Zonarol Protected Liver from Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of Zonarol
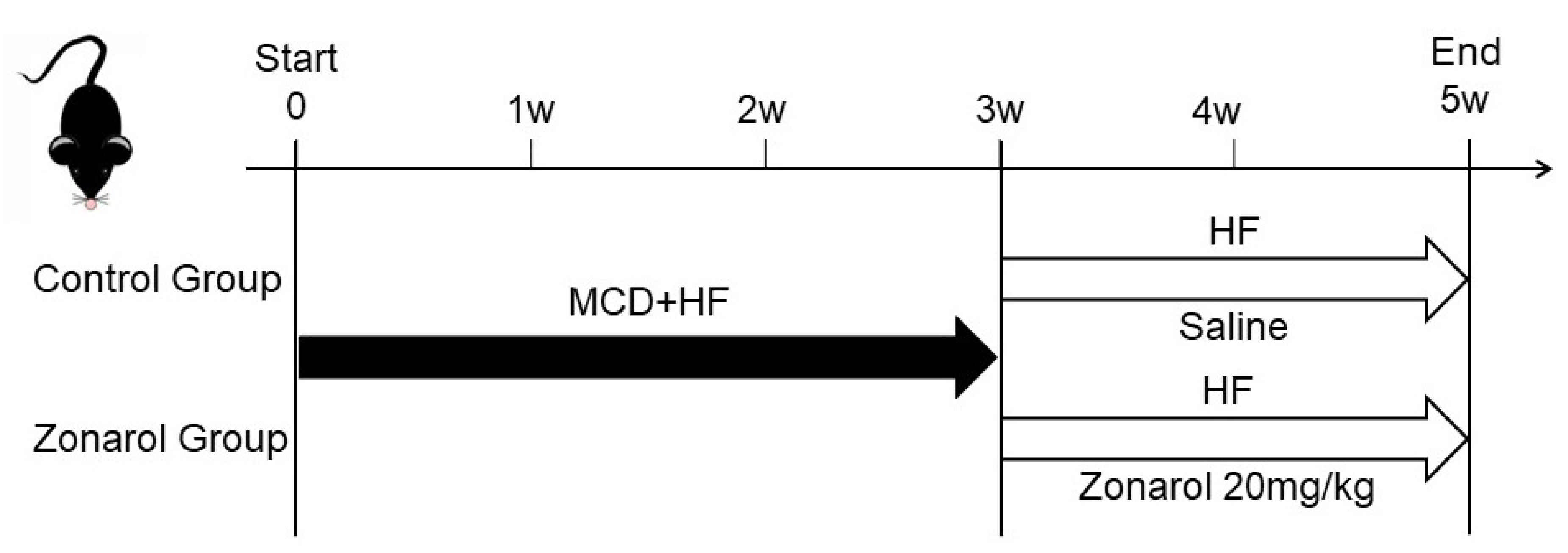
2.2. Experimental Animals
2.3. Ethics
2.4. Histopathology
2.5. IHC
2.6. Western Blotting
2.7. The Analysis of the Tissue Lipid Content
2.8. Quantitative Real-Time Polymerase Chain Reaction
2.9. Measurement of the TBARS Levels
2.10. Statistical Analyses
3. Results
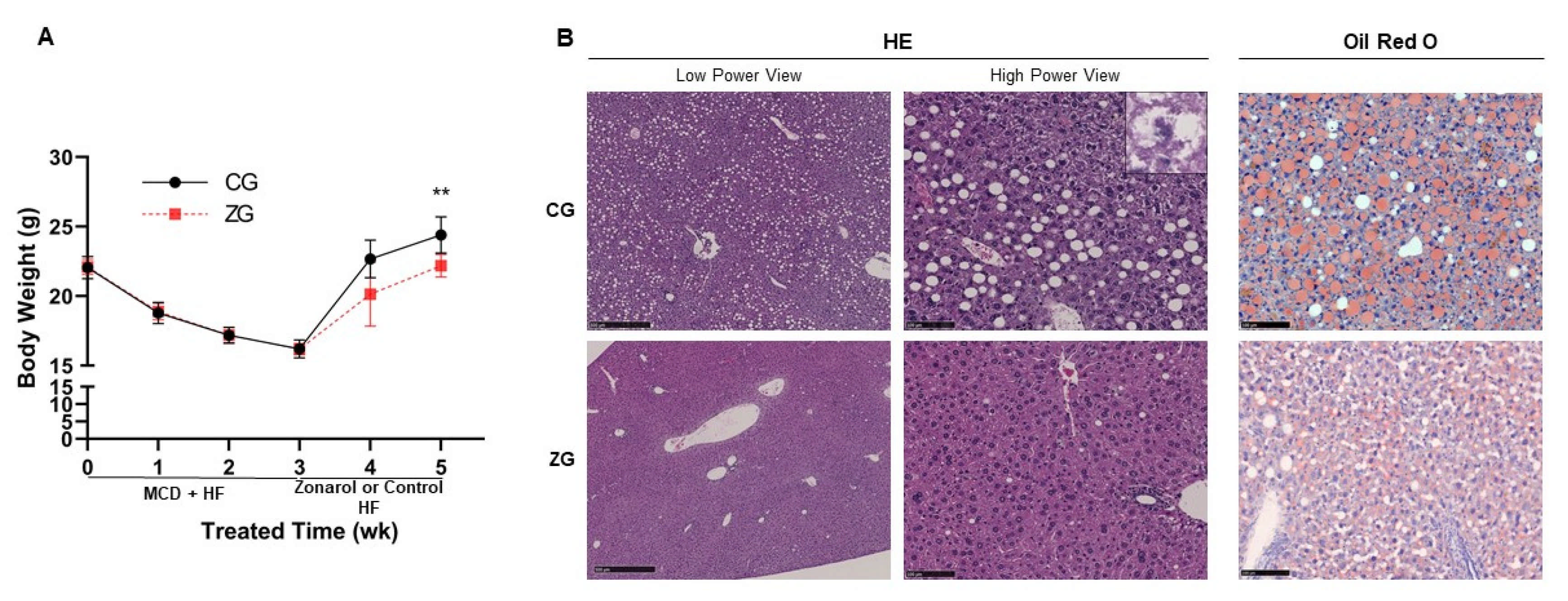
3.1. Gavage Administration of Zonarol Restrained the Body Weight Gain Induced by HF Diet Feeding, Meanwhile the Liver Condition Was Improved
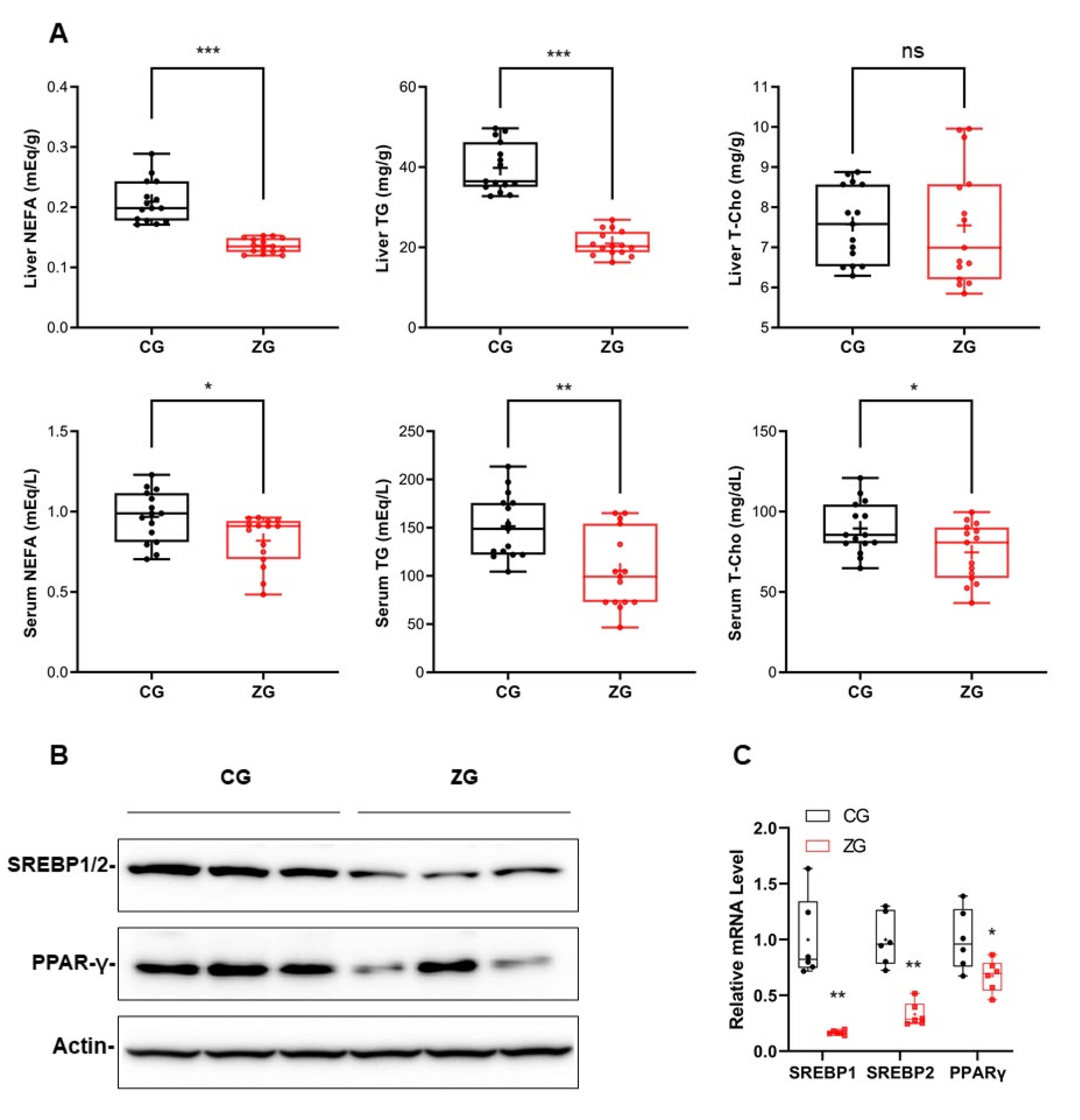
3.2. After Zonarol Gavage Administration, the Lipid Contents of the Liver and Serum of Mice Tended to Be Decreased, While the Lipid Metabolism Was Improved
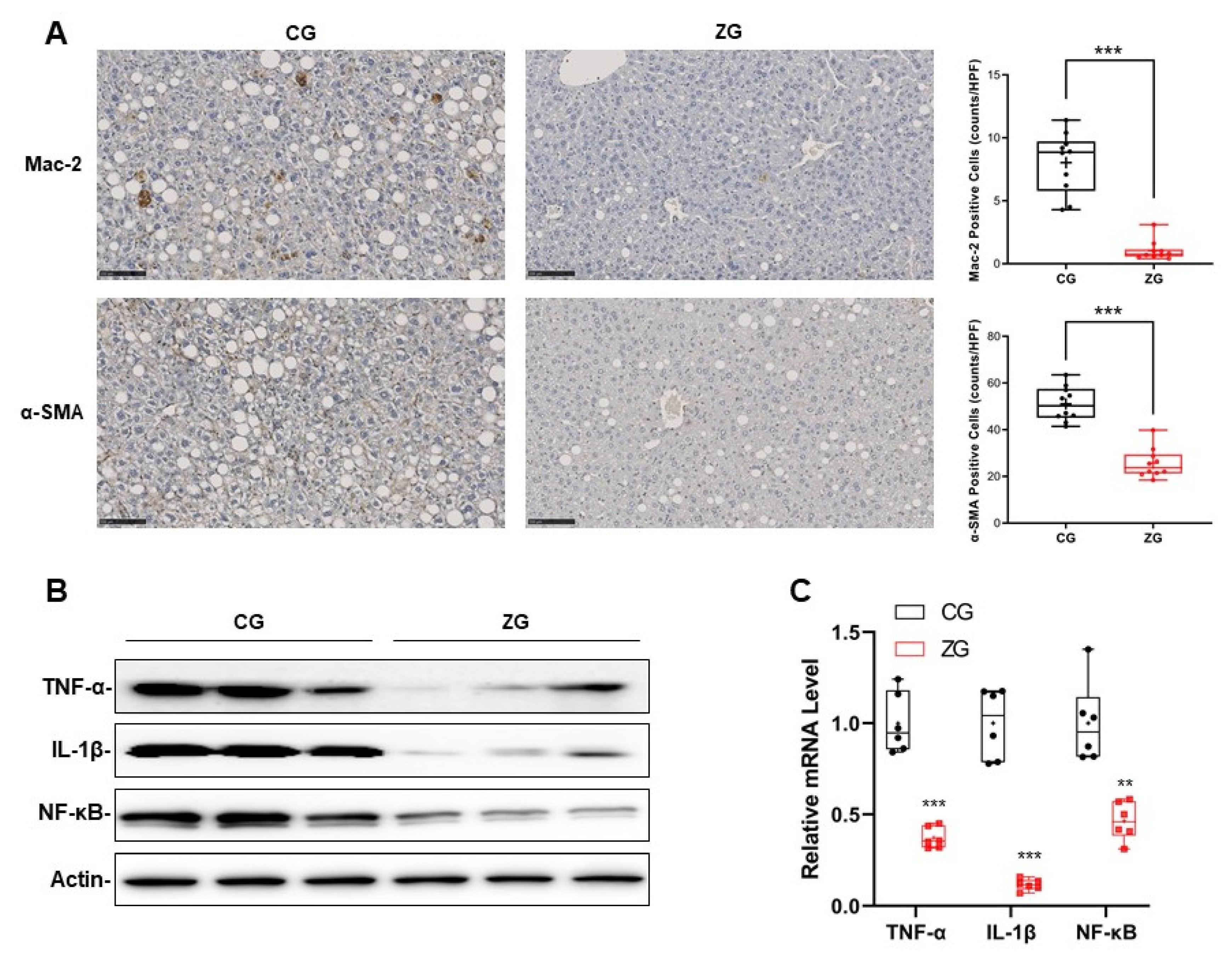
3.3. Inflammatory Reaction Was Significantly Suppressed in NAFLD Mice with Gavage Administration of Zonarol
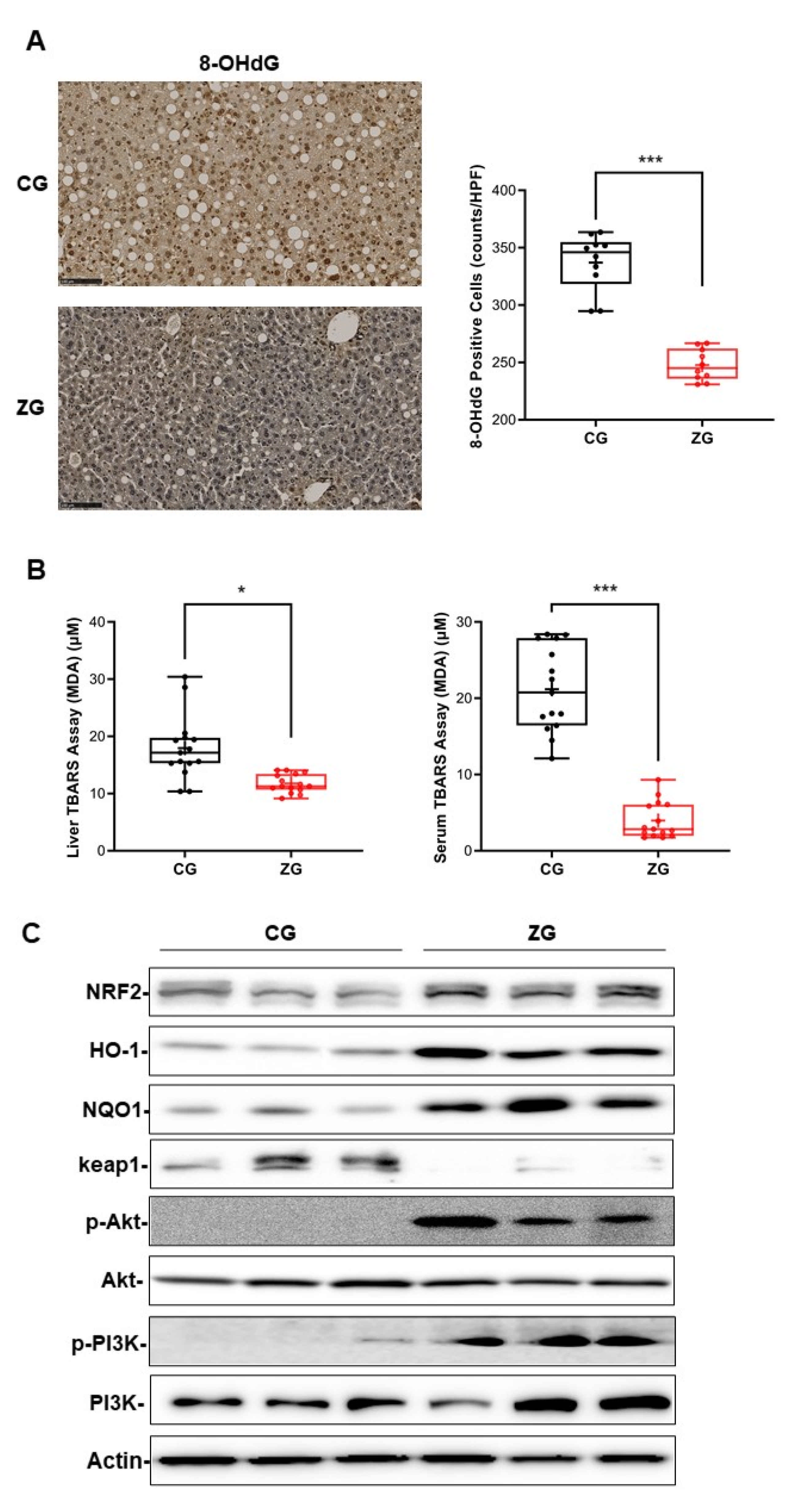
3.4. Oral Administration of Zonarol Protected the Liver from Oxidative Stress While the Nrf2 Pathway Played an Important Role
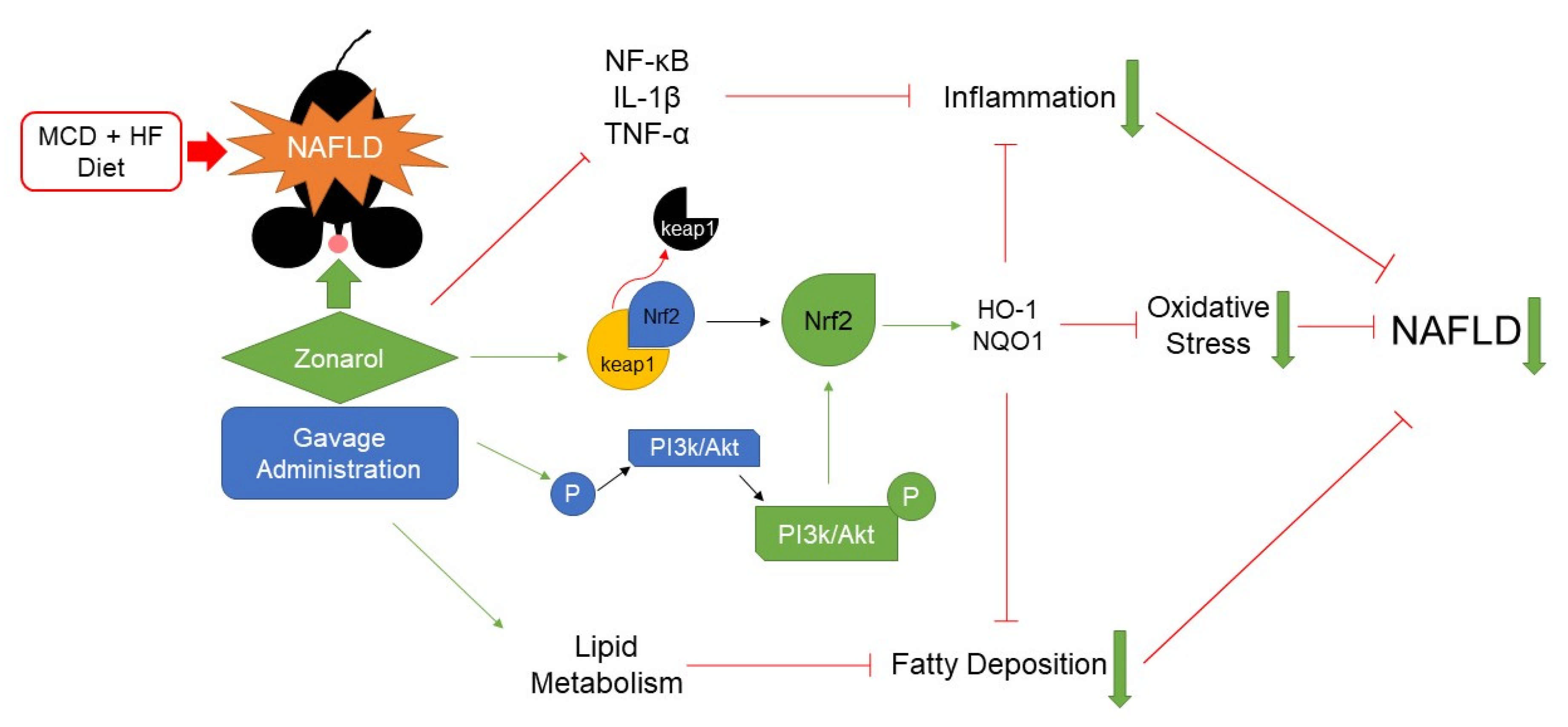
