Standardised Outcome Reporting for the Nutrition Management of Complex Chronic Disease: A Rapid Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.2.1. Study Population
2.2.2. Types of Interventions
2.3. Study Records
2.3.1. Data Management
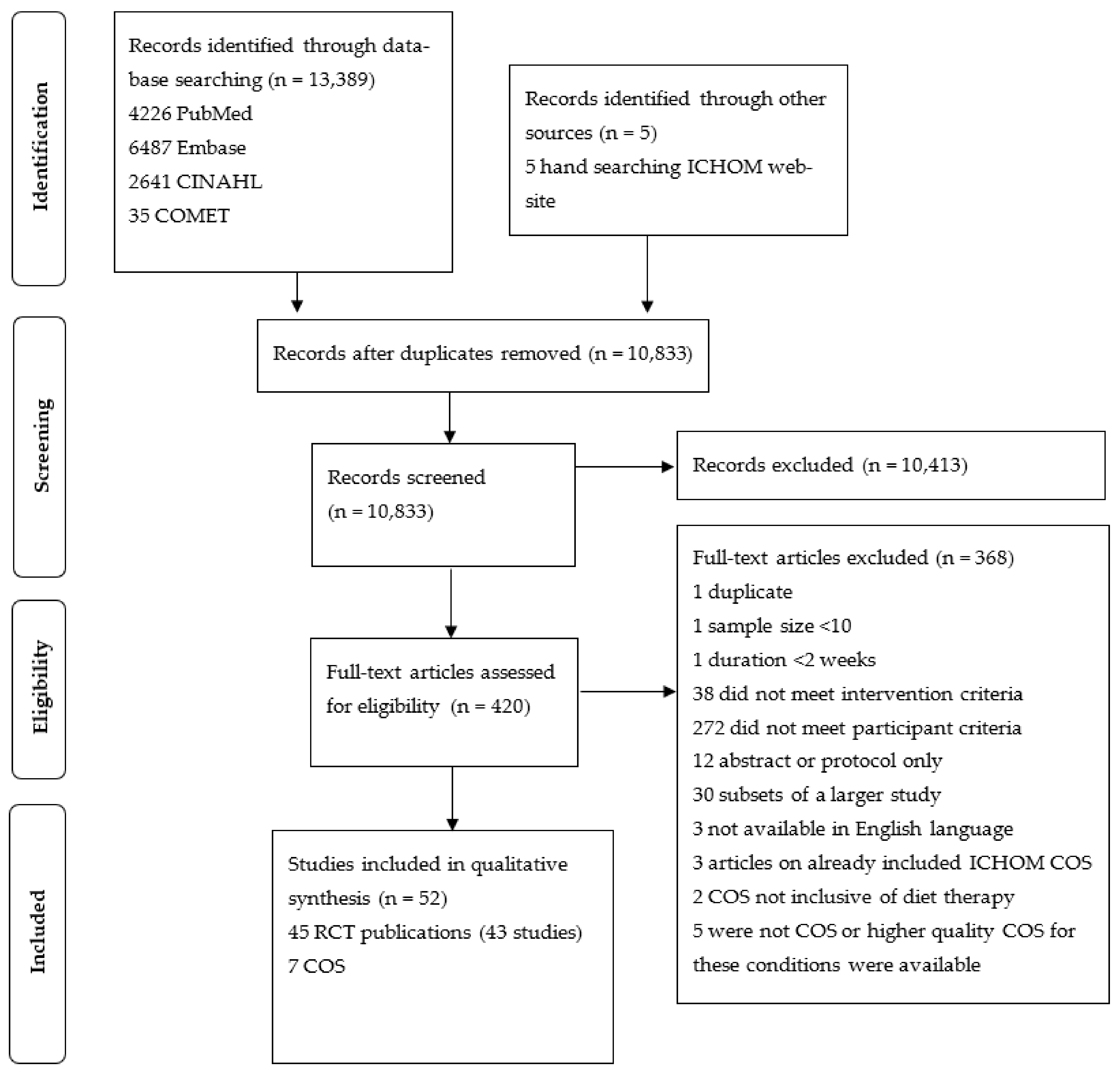
2.3.2. Selection Process
2.4. Data Extraction and Items
2.5. Data Analysis
2.6. Quality Assessment
3. Results
3.1. Study Characteristics
3.1.1. RCT Complex Chronic Disease Combinations
3.1.2. RCT Interventions
3.1.3. COS Included
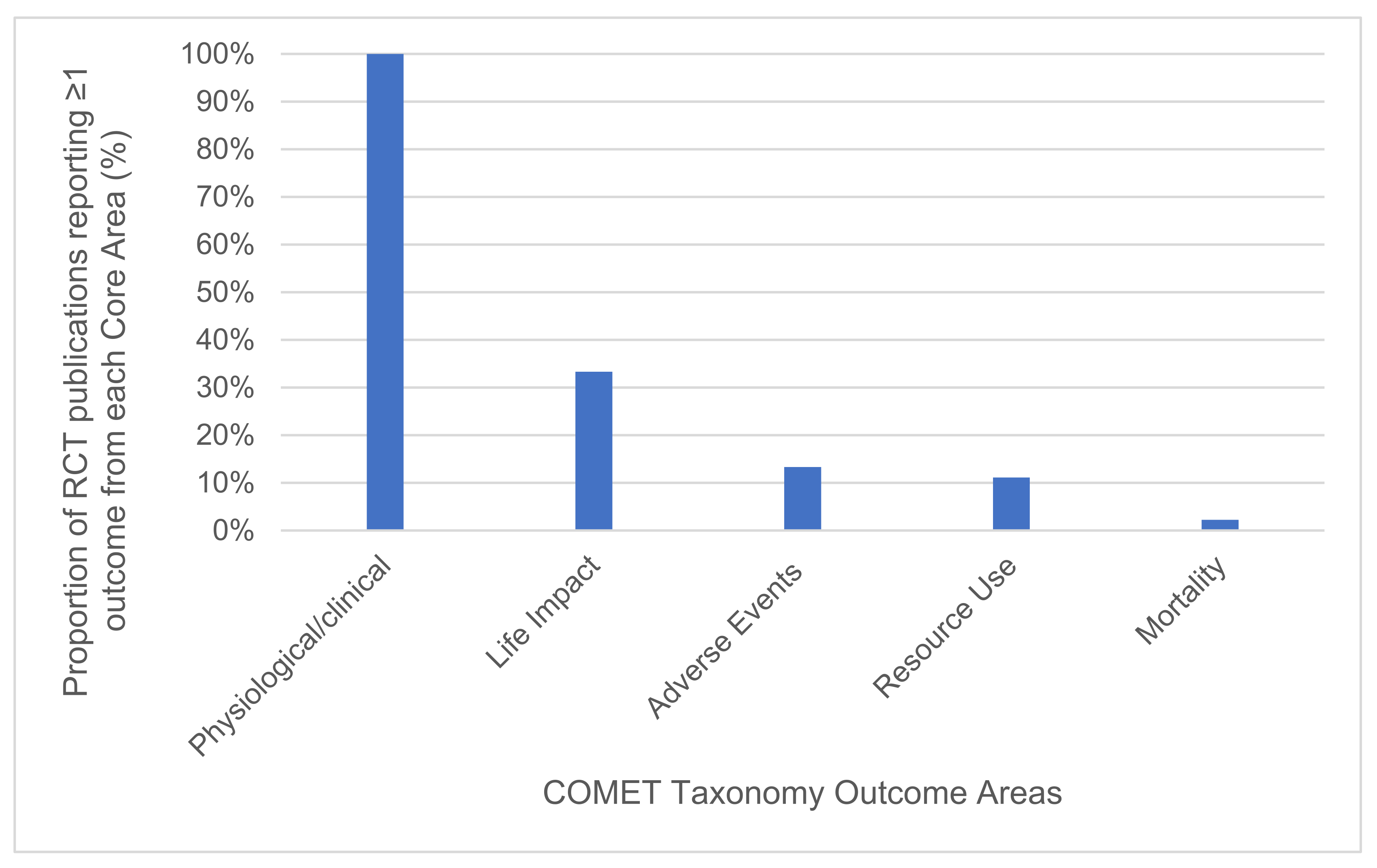
3.2. RCT Outcome Reporting Scope and Consistency
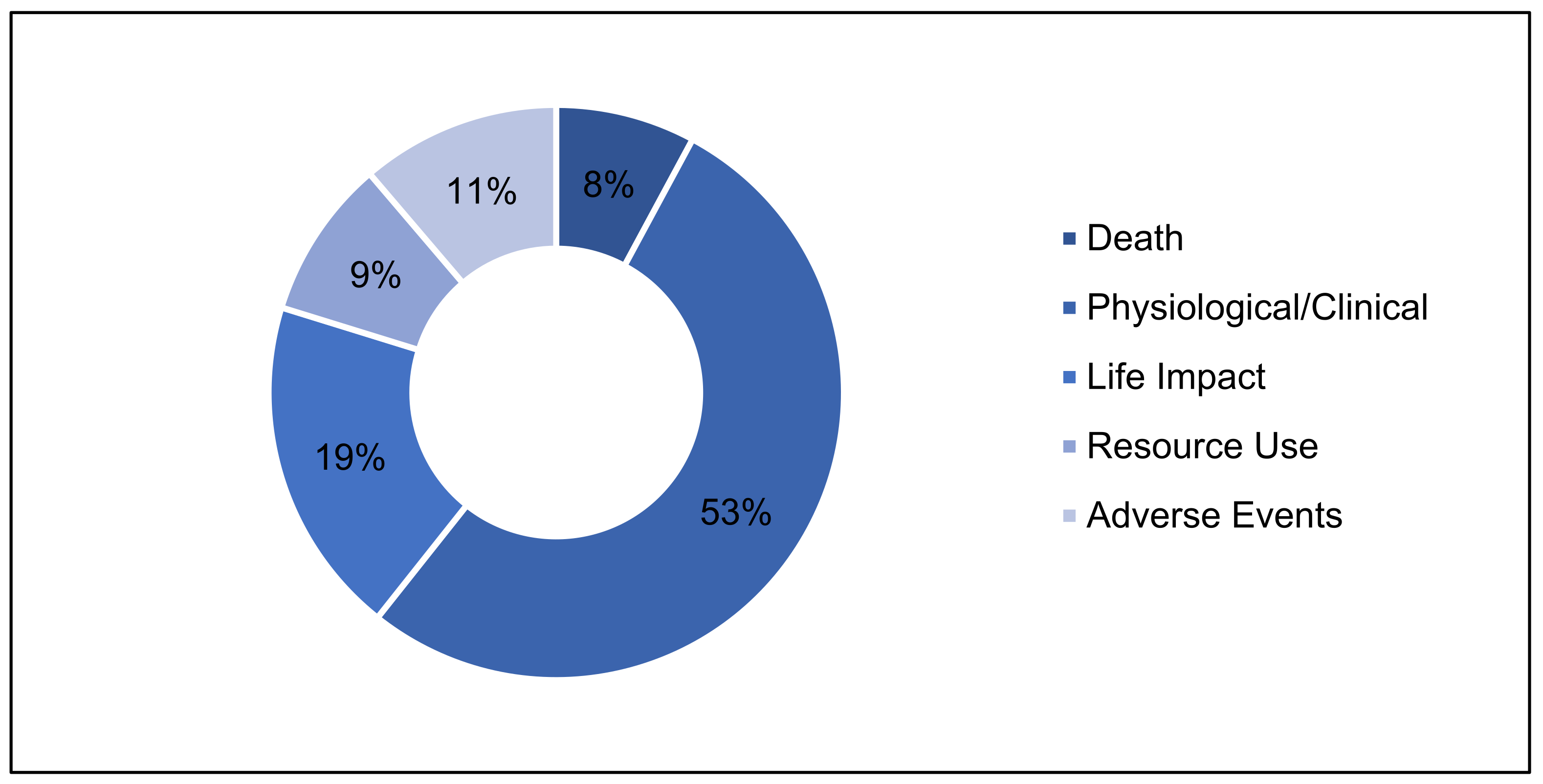
3.2.1. Outcome Areas
3.2.2. Outcome Domains
3.2.3. Individual Outcomes and Outcome Measures
3.3. COS Outcome Requesting
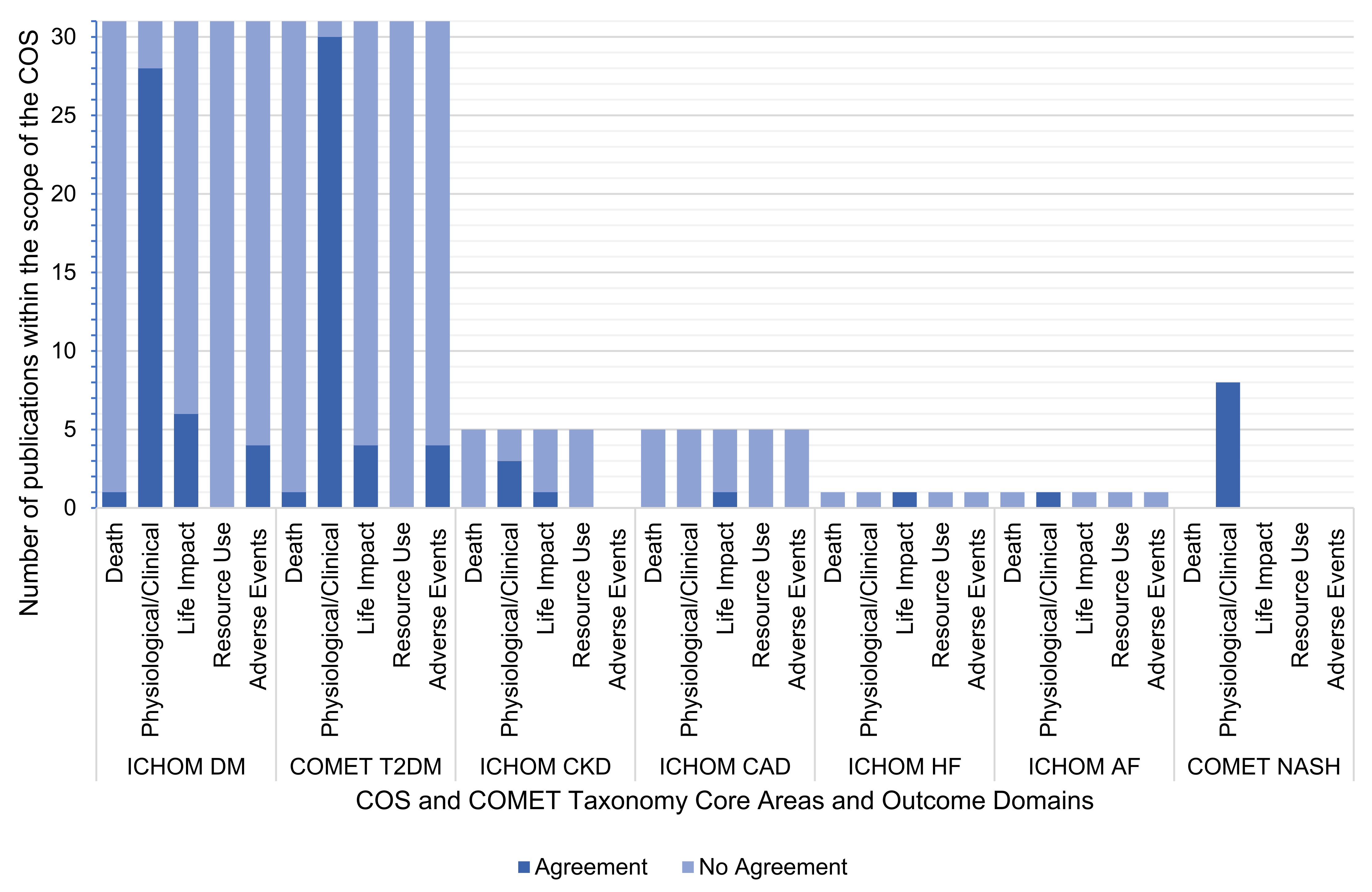
3.4. Agreement between Outcomes Reported by RCTs and Those Requested by COS
3.5. Quality Assessment Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.; Brookes, S.T.; Clarke, M.; Gargon, E.; Gorst, S.; Harman, N.; et al. The COMET Handbook: Version 1.0. Trials 2017, 18, 280. [Google Scholar] [CrossRef]
- Dodd, S.; Clarke, M.; Becker, L.; Mavergames, C.; Fish, R.; Williamson, P.R. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J. Clin. Epidemiol. 2018, 96, 84–92. [Google Scholar] [CrossRef]
- Sautenet, B.; Tong, A.; Williams, G.; Hemmelgarn, B.R.; Manns, B.; Wheeler, D.C.; Tugwell, P.; Van Biesen, W.; Winkelmayer, W.C.; Crowe, S.; et al. Scope and Consistency of Outcomes Reported in Randomized Trials Conducted in Adults Receiving Hemodialysis: A Systematic Review. Am. J. Kidney Dis. 2018, 72, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Harman, N.L.; Wilding, J.P.H.; Curry, D.; Harris, J.; Logue, J.; Pemberton, R.J.; Perreault, L.; Thompson, G.; Tunis, S.; Williamson, P.R. Selecting Core Outcomes for Randomised Effectiveness trials In Type 2 diabetes (SCORE-IT): A patient and healthcare professional consensus on a core outcome set for type 2 diabetes. BMJ Open Diabetes Res. Care 2019, 7, e000700. [Google Scholar] [CrossRef]
