women) with a 10.5-year follow-up, found that men in the highest quintile of red meat consumption had a higher risk of pancreatic cancer (HR: 1.35; 95% CI: 1.07, 1.70), which attenuated after adjustment for CML-AGE consumption (HR: 1.20; 95% CI: 0.95, 1.53) [22]. This study suggested that consumption of meat that is enriched in Nε-(carboxymethyllysine)-AGE might contribute to pancreatic cancer through mechanisms other than meat mutagens, such as increased AGEs’ formation [61]. There a potential mechanistic link reported between carbohydrate-derived metabolites and cancer, which may provide a biologic consequence of lifestyle that can directly affect tumor biology. Failure due to AGEs can lead to protein damage, aberrant cell signaling, increased stress responses, and decreased genetic fidelity [62].
12. Limitations of Animal Studies in dAGEs
Animal studies have many limitations in general. In particular, the animal studies that have investigated dAGEs’ effects in health and disease present a significant heterogenicity in study design, dietary patterns related to the AGEs’ content, AGEs’ measurements, and the presentation of results. In addition, the existing studies estimate some of the many AGEs compounds, although εN-carboxymethyl-lysine and methylglyoxal are good representatives, as they are increased in various pathologies and associated with markers of health and disease. The short follow-up duration is considered to be a problem, as the adverse consequences of an H-AGEs diet may arise over a period of years rather than weeks or months. However, the fact that H-AGEs and L-AGEs diets were associated with markers of disease in various experimental animal models support their role in various pathologies.
Furthermore, the existing food AGEs database also has limitations, including the fact that foods were selected from diets common in the U.S. population, and thus may not represent the international average. Another limitation is that only two of many AGEs were measured. However, the fact that both diets were associated with markers of disease in healthy subjects and were elevated in patients with diabetes and kidney disease lends credibility to their role as pathogens in foods consumed by the public and persons with certain chronic diseases.
There are also conflicting reports that present evidence against an association between intake of dAGEs and their presence in the body or their real pathological effects [63,64].
There are also studies supporting that dAGEs have no systemic effects. Semba et al. found that a high- or low-AGEs diet had no significant impact on peripheral arterial tonometry or any inflammatory mediators after 6 weeks of dietary intervention [65]. Kellow et al. concluded that there is insufficient evidence to recommend dietary AGEs’ restriction for the alleviation of the proinflammatory milieu in healthy individuals and patients with diabetes or renal failure, limiting the conclusions that can be drawn [66].
Future studies should continue to expand the dAGEs database and investigate additional methods for reducing AGEs’ generation during home cooking and food processing.
13. Conclusions
A large body of evidence from experimental in vivo, in vitro, as well as clinical studies have indicated the important role of AGEs in the pathogenesis of various pathologies and their complications. Animal studies, as well as in vitro and clinical data, further support the role of dAGEs in health and disease. The animal studies that have investigated the dAGEs’ effects on health and disease, besides the general limitations of this kind of study, presented a significant heterogenicity in study design, AGEs’ assessment, and the presentation of results, which makes it difficult to perform a metanalysis. Dietary AGEs intake was poorly reported in several articles. Moreover, it is likely that there may have been differences other than the AGEs’ content between interventions. Finally, the included studies had a relatively short follow-up duration. The adverse consequences of an H-AGEs diet may arise over a period of years rather than weeks or months, as AGEs accumulate, glycate other proteins, or are incorporated into tissues. Therefore, studies of longer duration are required. However, the fact that H-AGEs and L-AGEs diets were associated with markers of disease in various experimental models support their role in various pathologies.
In addition, the existing food database also has limitations, including the fact that foods were selected from diets common in the U.S. population, and thus may not represent the international average. Another limitation is that only two of many AGEs were measured. However, the fact that both diets were associated with markers of disease in healthy subjects and were elevated in patients with diabetes and kidney disease lends credibility to their role as pathogens in foods consumed by the public and persons with certain chronic diseases. However, ongoing studies are needed to further expand the dAGEs database and investigate additional methods for reducing AGEs’ generation during home cooking and food processing. Future studies should continue to investigate the health effects of AGEs and refine recommendations for safe dietary intakes. However, current data support the need for a paradigm shift that acknowledges that how we prepare and process food may be equally as important as nutrient composition.
However, increased dAGEs’ consumption, which is synonymous with the increased consumption of processed foods in terms of the “modern Western” diet, increases the total body AGEs’ burden. Most, but not all studies support that they are involved in the pathogenesis of various diseases and their complications, in a similar way and independently of those endogenously formed. These data may directly relate to humans, and dAGEs’ restriction seems to be a simple, safe alternative strategy for the prevention of various diseases or the delay of their progression. Ongoing studies are needed to further expand the dAGEs database and investigate additional methods for reducing AGEs’ generation during home cooking and food processing.