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, Y.-Y.; Yeh, M.M. Non-alcoholic fatty liver disease: A review with clinical and pathological correlation. J. Formos. Med. Assoc. 2021, 120, 68–77. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; LaVine, J.E.; Ratziu, V.; McCullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pathology and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 145–171. [Google Scholar] [CrossRef] [Green Version]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Cave, M.C.; Clair, H.B.; Hardesty, J.E.; Falkner, K.C.; Feng, W.; Clark, B.J.; Sidey, J.; Shi, H.; Aqel, B.A.; McClain, C.J.; et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2016, 1859, 1083–1099. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, M.; Zou, Y.; Guo, H.; Zhang, B.; Xia, C.; Zhang, H.; Yang, W.; Xu, C. NFκB/Orai1 facilitates endoplasmic reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Front Cell Dev Biol. 2019, 7, 202. [Google Scholar] [CrossRef]
- Joshi-Barve, S.; Barve, S.S.; Amancherla, K.; Gobejishvili, L.; Hill, D.; Cave, M.; Hote, P.; McClain, C.J. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 2007, 46, 823–830. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell–Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mito-chondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Meng, X.; Guo, X.; Zhang, J.; Moriya, J.; Kobayashi, J.; Yamaguchi, R.; Yamada, S. Acupuncture on ST36, CV4 and KI1 suppresses the progression of methionine- and cho-line-deficient diet-induced nonalcoholic fatty liver disease in mice. Metabolites 2019, 9, 299. [Google Scholar] [CrossRef] [Green Version]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)—Pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol Life Sci 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.; Vonghia, L. Pharmacological Treatment for Non-alcoholic Fatty Liver Disease. Adv. Ther. 2019, 36, 1052–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, T.T.; Tshabalala, T.; Dangarembizi, R.; Erlwanger, K.H.; Ndhlala, A.R. The Potential Therapeutic Value of Medicinal Plants in the Management of Metabolic Disorders. Molecules 2020, 25, 2669. [Google Scholar] [CrossRef]