- Williamson, P.R.; Altman, D.G.; Blazeby, J.M.; Clarke, M.; DeVane, D.; Gargon, E.; Tugwell, P. Developing core outcome sets for clinical trials: Issues to consider. Trials 2012, 13, 132. [Google Scholar] [CrossRef]
- ICHOM. International Consortium for Health Outcome Measurement. 2020. Available online: https://www.ichom.org/ (accessed on 11 July 2020).
- COMET Initiative. Core Outcome Measures in Effectiveness Trials. 2020. Available online: http://www.comet-initiative.org/ (accessed on 11 July 2020).
- ICHOM. Our Methodology. 2020. Available online: https://www.ichom.org/standard-sets/#methodology (accessed on 11 July 2020).
- Kirkham, J.; Gorst, S.; Altman, D.G.; Blazeby, J.; Clarke, M.; Devane, D.; Gargon, E.; Moher, D.; Schmitt, J.; Tugwell, P.; et al. Core Outcome Set–STAndards for Reporting: The COS-STAR Statement. PLoS Med. 2016, 13, e1002148. [Google Scholar] [CrossRef] [PubMed]
- COMET Initiative. Taxonomy Explanation Table. 2018. Available online: http://www.comet-initiative.org/assets/downloads/Taxonomy%20explanation%20table%202018.10.30.pdf (accessed on 11 July 2020).
- Islam, M.M.; Valderas, J.M.; Yen, L.; Dawda, P.; Jowsey, T.; McRae, I.S. Multimorbidity and Comorbidity of Chronic Diseases among the Senior Australians: Prevalence and Patterns. PLoS ONE 2014, 9, e83783. [Google Scholar] [CrossRef] [PubMed]
- Pefoyo, A.J.K.; E Bronskill, S.; Gruneir, A.; Calzavara, A.; Thavorn, K.; Petrosyan, Y.; Maxwell, C.J.; Bai, Y.; Wodchis, W.P. The increasing burden and complexity of multimorbidity. BMC Public Health 2015, 15, 415. [Google Scholar] [CrossRef]
- Cassell, A.; Edwards, D.; Harshfield, A.; Rhodes, K.; Brimicombe, J.; Payne, R.; Griffin, S. The epidemiology of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2018, 68, e245–e251. [Google Scholar] [CrossRef]