Future studies should continue to investigate the health effects of AGEs and refine recommendations for safe dietary intakes. However, current data support the need for a paradigm shift that acknowledges that how we prepare, and process food may be equally as important as nutrient composition.
Author Contributions
M.P. contributed to the paper by writing and editing the review. I.M. contributed by writing—original draft preparation. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peppa, M.; Raptis, S.A. Advanced glycation end products and cardiovascular disease. Curr. Diabetes Rev. 2008, 4, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Uribarri, J.; Vlassara, H. Aging and glycoxidant stress. Hormones 2008, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; Vlassara, H. Advanced glycation end products and diabetic complications: A General overview. Hormones 2005, 4, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.; Morrissey, P.A.; Ames, J.M. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 211–248. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Nonenzymatic Food Browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Tessier, F.J.; Niquet, C. The metabolic, nutritional and toxicological consequences of ingested dietary Maillard reaction products: A literature review. J. Soc. Biol. 2007, 201, 199–207. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315. [Google Scholar] [CrossRef]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glyco-toxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major con-tributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.U.; Pyzik, R.; Striker, G.E. AGE’s in Foods and practical ways to reduce them. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Thornton, K.; Merhi, Z.; Jindal, S.; Goldsammler, M.; Charron, M.J.; Buyuk, E. Dietary Advanced Glycation End Products (AGEs) could alter ovarian function in mice. Mol. Cell. Endocrinol. 2020, 510, 110826. [Google Scholar] [CrossRef]
- Sowndhar Rajan, B.; Manivasagam, S.; Dhanusu, S.; Chandrasekar, N.; Krishna, K.; Kalaiarasu, L.P.; Babu, A.A.; Vellaichamy, E. Diet with high content of advanced glycation end products induces systemic inflammation and weight gain in experimental mice: Protective role of curcumin and gallic acid. Food Chem. Toxicol. 2018, 114, 237–245. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, L.; Wang, C.; Wang, S.; Xu, J.; Dong, J.; Kang, Q.; Zhai, X.; Zhao, Y.; Shan, Z. AGEs-RAGE axis causes endothelial-to-mesenchymal transition in early calcific aortic valve disease via TGF-β1 and BMPR2 signaling. Exp. Gerontol. 2020, 141, 111088. [Google Scholar] [CrossRef]
- Mastrocola, R.; Bello, F.D.; Cento, A.S.; Gaens, K.; Collotta, D.; Aragno, M.; Medana, C.; Collino, M.; Wouters, K.; Schalkwijk, C.G. Altered hepatic sphingolipid metabolism in insulin resistant mice: Role of advanced glycation endproducts. Free Radic. Biol. Med. 2021, 169, 425–435. [Google Scholar] [CrossRef]
- Verboven, M.; Deluyker, D.; Ferferieva, V.; Lambrichts, I.; Hansen, D.; Eijnde, B.O.; Bito, V. Western diet given to healthy rats mimics the human phenotype of diabetic cardiomyopathy. J. Nutr. Biochem. 2018, 61, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lin, J.-A.; Lin, H.-T.; Chen, S.-Y.; Yen, G.-C. Potential effect of advanced glycation end products (AGEs) on spermatogenesis and sperm quality in rodents. Food Funct. 2019, 10, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ren, Y.; Zhang, Q.; Dong, S.; Han, K.; Feng, G.; Wu, H.; Zhao, Y. Glycated fish protein supplementation modulated gut microbiota composition and reduced inflammation but increased accumulation of advanced glycation end products in high-fat diet fed rats. Food Funct. 2019, 10, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Yap, F.Y.; Tong, D.C.; Andrikopoulos, S.; Gasser, A.; Thallas-Bonke, V.; Webster, D.E.; Miyazaki, J.I.; Kay, T.W.; Slattery, R.M.; et al. Advanced Glycation End Products Are Direct Modulators of β-Cell Function. Diabetes 2011, 60, 2523–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Sun, H.; Sun, Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: A pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis. 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, D.J.; Yap, F.Y.T.; Keshvari, S.; Simmons, D.; Gallo, L.A.; Fotheringham, A.; Zhuang, A.; Slattery, R.M.; Hasnain, S.; Coughlan, M.; et al. Perinatal exposure to high dietary advanced glycation end products in transgenic NOD8.3 mice leads to pancreatic beta cell dysfunction. Islets 2017, 10, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yu, S.C.; Lo, Y.C.; Lin, I.H.; Tung, T.H.; Huang, S.Y. A high linoleic acid diet exacerbates metabolic responses and gut mi-crobiota dysbiosis in obese rats with diabetes mellitus. Food Funct. 