- Brown, E.S.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Sims, J.J.; Squatrito, D.; Wing, R.M.; Radlick, P. Zonarol and isozonarol, fungitoxic hydroquinones from the brown seaweed Dictyopteris zonarioides. J. Org. Chem. 1973, 38, 2383–2386. [Google Scholar] [CrossRef]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.-Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 19, e113509. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neu-roprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Nawata, A.; Noguchi, H.; Mazaki, Y.; Kurahashi, T.; Izumi, H.; Wang, K.-Y.; Guo, X.; Uramoto, H.; Kohno, K.; Taniguchi, H.; et al. Overexpression of Peroxiredoxin 4 Affects Intestinal Function in a Dietary Mouse Model of Nonalcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0152549. [Google Scholar]
- Han, J.; Guo, X.; Meng, X.-J.; Zhang, J.; Yamaguchi, R.; Motoo, Y.; Yamada, S. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J. Gastroenterol. 2020, 26, 5118–5129. [Google Scholar] [CrossRef]
- Nabeshima, A.; Yamada, S.; Guo, X.; Tanimoto, A.; Wang, K.-Y.; Shimajiri, S.; Kimura, S.; Tasaki, T.; Noguchi, H.; Kitada, S.; et al. Peroxiredoxin 4 Protects Against Nonalcoholic Steatohepatitis and Type 2 Diabetes in a Nongenetic Mouse Model. Antioxid. Redox Signal. 2013, 19, 1983–1998. [Google Scholar] [CrossRef] [Green Version]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–22. [Google Scholar]