- Wei, M.Y.; Mukamal, K.J. Multimorbidity and Mental Health-Related Quality of Life and Risk of Completed Suicide. J. Am. Geratr. Soc. 2019, 67, 511–519. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Uttamacharya, U.; Jain, K.; Biritwum, R.B.; Yawson, A.E.; Wu, F.; Guo, Y.; Maximova, T.; Espinoza, B.M.; Rodríguez, A.S.; et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: What does the study on global ageing and adult health (SAGE) reveal? BMC Med. 2015, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Spaak, J. Novel combined management approaches to patients with diabetes, chronic kidney disease and cardiovascular disease. J. R. Coll. Physicians Edinb. 2017, 47, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, J.; Campbell, N.R.; Duhaney, T.; Mang, E.; Gelfer, M. Reducing deaths by diet: Call to action for a public policy agenda for chronic disease prevention. Can. Fam Physician 2016, 62, 469–470. [Google Scholar] [PubMed]
- Keaver, L.; O’Meara, C.; Mukhtar, M.; McHugh, C. Providing Nutrition Care to Patients with Chronic Disease: An Irish Teaching Hospital Healthcare Professional Study. J. Biomed. Educ. 2018. [Google Scholar] [CrossRef]
- Ojo, O. Nutrition and Chronic Conditions. Nutrients 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Haggerty, J.; Almirall, J.; Bouhali, T.; Sasseville, M.; Lemieux, M. Lifestyle factors and multimorbidity: A cross sectional study. BMC Public Health 2014, 14, 686. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Dekker, L.H.; De Borst, M.; Meems, L.M.G.; De Boer, R.A.; Bakker, S.J.L.; Navis, G.J. The association of multimorbidity within cardio-metabolic disease domains with dietary patterns: A cross-sectional study in 129 369 men and women from the Lifelines cohort. PLoS ONE 2019, 14, e0220368. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Clarivate Analytics. EndNoteX9. 2020. Available online: https://endnote.com/ (accessed on 11 July 2020).