2019, 10, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox Balance, Antioxidant Defense, and Oxidative Damage in the Hypothalamus and Cerebral Cortex of Rats with High Fat Diet-Induced Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Yuan, X.; Zhao, J.; Zhang, Y.; Hu, J.; Wang, J.; Li, J. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Illien-Jünger, S.; Palacio-Mancheno, P.; Kindschuh, W.F.; Chen, X.; Sroga, G.E.; Vashishth, D.; Iatridis, J. Dietary Advanced Glycation End Products Have Sex- and Age-Dependent Effects on Vertebral Bone Microstructure and Mechanical Function in Mice. J. Bone Miner. Res. 2017, 33, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; He, C.; Hattori, M.; McEvoy, R.; Zheng, F.; Vlassara, H. Fetal or Neonatal Low-Glycotoxin Environment Prevents Au-toimmune Diabetes in NOD Mice. Diabetes 2003, 52, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; Brem, H.; Ehrlich, P.; Zhang, J.G.; Cai, W.; Li, Z.; Croitoru, A.; Thung, S.; Vlassara, H. Adverse effects of dietary glycotoxins on wound healing in ge-netically diabetic mice. Diabetes 2003, 52, 2805–2813. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Fuh, H.; Makita, Z.; Krungkrai, S.; Cerami, A.; Bucala, R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc. Natl. Acad. Sci. USA 1992, 89, 12043–12047. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; Vlassara, H. Reduced Oxidant Stress and Extended Lifespan in Mice Exposed to a Low Glycotoxin Diet: Association with Increased AGER1 Expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.; Dong, H.H.; Li, Z.; Cai, W.; Altomonte, J.; Thung, S.N.; Zeng, F.; Fisher, E.; Vlassara, H. Improved Insulin Sensitivity Is Associated With Restricted Intake of Dietary Glycoxidation Products in the db/db Mouse. Diabetes 2002, 51, 2082–2089. [Google Scholar] [CrossRef] [Green Version]
- Sandu, O.; Song, K.; Cai, W.; Zheng, F.; Uribarri, J.; Vlassara, H. Insulin Resistance and Type 2 Diabetes in High-Fat-Fed Mice Are Linked to High Glycotoxin Intake. Diabetes 2005, 54, 2314–2319. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Zheng, F.; Striker, G.E.; Vlassara, H. Oral Glycotoxins Determine the Effects of Calorie Restriction on Oxidant Stress, Age-Related Diseases, and Lifespan. Am. J. Pathol. 2008, 173, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced Ovarian Glyoxalase-I Activity by Dietary Glycotoxins and Androgen Excess: A Causative Link to Polycystic Ovarian Syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; He, C.; Cai, W.; Hattori, M.; Steffes, M.; Vlassara, H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes/Metab. Res. Rev. 2002, 18, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Kanatsuna, T.; Nakamura, N.; Kitagawa, Y.; Mori, H.; Kajiyama, S.; Nakano, K.; Kondo, M. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes 1986, 35, 486–490. [Google Scholar] [CrossRef]
- Vlassara, H.; Striker, L.J.; Teichberg, S.; Fuh, H.; Li, Y.M.; Steffes, M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc. Natl. Acad. Sci. USA 1994, 91, 11704–11708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onorato, J.M.; Jenkins, A.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine, an Inhibitor of Advanced Glycation Reactions, Also Inhibits Advanced Lipoxidation Reactions. J. Biol. Chem. 2000, 275, 21177–21184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, T.O.; Alderson, N.L.; Chachich, M.E.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: Evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J. Biol. Chem. 2003, 278, 42012–42019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolffenbuttel, B.H.; Boulanger, C.M.; Crijns, F.R.L.; Huijberts, M.S.P.; Poitevin, P.; Swennen, G.N.M.; Vasan, S.; Egan, J.J.; Ulrich, P.; Cerami, A.; et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 4630–4634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, T.P.; Alderson, N.L.; Arrington, D.D.; Beattie, R.J.; Basgen, J.M.; Steffes, M.W.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002, 61, 939–950. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against Loss of Innate Defenses in Adulthood by Low Advanced Glycation End Products (AGE) Intake: Role of the Antiinflammatory AGE Receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, H.; Nakatani, Y.; Kawamori, D.; Miyatsuka, T.; Matsuoka, T.-A. Involvement of Oxidative Stress and the JNK Pathway in Glucose Toxicity. Rev. Diabet. Stud. 2004, 1, 165. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Helzlsouer, K.J.; Alberg, A.J.; Huang, H.Y.; Hoffman, S.C.; Strickland, P.T.; Brock, J.W.; Burse, V.W.; Needham, L.L.; Bell, D.A.; Lavigne, J.A.; et al. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol. Biomark. Prev. 1999, 8, 525–532. [Google Scholar]