- Marra, F.; Lotersztajn, S. Pathophysiology of nonalcoholic steatohepatitis: Perspectives for a targeted treatment. Curr. Pharm. Des. 2013, 19, 5250–5269. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Yao, M.; Yao, D.; Wang, L.; Yang, X.; Yao, D. Nonalcoholic Lipid Accumulation and Hepatocyte Malignant Transformation. J. Clin. Transl. Hepatol. 2016, 4, 123–130. [Google Scholar] [PubMed] [Green Version]
- Yamada, S.; Tanimoto, A.; Sasaguri, Y. Critical in vivo roles of histamine and histamine receptor signaling in animal models of metabolic syndrome. Pathol. Int. 2016, 66, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.U.; Sheikh, T.A. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of Non-alcoholic fatty liver disease. Free Radic. Res. 2015, 49, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Guo, X. Peroxiredoxin 4 (PRDX4): Its critical in vivo roles in animal models of metabolic syndrome ranging from atherosclerosis to nonalcoholic fatty liver disease. Pathol. Int. 2018, 68, 91–101. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Li, C.; Zhang, Y.; Xu, S.; Li, X.; Li, H.; Zhang, X. Nrf2 deletion causes “benign” simple steatosis to develop into nonalcoholic steatohepatitis in mice fed a high-fat diet. Lipids Health Dis. 2013, 12, 165. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.; Yao, W.; Yuan, D.; Zhou, S.; Chen, X.; Zhang, Y.; Li, H.; Xia, Z.; Hei, Z. Brg1-mediated Nrf2/HO-1 pathway activation alleviates hepatic ischemia–reperfusion injury. Cell Death Dis. 2017, 8, e2841. [Google Scholar] [CrossRef] [PubMed]
- Krycer, J.R.; Sharpe, L.J.; Luu, W.; Brown, A.J. The Akt-SREBP nexus: Cell signaling meets lipid metabolism. Trends Endocrinol. Metab. 2010, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Kushiyama, A.; Sakoda, H.; Nakatsu, Y.; Iizuka, M.; Taki, N.; Fujishiro, M.; Fukushima, T.; Kamata, H.; Nagamachi, A.; et al. Involvement of resistin-like molecule β in the development of methionine-choline deficient diet-induced non-alcoholic steatohepatitis in mice. Sci. Rep. 2016, 6, 20157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, M.L.; Oró, D.; Tølbøl, K.S.; Thrane, S.T.; Nielsen, J.C.; Cohen, T.S.; Tabor, D.E.; Fernandes, F.; Tovchigrechko, A.; Veidal, S.S.; et al. Towards a standard diet-induced and biopsy-confirmed mouse model of non-alcoholic steatohepatitis: Impact of dietary fat source. World J. Gastroenterol. 