- Smith, S.M.; Wallace, E.; Salisbury, C.; Sasseville, M.; Bayliss, E.; Fortin, M. A Core Outcome Set for Multimorbidity Research (COSmm). Ann. Fam. Med. 2018, 16, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Kottner, J.; Coleman, S.; Muir, D.; Beeckman, D.; Chaboyer, W.; Cuddigan, J.; Moore, Z.; Rutherford, C.; Schmitt, J.; et al. Outcomes for Pressure Ulcer Trials (OUTPUTs) project: Review and classification of outcomes reported in pressure ulcer prevention research. Br. J. Dermatol. 2021, 184, 617–626. [Google Scholar] [CrossRef]
- Chow, A.; Soon, C.; Smith, H.; Apfelbacher, C. Outcome Measurements Used in Randomized Controlled Trials of Teledermatology: A Systematic Mapping Review. Acta Derm. Venereol. 2019, 99, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Rönsch, H.; Apfelbacher, C.; Brans, R.; Matterne, U.; Molin, S.; Ofenloch, R.; Oosterhaven, J.A.F.; Schuttelaar, M.L.A.; Weisshaar, E.; Yew, Y.W.; et al. Which outcomes have been measured in hand eczema trials? A systematic review. Contact Dermat. 2019, 80, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Methods Bias. RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. 2021. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 11 July 2020).
- Higgins, J.P.T.; Savovic, J.; Page, M.J.; Sterne, J.A.C. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) SHORT VERSION (CRIBSHEET). 2019. Available online: https://drive.google.com/file/d/1Q4Fk3HCuBRwIDWTGZa5oH11OdR4Gbhdo/view (accessed on 11 July 2021).
- Abd El-Kader, S.M.; Al-Shreef, F.M.; Al-Jiffri, O.H. Biochemical parameters response to weight loss in patients with non-alcoholic steatohepatitis. Afr. Health Sci. 2016, 16, 242–249. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Impact of weight reduction on insulin resistance, adhesive molecules and adipokines dysregulation among obese type 2 diabetic patients. Afr. Health Sci. 2018, 18, 873–883. [Google Scholar] [CrossRef]
- Abed, H.S.; Wittert, G.; Leong, D.P.; Shirazi, M.G.; Bahrami, B.; Middeldorp, M.; Lorimer, M.; Lau, D.H.; Antic, N.A.; Brooks, A.G.; et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA 2013, 310, 2050–2060. [Google Scholar] [CrossRef]
- Aller, R.; A De Luis, D.; Izaola, O.; De La Fuente, B.; Bachiller, R. Effect of a high monounsaturated vs high polyunsaturated fat hypocaloric diets in nonalcoholic fatty liver disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1041–1047. [Google Scholar]
- Brown, A.; Dornhorst, A.; McGowan, B.; Omar, O.; Leeds, A.R.; Taheri, S.; Frost, G.S. Low-energy total diet replacement intervention in patients with type 2 diabetes mellitus and obesity treated with insulin: A randomized trial. BMJ Open Diabetes Res. Care 2020, 8, e001012. [Google Scholar] [CrossRef]
- Campos, R.M.S.; De Piano, A.; Da Silva, P.L.; Carnier, J.; Sanches, P.L.; Corgosinho, F.C.; Masquio, D.C.L.; Lazaretti-Castro, M.; Oyama, L.M.; Nascimento, C.M.O.; et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine 2012, 42, 146–156. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Manzoni, G.M.; Cuzziol, P.; Cesa, G.L.; Corti, S.; Tuzzi, C.; Villa, V.; Liuzzi, A.; Petroni, M.L.; Molinari, E. TECNOB study: Ad interim results of a randomized controlled trial of a multidisciplinary telecare intervention for obese patients with type-2 diabetes. Clin. Pract. Epidemiol. Ment. Health 2011, 7, 44–50. [Google Scholar] [CrossRef][Green Version]
- Chan, D.F.Y.; So, H.K.; Hui, S.C.N.; Chan, R.; Li, A.M.; Sea, M.M.; Chu, W.; Chan, M.; Woo, J.; Nelson, E.A.S. Dietitian-led lifestyle modification programme for obese Chinese adolescents with non-alcoholic fatty liver disease: A randomized controlled study. Int. J. Obes. 2018, 42, 1680–1690. [Google Scholar] [CrossRef]
- Ciarambino, T.; Ferrara, N.; Castellino, P.; Paolisso, G.; Coppola, L.; Giordano, M. Effects of a 6-days-a-week low protein diet regimen on depressive symptoms in young-old type 2 diabetic patients. Nutrition 2011, 27, 46–49. [Google Scholar] [CrossRef]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.-A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.; Kirkness, A.; Zelestis, E.; Hollams, D.; Kneale, C.; Armari, E.; Bennett, T.; Daly, J.; Tofler, G. A randomised trial of a weight loss intervention for overweight and obese people diagnosed with coronary heart disease and/or type 2 diabetes. Ann. Behav. Med. 2012, 44, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.; Kark, J.D.; Berry, E.M.; Adler, B.; Ziv, E.; Raz, I. The effect of a low carbohydrate energy-unrestricted diet on weight loss in obese type 2 diabetes patients—A randomized controlled trial. e-SPEN 2011, 6, e178–e186. [Google Scholar] [CrossRef]
- Holland-Carter, L.; Tuerk, P.W.; Wadden, T.A.; Fujioka, K.N.; Becker, L.E.; Miller-Kovach, K.; Hollander, P.L.; Garvey, W.T.; Weiss, D.; Rubino, D.M.; et al. Impact on psychosocial outcomes of a nationally available weight management program tailored for individuals with type 2 diabetes: Results of a randomized controlled trial. J. Diabetes Complicat. 2017, 31, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Howden, E.J.; Leano, R.; Petchey, W.; Coombes, J.S.; Isbel, N.M.; Marwick, T.H. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1494–1501. [Google Scholar] [CrossRef]
- Iqbal, N.; Vetter, M.L.; Moore, R.H.; Chittams, J.L.; Dalton-Bakes, C.V.; Dowd, M.; Williams-Smith, C.; Cardillo, S.; Wadden, T.A. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity (Silver Spring) 2010, 18, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, T.; Granfeldt, Y.; Erlanson-Albertsson, C.; Ahrén, B.; Lindeberg, S. A paleolithic diet is more satiating per calorie than a mediterranean-like diet in individuals with ischemic heart disease. Nutr. Metab. (Lond.) 2010, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, D.R.; Pedersen, E.; Clifton, P.M. Weight-loss diets in people with type 2 diabetes and renal disease: A randomized controlled trial of the effect of different dietary protein amounts. Am. J. Clin. Nutr. 2013, 98, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-J.; Park, S.-H.; Choi, E.-K.; Cha, Y.-S.; Cho, B.-H.; Kim, Y.-G.; Kim, M.-G.; O Song, W.; Park, T.-S.; Ko, J.-K.; et al. Beneficial effects of Korean traditional diets in hypertensive and type 2 diabetic patients. J. Med. Food 2014, 17, 161–171. [Google Scholar] [CrossRef]
- Karusheva, Y.; Kunstein, L.; Bierwagen, A.; Nowotny, B.; Kabisch, S.; Groener, J.B.; Fleitmann, A.K.; Herder, C.; Pacini, G.; Strassburger, K.; et al. An 8-week diet high in cereal fiber and coffee but free of red meat does not improve beta-cell function in patients with type 2 diabetes mellitus: A randomized controlled trial. Nutr. Metab. 2018, 15, 90. [Google Scholar] [CrossRef]
- Khoo, J.; Piantadosi, C.; Duncan, R.; Worthley, S.G.; Jenkins, A.; Noakes, M.; Worthley, M.I.; Lange, K.; Wittert, G.A. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J. Sex. Med. 2011, 8, 2868–2875. [Google Scholar] [CrossRef]