- Lin, R.-Y.; Choudhury, R.P.; Cai, W.; Lu, M.; Fallon, J.; Fisher, E.; Vlassara, H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2003, 168, 213–220. [Google Scholar] [CrossRef]
- Lin, R.-Y.; Reis, E.D.; Dore, A.T.; Lu, M.; Ghodsi, N.; Fallon, J.; Fisher, E.; Vlassara, H. Lowering of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis 2002, 163, 303–311. [Google Scholar] [CrossRef]
- Haesen, S.; Cöl, Ü.; Schurgers, W.; Evens, L.; Verboven, M.; Driesen, R.B.; Bronckaers, A.; Lambrichts, I.; Deluyker, D.; Bito, V. Glycolaldehyde-modified proteins cause adverse functional and structural aortic remodeling leading to cardiac pressure overload. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Stavroulakis, P.; Raptis, S.A. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009, 17, 461–472. [Google Scholar] [CrossRef] [PubMed]
- West, R.K.; Moshier, E.; Lubitz, I.; Schmeidler, J.; Godbold, J.; Cai, W.; Uribarri, J.; Vlassara, H.; Silverman, J.M.; Beeri, M.S. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech. Ageing Dev. 2014, 140, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-H.; Xie, J.-Z.; Jiang, X.; Lv, B.-L.; Cheng, X.-S.; Du, L.-L.; Zhang, J.-Y.; Wang, J.-Z.; Zhou, X.-W. Methylglyoxal Induces Tau Hyperphosphorylation via Promoting AGEs Formation. NeuroMol. Med. 2012, 14, 338–348. [Google Scholar] [CrossRef]
- Li, J.J.; Dickson, D.; Hof, P.R.; Vlassara, H. Receptors for advanced glycosylation endproducts in human brain: Role in brain ho-meostasis. Mol. Med. 1998, 4, 46–60. [Google Scholar] [CrossRef]
- Egawa, T.; Tsuda, S.; Goto, A.; Ohno, Y.; Yokoyama, S.; Goto, K.; Hayashi, T. Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. Br. J. Nutr. 2017, 117, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dongen, K.C.; Linkens, A.M.; Wetzels, S.M.; Wouters, K.; Vanmierlo, T.; van de Waarenburg, M.P.; Scheijen, J.L.; de Vos, W.M.; Belzer, C.; Schalkwijk, C.G. Dietary advanced glycation endproducts (AGEs) increase their concentration in plasma and tissues, result in inflammation and modulate gut microbial composition in mice; evidence for reversibility. Food Res. Int. 2021, 147, 110547. [Google Scholar] [CrossRef] [PubMed]
- Janšáková, K.; Lengyelová, E.; Pribulová, N.; Somoza, V.; Celec, P.; Šebeková, K.; Ostatníková, D.; Tóthová, Ľ. Metabolic and Renal Effects of Dietary Advanced Glycation end Products in Pregnant Rats—A Pilot Study. Physiol. Res. 2019, 68, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, F.; Rahmani, M.; Tavalaee, M. Effect of Different High-Fat and Advanced Glycation End-Products Diets in Obesity and Diabetes-Prone C57BL / 6 Mice on Sperm Function. Int. J. Fertil. Steril. 2021, 15, 226–233. [Google Scholar]
- Van Heijst, J.W.J.; Niessen, H.W.M.; Hoekman, K.; Schalkwijk, C.G. Advanced glycation end products in human cancer tissues: Detection of Nε-(carboxymethyl)lysine and argpyrimidine. Ann. N. Y. Acad. Sci. 2005, 1043, 725–733. [Google Scholar] [CrossRef]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2014, 101, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.P. Advanced Glycation End-Products: A Biological Consequence of Lifestyle Contributing to Cancer Disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef] [Green Version]
- Tessier, F.J.; Boulanger, E.; Howsam, M. Metabolic transit of dietary advanced glycation end-products—The case of N(Ɛ)-carboxymethyllysine. Glycoconj. J. 2021, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, A.; Niquet-Leridon, C.; Boulanger, E.; Tessier, F.J. How Can Diet Affect the Accumulation of Advanced Glycation End-Products in the Human Body? Foods 2016, 5, 84. Available online: https://pubmed.ncbi.nlm.nih.gov/28231179 (accessed on 6 December 2019). [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Gebauer, S.K.; Baer, D.J.; Sun, K.; Turner, R.; Silber, H.A.; Talegawkar, S.; Ferrucci, L.; Novotny, J.A. Dietary Intake of Advanced Glycation End Products Did Not Affect Endothelial Function and Inflammation in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2014, 144, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Kellow, N.J.; Savige, G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: A systematic review. Eur. J. Clin. Nutr. 2013, 67, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).