2019, 25, 4904–4920. [Google Scholar] [CrossRef] [PubMed]
| Steatosis Score | Inflammatory Score | Ballooning Score | |||
|---|---|---|---|---|---|
| 0 | No lipid droplets. | 0 | No inflammation. | 0 | None. |
| 1 | Lipid droplets in <33% of hepatocytes. | 1 | <10 inflammatory foci, each consisting of >5 inflammatory cells. | 1 | Few ballooned cells. |
| 2 | Lipid droplets 33%–66% of hepatocytes. | 2 | ≥10 inflammatory foci. | 2 | Many ballooned cells or prominent ballooning of cells |
| 3 | Lipid droplets in >66% of hepatocytes. | 3 | uncountable diffuse or fused inflammatory foci. | ||
| Steatosis Score | Inflammation Score | Ballooning Score | NAFLD Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | CG | ZG | p | Score | CG | ZG | p | Score | CG | ZG | p | Score | CG | ZG | p |
| 0 | 0 | 0 | 0.00904 | 0 | 0 | 4 | 0.00898 | 0 | 2 | 9 | 0.0155 | 0–3 | 1 | 9 | 0.00418 |
| 1 | 1 | 9 | 1 | 1 | 5 | 1 | 10 | 6 | 4–6 | 11 | 6 | ||||
| 2 | 8 | 4 | 2 | 12 | 6 | 2 | 3 | 0 | 7–8 | 3 | 0 | ||||