- Kim, C.J.; Kim, D.J.; Park, H.R. Effects of a cardiovascular risk reduction intervention with psychobehavioral strategies for Korean adults with type 2 diabetes and metabolic syndrome. J. Cardiovasc. Nurs. 2011, 26, 117–128. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Brubaker, P.H.; Morgan, T.M.; Haykowsky, M.J.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef]
- Krebs, J.D.; Elley, C.R.; Strong, A.P.; Lunt, H.; Drury, P.L.; Bell, D.A.; Robinson, E.; Moyes, S.A.; Mann, J.I. The Diabetes Excess Weight Loss (DEWL) Trial: A randomised controlled trial of high-protein versus high-carbohydrate diets over 2 years in type 2 diabetes. Diabetologia 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Luger, M.; Holstein, B.; Schindler, K.; Kruschitz, R.; Ludvik, B. Feasibility and efficacy of an isocaloric high-protein vs. standard diet on insulin requirement, body weight and metabolic parameters in patients with type 2 diabetes on insulin therapy. Exp. Clin. Endocrinol. Diabetes 2013, 121, 286–294. [Google Scholar] [CrossRef]
- Lynch, E.B.; Liebman, R.; Ventrelle, J.; Avery, E.F.; Richardson, D. A self-management intervention for African Americans with comorbid diabetes and hypertension: A pilot randomized controlled trial. Prev. Chronic Dis. 2014, 11, e90. [Google Scholar] [CrossRef] [PubMed]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. The metabolic and hepatic impact of two personalized dietary strategies in subjects with obesity and nonalcoholic fatty liver disease: The fatty liver in obesity (FLiO) randomized controlled trial. Nutrients 2019, 11, 2543. [Google Scholar] [CrossRef]
- Mayer, S.; Jeffreys, A.S.; Olsen, M.K.; McDuffie, J.R.; Feinglos, M.N.; Yancy, W.S. Two diets with different haemoglobin A1c and antiglycaemic medication effects despite similar weight loss in type 2 diabetes. Diabetes Obes. Metab. 2014, 16, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Mekki, K.; Bouzidi-Bekada, N.; Kaddous, A.; Bouchenak, M. Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct. 2010, 1, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mollentze, W.F.; Joubert, G.; Prins, A.; Van Der Linde, S.; Marx, G.M.; Tsie, K.G. The safety and efficacy of a low-energy diet to induce weight loss, improve metabolic health, and induce diabetes remission in insulin-treated obese men with type 2 diabetes: A pilot RCT. Int. J. Diabetes Dev. Ctries 2019, 39, 618–625. [Google Scholar] [CrossRef]
- Morris, E.; Aveyard, P.; Dyson, P.; Noreik, M.; Bailey, C.; Fox, R.; Jerome, D.; Tan, G.D.; A Jebb, S. A food-based, low-energy, low-carbohydrate diet for people with type 2 diabetes in primary care: A randomized controlled feasibility trial. Diabetes Obes. Metab. 2020, 22, 512–520. [Google Scholar] [CrossRef]
- Nowotny, B.; Zahiragic, L.; Bierwagen, A.; Kabisch, S.; Groener, J.B.; Nowotny, P.J.; Fleitmann, A.K.; Herder, C.; Pacini, G.; Erlund, I.; et al. Low-energy diets differing in fibre, red meat and coffee intake equally improve insulin sensitivity in type 2 diabetes: A randomised feasibility trial. Diabetologia 2015, 58, 255–264. [Google Scholar] [CrossRef]
- Orazio, L.K.; Isbel, N.M.; Armstrong, K.A.; Tarnarskyj, J.; Johnson, D.W.; Hale, R.E.; Kaisar, M.; Banks, M.D.; Hickman, I.J. Evaluation of dietetic advice for modification of cardiovascular disease risk factors in renal transplant recipients. J. Ren. Nutr. 2011, 21, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Oshakbayev, K.; Bimbetov, B.; Manekenova, K.; Bedelbayeva, G.; Mustafin, K.; Dukenbayeva, B. Severe nonalcoholic steatohepatitis and type 2 diabetes: Liver histology after weight loss therapy in a randomized clinical trial. Curr. Med. Res. Opin. 2019, 35, 157–165. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Triantafillidou, D.; Panagiotakos, D.B.; Koutsovasilis, A.; Saliaris, M.; Manolis, A.; Melidonis, A.; Zampelas, A. A high-protein low-fat diet is more effective in improving blood pressure and triglycerides in calorie-restricted obese individuals with newly diagnosed type 2 diabetes. Eur. J. Clin. Nutr. 2010, 64, 595–602. [Google Scholar] [CrossRef]
- Patil, M.R.; Mishra, A.; Jain, N.; Gutch, M.; Tewari, R. Weight loss for reduction of proteinuria in diabetic nephropathy: Comparison with angiotensin-converting enzyme inhibitor therapy. Indian J. Nephrol. 2013, 23, 108–113. [Google Scholar] [CrossRef]
- Paula, T.P.; Viana, L.V.; Neto, A.T.Z.; Leitao, C.; Gross, J.L.; Azevedo, M.J. Effects of the DASH Diet and Walking on Blood Pressure in Patients With Type 2 Diabetes and Uncontrolled Hypertension: A Randomized Controlled Trial. J. Clin. Hypertens. 2015, 17, 895–901. [Google Scholar] [CrossRef]
- Raygan, F.; Bahmani, F.; Kouchaki, E.; Aghadavod, E.; Sharifi, S.; Akbari, E.; Heidari, A.; Asemi, Z. Comparative effects of carbohydrate versus fat restriction on metabolic profiles, biomarkers of inflammation and oxidative stress in overweight patients with type 2 diabetic and coronary heart disease: A randomized clinical trial. ARYA Atheroscler. 2016, 12, 266–273. [Google Scholar] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.M.; Tuerk, P.W.; Wadden, T.A.; Garvey, W.T.; Weiss, D.; Hermayer, K.L.; Aronne, L.J.; Becker, L.E.; Fujioka, K.; Miller-Kovach, K.; et al. Changes in weight control behaviors and hedonic hunger in a commercial weight management program adapted for individuals with type 2 diabetes. Int. J. Obes. 2020, 44, 990–998. [Google Scholar] [CrossRef]
- Sixt, S.; Beer, S.; Blüher, M.; Korff, N.; Peschel, T.; Sonnabend, M.; Teupser, D.; Thiery, J.; Adams, V.; Schuler, G.; et al. Long- but not short-term multifactorial intervention with focus on exercise training improves coronary endothelial dysfunction in diabetes mellitus type 2 and coronary artery disease. Eur. Heart J. 2010, 31, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Utari, A.; Maududi, M.S.; Kusumawati, N.R.D.; Mexitalia, M. Effects of low glycemic index diet on insulin resistance among obese adolescent with non-alcoholic fatty liver disease: A randomized controlled trial. Med. J. Indones. 2019, 28, 123–128. [Google Scholar] [CrossRef]
- von Haehling, S.; Stellos, K.; Qusar, N.; Gawaz, M.; Bigalke, B. Weight reduction in patients with coronary artery disease: Comparison of Traditional Tibetan Medicine and Western diet. Int. J. Cardiol. 2013, 168, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- The Look AHEAD Research Group; Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Ziegler, D.; Strom, A.; Nowotny, B.; Zahiragic, L.; Nowotny, P.J.; Carstensen-Kirberg, M.; Herder, C.; Roden, M. Effect of Low-Energy Diets Differing in Fiber, Red Meat, and Coffee Intake on Cardiac Autonomic Function in Obese Individuals With Type 2 Diabetes. Diabetes Care 2015, 38, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- ICHOM. ICHOM Standard Set for Diabetes in Adults. 2018. Available online: https://www.ichom.org/portfolio/diabetes/ (accessed on 7 July 2020).