| 3 | 6 | 2 | 3 | 2 | 0 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Guo, X.; Koyama, T.; Kawai, D.; Zhang, J.; Yamaguchi, R.; Zhou, X.; Motoo, Y.; Satoh, T.; Yamada, S. Zonarol Protected Liver from Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients 2021, 13, 3455. https://doi.org/10.3390/nu13103455
Han J, Guo X, Koyama T, Kawai D, Zhang J, Yamaguchi R, Zhou X, Motoo Y, Satoh T, Yamada S. Zonarol Protected Liver from Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients. 2021; 13(10):3455. https://doi.org/10.3390/nu13103455
Chicago/Turabian StyleHan, Jia, Xin Guo, Tomoyuki Koyama, Daichi Kawai, Jing Zhang, Reimon Yamaguchi, Xiaolei Zhou, Yoshiharu Motoo, Takumi Satoh, and Sohsuke Yamada. 2021. "Zonarol Protected Liver from Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in a Mouse Model" Nutrients 13, no. 10: 3455. https://doi.org/10.3390/nu13103455
APA StyleHan, J., Guo, X., Koyama, T., Kawai, D., Zhang, J., Yamaguchi, R., Zhou, X., Motoo, Y., Satoh, T., & Yamada, S. (2021). Zonarol Protected Liver from Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients, 13(10), 3455. https://doi.org/10.3390/nu13103455