- ICHOM. ICHOM Standard Set for Chronic Kidney Disease. 2017. Available online: https://www.ichom.org/portfolio/chronic-kidney-disease/ (accessed on 7 July 2020).
- ICHOM. ICHOM Standard Set for Coronary Artery Disease. 2015. Available online: https://www.ichom.org/portfolio/coronary-artery-disease/ (accessed on 7 July 2020).
- ICHOM. ICHOM Standard Set for Heart Failure. 2016. Available online: https://www.ichom.org/portfolio/heart-failure/ (accessed on 7 July 2020).
- ICHOM. ICHOM Standard Set for Atrial Fibrilation. 2019. Available online: https://www.ichom.org/portfolio/atrial-fibrillation/ (accessed on 7 July 2020).
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; LaVine, J.E.; Ratziu, V.; McCullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef]
- Dodd, S.; Harman, N.; Taske, N.; Minchin, M.; Tan, T.; Williamson, P.R. Core outcome sets through the healthcare ecosystem: The case of type 2 diabetes mellitus. Trials 2020, 21, 570. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Hirsch, M.; Kawsar, A.; Gale, C.; Pealing, L.; Plana, M.N.; Showell, M.; Williamson, P.R.; Khan, K.S.; Ziebland, S.; et al. Outcome reporting across randomised controlled trials evaluating therapeutic interventions for pre-eclampsia. BJOG 2017, 124, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.; Brigic, A.; Whiting, P.; Cawthorn, S.J.; Avery, K.N.L.; Donovan, J.L.; Blazeby, J. Reporting Clinical Outcomes of Breast Reconstruction: A Systematic Review. J. Natl. Cancer Inst. 2011, 103, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, L.; Perkins, G.; Clarey, A.; Haywood, K. A systematic review of the outcomes reported in cardiac arrest clinical trials: The need for a core outcome set. Resuscitation 2015, 88, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Whistance, R.N.; Forsythe, R.O.; McNair, A.G.K.; Brookes, S.T.; Avery, K.; Pullyblank, A.M.; Sylvester, P.A.; Jayne, D.G.; Jones, J.E.; Brown, J.; et al. A systematic review of outcome reporting in colorectal cancer surgery. Colorectal Dis. 2013, 15, e548–e560. [Google Scholar] [CrossRef] [PubMed]
- Rogozińska, E.; Gargon, E.; Olmedo-Requena, R.; Asour, A.; Cooper, N.A.M.; Vale, C.L.; Hooft, J.V. Methods used to assess outcome consistency in clinical studies: A literature-based evaluation. PLoS ONE 2020, 15, e0235485. [Google Scholar] [CrossRef]
- Gandhi, G.Y.; Murad, M.H.; Fujiyoshi, A.; Mullan, R.J.; Flynn, D.N.; Elamin, M.B.; Swiglo, B.A.; Isley, W.L.; Guyatt, G.H.; Montori, V.M. Patient-Important Outcomes in Registered Diabetes Trials. JAMA 2008, 299, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Malhotra, A.; Banning, A.; Jenkinson, C. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: Systematic review. BMJ 2010, 341, c5707. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; King, M.T.; Calvert, M.J.; Stockler, M.R.; Friedlander, M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 2018, 9, 353–367. [Google Scholar] [CrossRef]
- Desmet, P.M.A.; Schifferstein, H.N.J. Sources of positive and negative emotions in food experience. Appetite 2008, 50, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Woolley, K.; Fishbach, A.; Wang, R. Food restriction and the experience of social isolation. J. Pers. Soc. Psychol. 2019, 119, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Carson, T.L.; Hidalgo, B.; Ard, J.; Affuso, O. Dietary interventions and quality of life: A systematic review of the literature. J. Nutr. Educ. Behav. 2014, 46, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Moza, A.; Autschbach, R.; Stoppe, C.; Goetzenich, A. Evaluating outcomes used in cardiothoracic surgery interventional research: A systematic review of reviews to develop a core outcome set. PLoS ONE 2015, 10, e0122204. [Google Scholar] [CrossRef]
- Boers, M.; Kirwan, J.; Wells, G.; Beaton, D.; Gossec, L.; D’Agostino, M.-A.; Conaghan, P.; Bingham, C.O.; Brooks, P.; Landewé, R.; et al. Developing Core Outcome Measurement Sets for Clinical Trials: OMERACT Filter 2.0. J. Clin. Epidemiol. 2014, 67, 745–753. [Google Scholar] [CrossRef] [PubMed]
- McPhail, S.M. Multimorbidity in chronic disease: Impact on health care resources and costs. Risk Manag. Healthc. Policy 2016, 9, 143–156. [Google Scholar] [CrossRef]
- Wiebe, S.; Guyatt, G.; Weaver, B.; Matijevic, S.; Sidwell, C. Comparative responsiveness of generic and specific quality-of-life instruments. J. Clin. Epidemiol. 2003, 56, 52–60. [Google Scholar] [CrossRef]
- Ware, J.E.; Gandek, B.; Guyer, R.; Deng, N. Standardising disease-specific quality of life measures across multiple chronic conditions: Development and initial evaluation of the QOL Disease Impact Scale (QDIS®). Health Qual. Life Outcomes 2016, 14, 84. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Gandek, B.; Allison, J. The validity of disease-specific quality of life attributions among adults with multiple chronic conditions. Int. J. Stat. Med. 2016, 5, 17. [Google Scholar] [CrossRef]
- Berard, L.D.; Siemens, R.; Woo, V. Monitoring Glycemic Control. Can. J. Diabetes 2013, 37, S35–S39. [Google Scholar] [CrossRef]
- Guan, V.X.; Probst, Y.C.; Neale, E.P.; Tapsell, L.C. Evaluation of the dietary intake data coding process in a clinical setting: Implications for research practice. PLoS ONE 2019, 14, e0221047. [Google Scholar] [CrossRef]
- Kim, O.Y.; Lee, J.H.; Sweeney, G. Metabolomic profiling as a useful tool for diagnosis and treatment of chronic disease: Focus on obesity, diabetes and cardiovascular diseases. Expert Rev. Cardiovasc. Ther. 2013, 11, 61–68. [Google Scholar] [CrossRef]
- Gu, Q.; Spinelli, J.J.; Dummer, T.B.J.; McDonald, T.E.; Moore, S.C.; Murphy, R.A. Metabolic profiling of adherence to diet, physical activity and body size recommendations for cancer prevention. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Tong, T.Y.N.; Koulman, A.; Griffin, J.L.; Wareham, N.J.; Forouhi, N.G.; Imamura, F. A combination of metabolites predicts adherence to the Mediterranean diet pattern and its associations with insulin sensitivity and lipid homeostasis in the general population: The Fenland study, United Kingdom. J. Nutr. 2020, 150, 568–578. [Google Scholar] [CrossRef]
- Kim, H.; Rebholz, C.M. Metabolic biomarkers of healthy diet patterns and cardiovascular outcomes. Curr. Atheroscler. Rep. 2021, 26. [Google Scholar] [CrossRef]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Szatmari, P.; Pierro, A.; Kelly, L.E.; Farid-Kapadia, M.; Chee-A-Tow, A.; Saeed, L.; Monga, S.; et al. Improving outcome reporting in clinical trial reports and protocols: Study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT). Trials 2019, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, N.S.; Strong, S.; McNair, A.; Brookes, S.T.; Crosby, T.; Griffin, S.M.; Blazeby, J.M. Reporting of Short-Term Clinical Outcomes After Esophagectomy A Systematic Review. Ann. Surg. 2012, 255, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.; Embleton, N.; van Goudoever, J.B.; Hay, W.W.; Bloomfield, F.H. Comparing apples with apples: It is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016, 79, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.L.; Kirkham, J.J.; Clarke, M.; Williamson, P.R. Assessing the impact of a research funder’s recommendation to consider core outcome sets. PLoS ONE 2019, 14, e0222418. [Google Scholar] [CrossRef]
- Akinremi, A.; Turnbull, A.E.; Chessare, C.M.; Bingham, C.O.; Needham, D.M.; Dinglas, V.D. Delphi panelists for a core outcome set project suggested both new and existing dissemination strategies that were feasibly implemented by a research infrastructure project. J. Clin. Epidemiol. 2019, 114, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Garritty, C.; Gartlehner, G.; Kamel, C.; King, V.J.; Nussbaumer-Streit, B.; Stevens, A. Interim Guidance from the Cochrane Rapid Reviews Methods Groups. 2020. Available online: https://methods.cochrane.org/rapidreviews/sites/methods.cochrane.org.rapidreviews/files/public/uploads/cochrane_rr_-_guidance-23mar2020-final.pdf (accessed on 28 August 2021).
- Tricco, A.C.; Antony, J.; Zarin, W.; Strifler, L.; Ghassemi, M.; Ivory, J.; Perrier, L.; Hutton, B.; Moher, D.; Straus, S.E. A scoping review of rapid review methods. BMC Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]


| Author | Year of Publication | Country of Publication | Chronic Disease (1) | Chronic Disease (2) | Dietary Intervention | Duration of Intervention |
|---|---|---|---|---|---|---|
| Abd El-Kader [34] | 2016 | Saudi Arabia | NASH | Obesity | Energy restricted weight loss diet, standard macronutrient distribution | 3 months |
| Abd El-Kader [35] | 2018 | Saudi Arabia | T2DM | Obesity | Energy restricted weight loss diet, standard macronutrient distribution | 12 weeks |
| Abed [36] | 2013 | Australia | AF | Obesity | Very low energy diet with exercise | 8-week diet and 15-month follow-up |
| Aller [37] | 2014 | Spain | NAFLD | Obesity | Diet enriched with MUFA vs. diet enriched with PUFA | 3 months |
| Brown [38] | 2020 | United Kingdom | T2DM | Obesity | Low energy diet with liquid total diet replacement formula | 3 months |
| Campos [39] | 2012 | Brazil | NAFLD | Obesity | Multidisciplinary weight loss program | 12 months |
| Castelnuvo [40] | 2011 | Italy | T2DM | Obesity | Multidisciplinary weight loss program | 4 weeks |
| Chan [41] | 2018 | Hong Kong | NAFLD | Obesity | Dietitian led lifestyle modification program | 16 weeks, with 52-week maintenance |
| Ciarambino [42] | 2011 | Italy | T2DM | CKD | Low protein diet | 4 weeks |
| Corley [43] | 2018 | New Zealand | T2DM | Obesity | Two consecutive days of VLED (2:5 pattern) | 4 weeks |
| Daniels [44] | 2014 | United Kingdom | T2DM | Obesity | Diet high in fruit and vegetables | 8 weeks + 4-week run-in period |
| Gallagher [45] | 2012 | Australia | T2DM/CHD | Obesity | Group multidisciplinary weight loss program | 16 weeks |
| Goldstein [46] | 2011 | Israel | T2DM | Obesity | Atkins diet | 52 weeks |
| Holland-Carter [47] | 2017 | United States of America | T2DM | Obesity | Weight watchers weight loss program | 52 weeks |
| Howden [48] | 2013 | Australia | CKD (St3-4) | MetS component | Multidisciplinary lifestyle modification program | 52 weeks |
| Iqbal [49] | 2010 | United States | T2DM | Obesity | Low carbohydrate vs. low-fat diet | 24 months |
| Jonsson [50] | 2010 | Sweden | IHD | Obesity | Paleolithic diet | 12 weeks |
| Jesudason [51] | 2013 | Australia | T2DM | Obesity/early renal failure | Moderate versus high protein diet | |
| Jung [52] | 2014 | South Korea | T2DM | Hypertension | Korean traditional diet | 12 weeks |
| Karusheva [53] * | 2018 | Germany | T2DM | Obesity | Diet high in cereal fiber and coffee but free of red meat | 8 weeks |
| Khoo [54] | 2011 | Australia | T2DM | Obesity | Energy restricted diet versus high protein, low carbohydrate | 8 weeks with 12-month follow-up |
| Kim [55] | 2011 | South Korea | T2DM | Metabolic syndrome | CVD risk reduction program | 16 weeks |
| Kitzman [56] | 2016 | Germany | Heart failure | Obesity | Energy-restricted diet high in fiber and coffee, but free of red meat | 8 weeks |
| Krebs [57] | 2012 | New Zealand | T2DM | Obesity | Low fat, high-protein diet | 24 months |
| Luger [58] | 2013 | Austria | T2DM | Obesity | High protein diet | 12 weeks |
| Lynch [59] | 2014 | United States of America | T2DM | Hypertension | Dietitian led intensive, group-based diabetes self-management classes | 6 months |
| Martin Alejandre [60] | 2019 | Spain | NAFLD | Obesity | Energy-restricted diet with high adherence to Mediterranean diet | 6 months |
| Mayer [61] | 2014 | United States of America | T2DM | Obesity | Low-carbohydrate diet | 48 weeks |
| Mekki [62] | 2010 | Algeria | CKD | Dyslipidemia/hypertriglyceridemia/hypercholesterolemia | Mediterranean diet | 90 days |
| Mollentze [63] | 2019 | South Africa | T2DM | Obesity | Energy-restricted, low-fat diet | 6 months |
| Morris [64] | 2020 | United Kingdom | T2DM | Obesity | Energy-restricted, low-carbohydrate diet | 12 weeks |
| Nowotny [65] * | 2015 | Germany | T2DM | Obesity | Energy-restricted diet high in fibre and coffee, but low in red meat | 8 weeks |
| Orazio [66] | 2011 | Australia | Renal transplant | Impaired glucose tolerance | Energy-restricted Mediterranean-style, low GI diet. | 2 years |
| Oshakbayev [67] | 2019 | Kazakhstan | T2DM | NAFLD | VLED | 24 weeks with 24 week follow up |
| Papakonstantinou [68] | 2010 | Greece | T2DM | Obesity | Energy-restricted, high-protein low-fat diet | 4 weeks for each diet, with 3-week washout |
| Patil [69] | 2013 | India | T2DM | Nephropathy | Energy-restricted weight-loss diet | 6 months |
| Paula [70] | 2015 | Brazil | T2DM | Hypertension | DASH diet | 4 weeks |
| Raygan [71] | 2016 | Iran | T2DM | CHD | Energy-restricted high- versus low-carbohydrate diet | 8 weeks |
| Ryan [72] | 2013 | Australia | NAFLD | Metabolic syndrome | Mediterranean diet | 2 × 6-week diet periods, plus 6-week washout |
| Schulte [73] | 2020 | United States of America | T2DM | Obesity | Weight Watchers diet | 12 months |
| Sixt [74] | 2010 | Germany | T2DM | CAD | Energy-restricted heart-healthy diet | 4 weeks inpatient, plus 5 months outpatient |
| Utari [75] | 2019 | Indonesia | NAFLD | Obesity | Energy-restricted, low-fat, low-GI diet | 12 weeks |
| von Haehling [76] | 2013 | Germany | CAD | Metabolic syndrome | Tibetan diet | 12 months |
| Wing [77] | 2013 | United States of America | T2DM | Metabolic syndrome | Lifestyle modification program | Median follow-up 9.6 years |
| Zeigler [78] * | 2015 | Germany | T2DM | Obesity | Energy-restricted diet high in fiber and coffee but low in red meat | 8 weeks |
| Author, Date | Date | Database | Clinical Area | Study Type | Methods | Treatment Approaches | Target Population |
|---|---|---|---|---|---|---|---|
| ICHOM [80] | 2017 | ICHOM | Chronic kidney disease | COS standard set | Systematic review, multiple modified Delphi surveys, stakeholder consultation, open review | Pre-RRT patients, HD patients, PD patients, transplant patients, conservative care patients | Stage 3a to Stage 5 CKD |
| ICHOM [79] | 2018 | ICHOM | Diabetes mellitus, type 1 and type 2 | COS standard set | Non-pharmacological therapy, non-insulin-based pharmacological therapy, insulin-based pharmacological therapy | Adults | |
| ICHOM [81] | 2015 | ICHOM | Coronary artery disease | COS standard set | Lifestyle modification, drug therapy, percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) | Asymptomatic coronary artery disease, stable angina, acute coronary syndrome (including acute myocardial infarction) | |
| ICHOM [82] | 2016 | ICHOM | Heart failure | COS standard set | Pharmacotherapy, intensive therapy, rehabilitation | Not further defined | |
| ICHOM [83] | 2019 | ICHOM | Atrial fibrillation | COS standard set | Management of cardiovascular risk factors, pharmacological management, non-pharmacological management | Not further defined | |
| Harman et al. [4] | 2019 | COMET | Diabetes mellitus, Type 2 | COS for clinical trials or clinical research | Online Delphi survey, face to face consensus meeting | Glucose lowering interventions | Not further defined |
| Sanyal et al. [84] | 2011 | COMET | NASH and NAFLD | Classified as COS for clinical trials or clinical research however not explicitly labelled as a COS | Summary of a 2009 workshop on endpoints in NASH | Not stated | Not further defined |
| COMET Core Area | Outcomes | ICHOM DM [79] | COMET T2DM [4] | ICHOM CKD [80] | ICHOM CAD [81] | ICHOM HF [82] | ICHOM AF [83] | COMET NASH/ NAFLD [84] |
|---|---|---|---|---|---|---|---|---|
| Death | Non-specific death outcomes | ✔□ | ✔□ | ✔□ | ✔□ | ✔□ | ✔□ | |
| Disease specific mortality | ✔□ | |||||||
| Physiological/Clinical | Cardiovascular event outcomes | ✔□ | ✔□ | ✔□ | ✔□ | |||
| Cerebrovascular outcomes | ✔□ | ✔□ | ✔□ | |||||
| Renal outcomes | ✔□ | ✔□ | ||||||
| Glycaemic outcomes | ✔□ | ✔□ | ✔□ | |||||
| Diabetes events outcomes | ✔□ | ✔□ | ✔□ | ✔□ | ||||
| Symptom control outcomes | ✔□ | ✔□ | ✔□ | ✔□ | ||||
| Hepatic outcomes | ✔□ | |||||||
| Dietary outcomes | ✔□ | |||||||
| Body composition outcomes | ✔□ | ✔□ | ||||||
| Physical activity outcomes | ✔□ | |||||||
| Oxidative stress outcomes | ✔□ | |||||||
| Lipid profile outcomes | ✔□ | |||||||
| Life Impact | Physical function outcomes | ✔□ | ✔□ | ✔□ | ✔□ | ✔□ | ||
| Role outcomes | ✔□ | |||||||
| Emotional wellbeing outcomes | ✔□ | ✔□ | ✔□ | |||||
| Quality of life | ✔□ | ✔□ | ✔□ | ✔□ | ||||
| Resource Use | Economic outcomes | ✔□ | ||||||
| Health care use outcomes | ✔□ | ✔□ | ✔□ | ✔□ | ✔□ | |||
| Further intervention | ✔□ | ✔□ | ||||||
| Adverse Events | Adverse outcomes | ✔□ | ✔□ | ✔□ | ✔□ | |||
| CV related adverse outcomes | ✔□ | ✔□ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, S.A.; Angel, C.A.; Campbell, K.L.; Hickman, I.J.; MacLaughlin, H.L. Standardised Outcome Reporting for the Nutrition Management of Complex Chronic Disease: A Rapid Review. Nutrients 2021, 13, 3388. https://doi.org/10.3390/nu13103388
Sandhu SA, Angel CA, Campbell KL, Hickman IJ, MacLaughlin HL. Standardised Outcome Reporting for the Nutrition Management of Complex Chronic Disease: A Rapid Review. Nutrients. 2021; 13(10):3388. https://doi.org/10.3390/nu13103388
Chicago/Turabian StyleSandhu, Savita A, Chloe A Angel, Katrina L Campbell, Ingrid J Hickman, and Helen L MacLaughlin. 2021. "Standardised Outcome Reporting for the Nutrition Management of Complex Chronic Disease: A Rapid Review" Nutrients 13, no. 10: 3388. https://doi.org/10.3390/nu13103388
APA StyleSandhu, S. A., Angel, C. A., Campbell, K. L., Hickman, I. J., & MacLaughlin, H. L. (2021). Standardised Outcome Reporting for the Nutrition Management of Complex Chronic Disease: A Rapid Review. Nutrients, 13(10), 3388. https://doi.org/10.3390/nu13103388






